To describe the impact of the introduction of the viral tetra vaccine in the National Immunization Program in 2013 for 15-month-old children in mortality rates and hospitalization associated with varicella in Brazil.
MethodsMortality rates and hospitalizations rates associated with varicella were evaluated between 2010 and 2016 and described according to Brazilian macro regions and age. The population was stratified into age groups: < 1year, 1–4 years, and 5–14 years. Data were collected from the Informatics Department of the Unified Health System. A percentage difference was calculated between rates of hospitalizations and mortality in the pre (2010–2012) and post-vaccination periods (2014–2016) to estimate the approximate effectiveness of the vaccine.
Data synthesisAt the national level, vaccination significantly reduced the mortality rates and hospitalizations rates in all age groups analyzed. Among those under 5 years of age, mortality rates and hospitalizations rates decreased 57–49% and 40–47%, respectively. There was a national decrease of up to 57% in the mortality rates due to the disease, with smaller decreases seen in the North and Northeast regions and the largest in the South and Southeast regions. The hospitalizations rates for varicella reached a maximum national decline of 47%. In children aged 1–4 years, with higher vaccination coverage, the highest reduction was observed in both mortality rates and hospitalizations rates, which decreased from 2.6 to 0.4/100,000/year.
ConclusionsThe tetra vaccine proved to be effective in reducing both mortality and hospitalizations of children and adolescents up to 15 years of age in the 2014–2016 triennium.
Descrever o impacto da introdução da vacina tetra viral no Programa Nacional de Imunização em 2013 para crianças de 15 meses nas taxas de mortalidade e de internação hospitalar associadas à varicela no Brasil.
MétodosAs taxas de mortalidade e de internação hospitalar associadas à varicela foram avaliadas entre 2010 e 2016 e descritas conforme macrorregiões brasileiras e idade. A população foi estratificada em grupos etários: <1 ano; 1–4 e 5–14 anos. Os dados foram coletados do Departamento de Informática do Sistema Unificado de Saúde. Foi realizado um cálculo de diferença percentual entre taxas de internações e mortalidade nos períodos pré (2010–2012) e pós-vacinal (2014–2016) para estimativa de impacto da vacina.
ResultadosNo nível nacional, a vacinação reduziu significativamente as taxas de mortalidade e de internação hospitalar em todas faixas etárias analisadas. Entre os menores de 5 anos, a taxas de mortalidade e de internação hospitalar diminuíram 57-49% e 40–47%, respectivamente. Houve uma queda nacional de até 57% nos índices de mortalidade pela doença, com menores quedas vistas nas regiões Norte e Nordeste e as maiores nas regiões Sul e Sudeste. As taxas de internação hospitalar por varicela atingiram queda nacional máxima de 47%. Em crianças de 1–4 anos, com maior cobertura vacinal, foi observada a maior redução tanto na taxa de internação hospitalar como na taxa de mortalidade, a qual passou de 2,6 para 0,4/100.000/ano.
ConclusõesA vacinação se mostrou efetiva em reduzir tanto mortalidade quanto hospitalizações das crianças e adolescentes de até 15 anos no triênio 2014–2016.
The Brazilian National Immunization Program (Programa Nacional de Imunizações [PNI]) is an international reference for public health policy. The country has already eradicated diseases with a worldwide dissemination, such as smallpox and polio, through vaccination. New to the vaccination calendar was the introduction of the varicella zoster virus (VZV) vaccine, which took place in September 2013 as a tetravalent viral vaccine – measles, mumps, rubella, and varicella (MMRV) – for infants at 15 months and more recently, in 2018, the introduction of a second dose of varicella vaccine for children aged 4–6 years.1 Estimates for 2014 show that varicella causes approximately four million hospitalizations for disease-related complications and 4000 deaths worldwide each year.2 In Brazil, data from the Unified Health System indicate varicella as the cause of 1800 deaths between 2003–2013, more than 36% of them among children aged 1–4 years.3
Caused by a DNA virus belonging to the order Herpesvirales, family Herpesviridae, subfamily Alphaherpesvirinae, genus Varicellovirus, and species human alphaherpesvirus 3, varicella is characterized by its high transmissibility and characteristic pruritic rash lesions. Even though its evolution is mostly self-limiting, lasting around one week and evolving with spontaneous cure, it brings risks inherent to viral spread and complications due to secondary skin and soft tissue infections, which can become fatal when they reach joints, bones, lungs, and the neurological system.4
The varicella vaccine was developed in Japan in the 1970s, but it started to be used in most western countries only in the 1990s.5 It induces immunity through the attenuated live virus and its efficacy is high: 70%–90% in preventing any form of varicella and 95%–100% in preventing severe illness.6
The effectiveness of the vaccine introduction has already been demonstrated in several countries around the world, such as Australia and the United States.7 Currently, five years after the introduction of the vaccine in Brazil and one year after the introduction of the second dose, it is in public health interest to verify the disease scenario around the country, aiming to assess its epidemiological alterations and make adjustments to vaccination programs, if necessary. This study aims to compare, at the national and macroregional levels, the frequency of hospitalizations and deaths related to the disease before and after the vaccination introduction.
MethodsData on mortality and hospitalizations related to VZV were collected from the Department of Informatics of the Unified Health System (DataSUS) http://www2.datasus.gov.br/DATASUS/index.php?area=02. the Health Information page, the Mortality section in Vital Statistics was accessed to evaluatevaricella-related deaths, the Hospital Morbidity section in the Epidemiological Information and Morbidity section toevaluate VZV-related hospitalizations, and the Health Care Immunizations section for vaccination coverage data collection. Varicella-related deaths were verified according to the International Classification of Diseases, 10th Revision (ICD-10). Only deaths caused by varicella zoster (B01) were considered in the calculation of mortality, due to the low prevalence of herpes zoster in the studied age group.
Attention was focused on the three age groups (<1, 1–4, 5–14 years) of greatest interest for vaccine impact assessment in the short term. The mortality rate (MR) for varicella was calculated by dividing the number of deaths from varicella by the population in each age group and then multiplied by 100,000. The same calculation was applied to determine the hospitalization rate (HR), which was calculated by dividing the number of hospitalizations from varicella by the population in each age group, and then multiplied by 10,000.
The size of the population used in the denominators was obtained from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística [IBGE]) based on the 2010 census and 2011–2016 population estimates. There was an irregular loss of mortality data due to lack of state notification in all assessed years, with the states of Rondônia (RO), Roraima (RR), Sergipe (SE), Tocantins (TO), Acre (AC), and MatoGrosso do Sul (MS) being the most affected.
The evolution of annual rates by age group and by Brazilian macro regions was shown graphically. The impact of vaccination was calculated as the percentage difference in pre- and post-vaccination rates. The statistical analysis included absolute numbers and rates by region and age group. The rates and their 95% confidence intervals were calculated assuming the Poisson distribution of outcomes (deaths and hospitalizations). Hierarchical Poisson regression was used, which took into account the possible random effects of the impact of vaccination between the states of the same macroregion and the correlation (intraclass) between the successive annual rates of each state. All analyses were performed using Stata statistical software (Stata Statistical Software: Release 12. College Station, TX, USA).
As this study used a public domain secondary data source and did not involve human subjects, it was not necessary to obtain approval from the ethics committee.
ResultsIn 2012, the year before the vaccine introduction, 967 hospitalizations for varicella were recorded in children under 1year in the country. In the same year, complications related to varicella were reported to be the cause of death of 26 patients. In 2016, the last year analyzed, 597 hospitalizations and eight deaths were recorded.
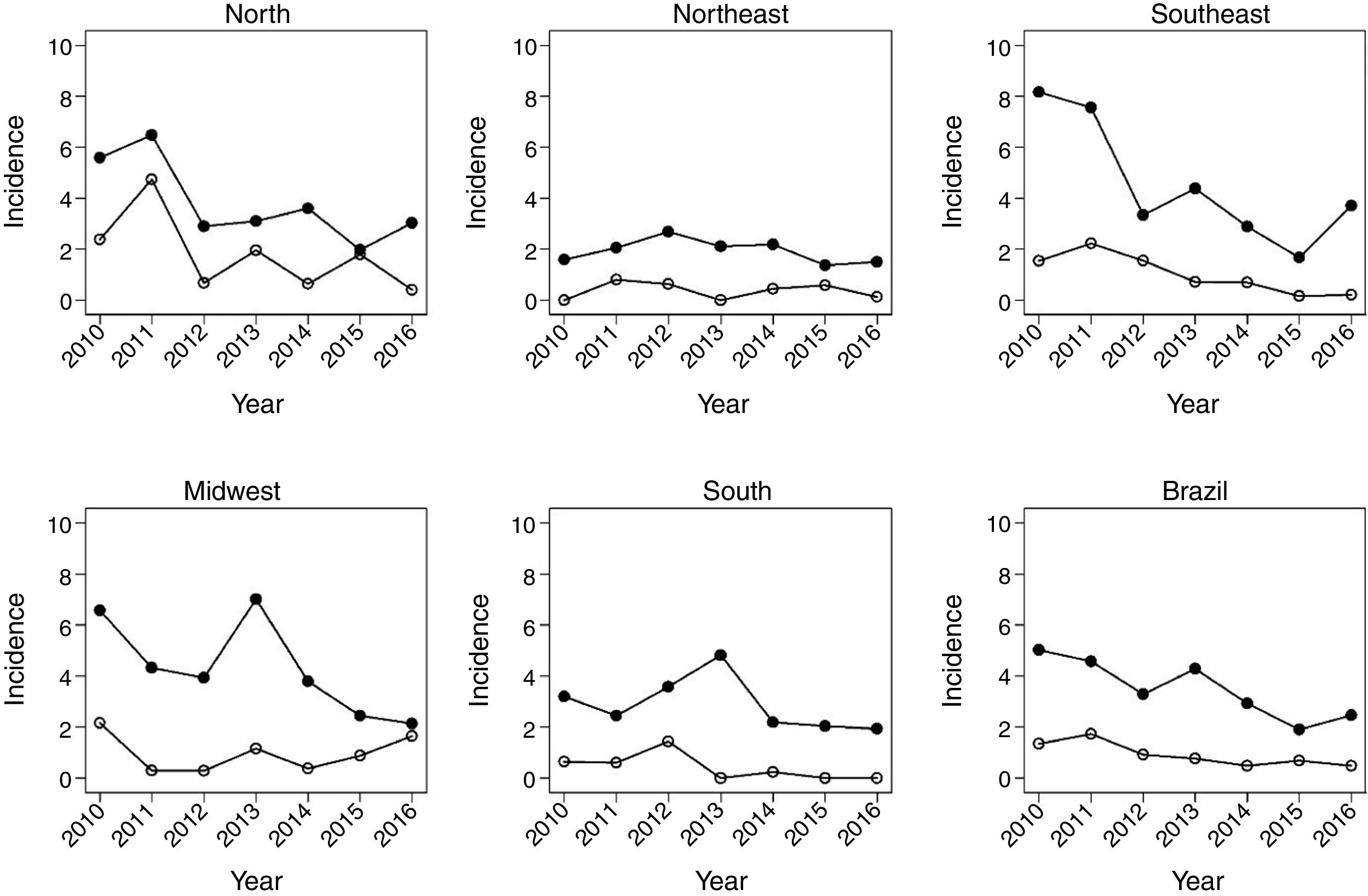
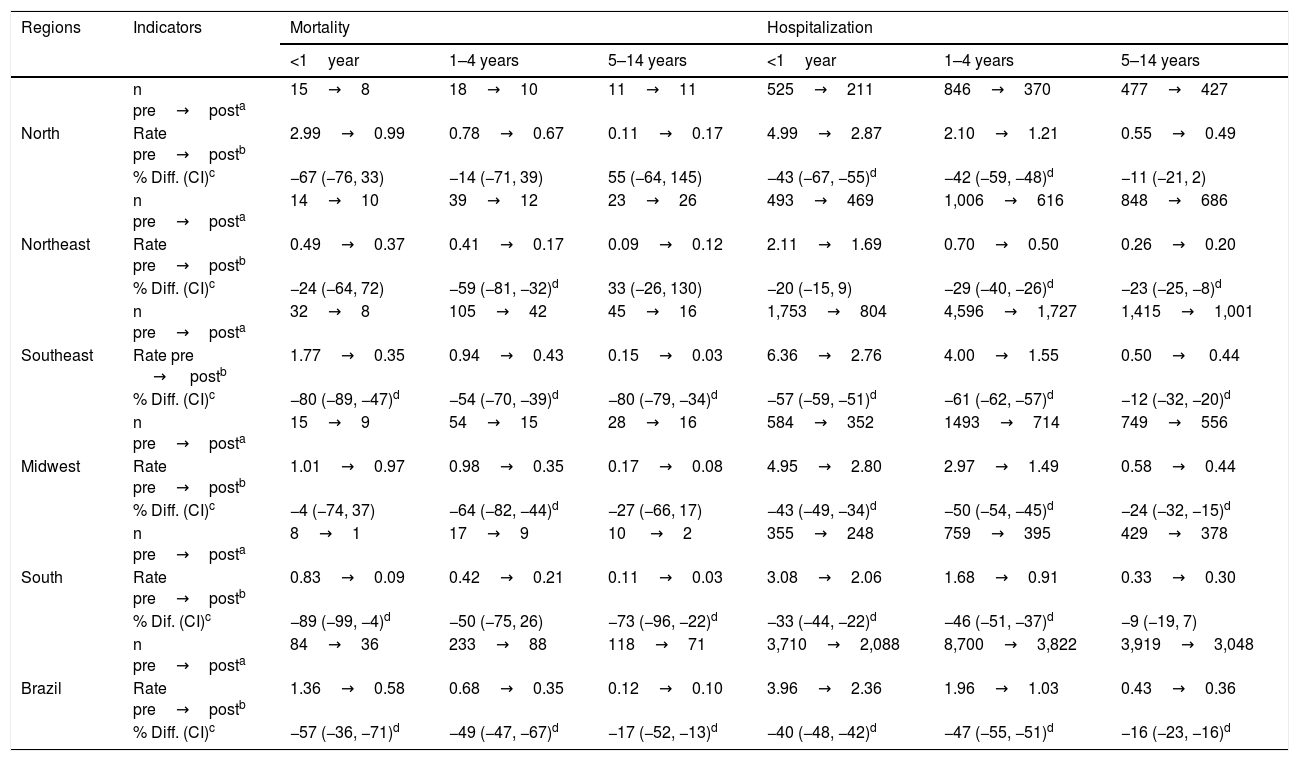
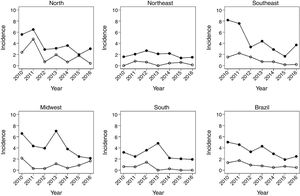
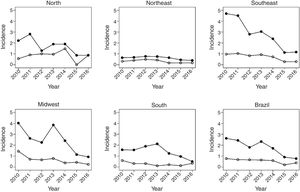
Fig. 1 shows the evolution of rates in children under 1year, between 2010 and 2016. There was a decrease in HR in all regions of the country. In Brazil, the HR decreased from 3.6 in 2012 to 2.5 in 2016. There was also a significant reduction in mortality due to the disease in the same age group. The most significant decrease occurred in the North region, followed by the Southeast.
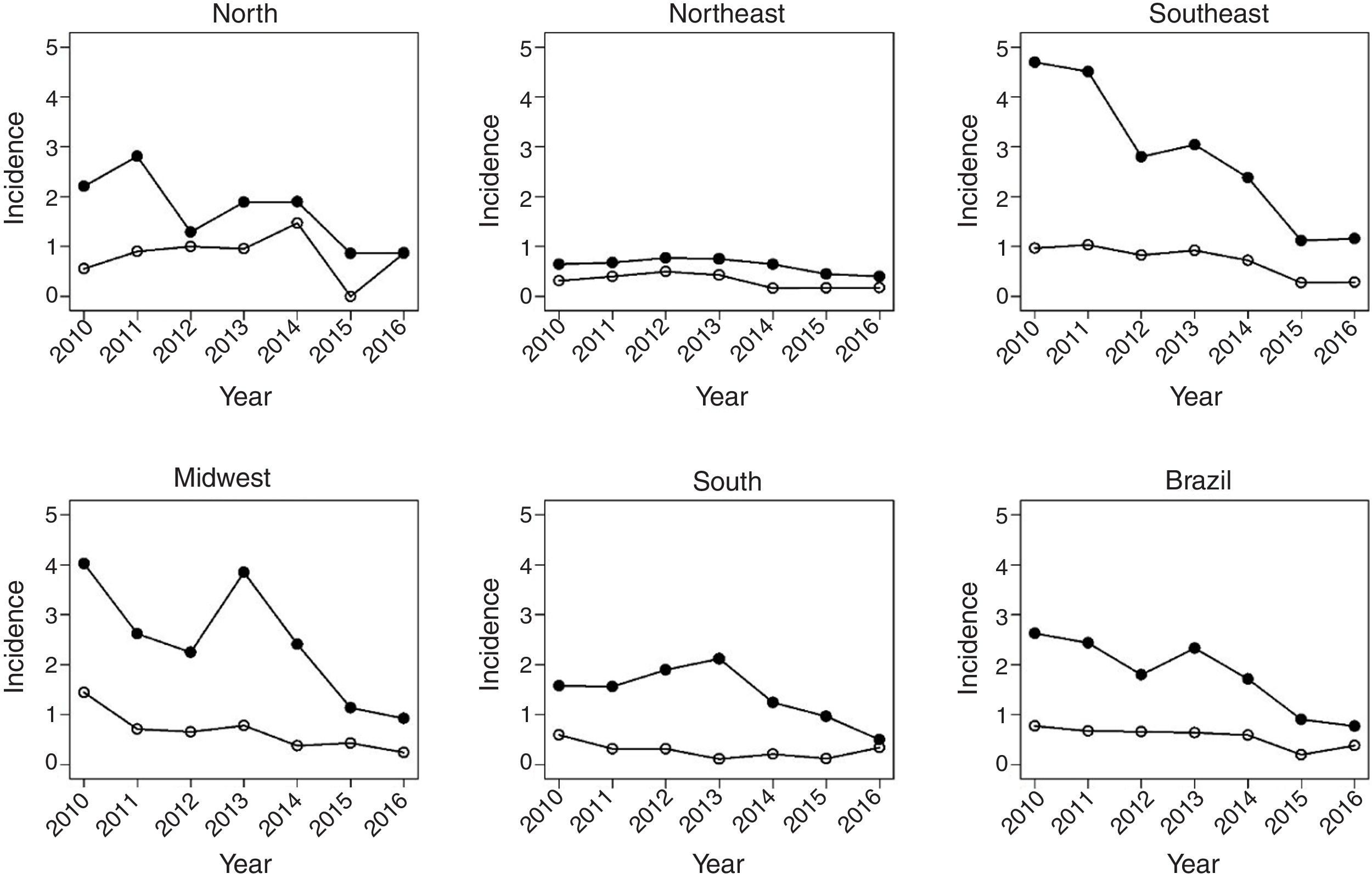
Fig. 2 shows the same temporal analysis for the age group from 1 to 4 years, the largest vaccination coverage group, which shows a greater decrease in hospitalization rates in the Southeast, from 2.8 in 2012 to 1.4 in 2016, and in the Midwest, from 2.3 to 1.2. Regarding deaths in this age group, there was also a decrease in mortality in the national scenario, from 0.9 deaths in 2010 to 0.4 per 100,000 children in 2016.
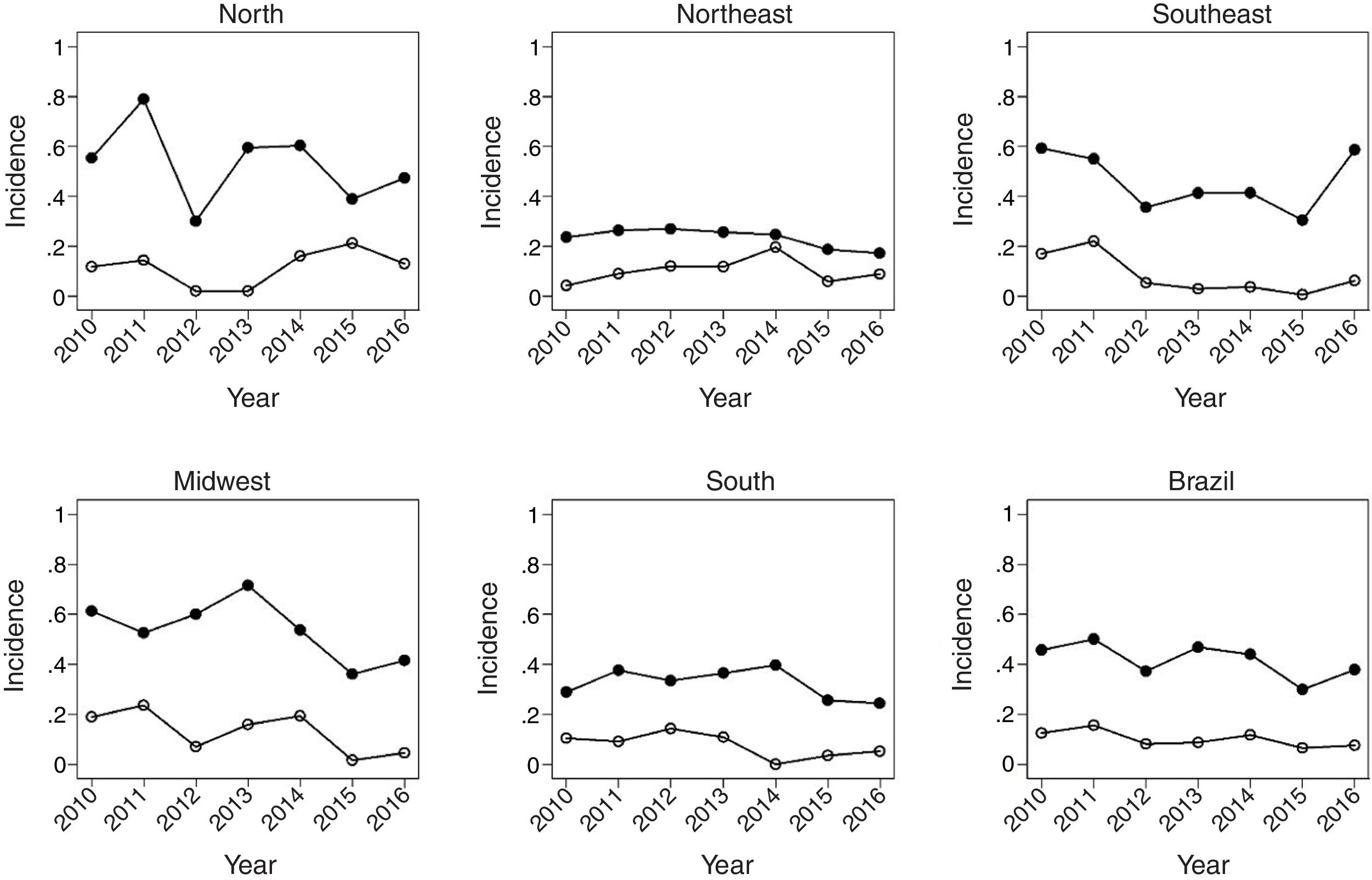
Fig. 3 covers the largest age group of the study, 5–14 years, showing a greater variation of incidence over the studied years. The Southeast region maintained the same MR in the years 2010 and 2016, after a sudden increase between 2015 and 2016 in relation to the previous post-vaccination years. Mortality showed a reduction in all regions, being more pronounced in the Midwest region.
Table 1 shows that, at the national level, varicella vaccination significantly reduced mortality and hospitalization rates in all analyzed age groups, although this effect was smaller in the age group of 5–14 years (MR, 17%; HR, 16%). In children under 5 years of age, there was a 57% decrease in MR in infants and a 49% decrease in children aged 1–4 years, while the decrease in hospitalizations was 40% and 47%, respectively.
Percentage difference in mortality and hospitalization rates during the period of three years after (2014–2016) vs. three years before (2010–2012) the introduction of varicella vaccination by age group.
| Regions | Indicators | Mortality | Hospitalization | ||||
|---|---|---|---|---|---|---|---|
| <1year | 1–4 years | 5–14 years | <1year | 1–4 years | 5–14 years | ||
| North | n pre→posta | 15→8 | 18→10 | 11→11 | 525→211 | 846→370 | 477→427 |
| Rate pre→postb | 2.99→0.99 | 0.78→0.67 | 0.11→0.17 | 4.99→2.87 | 2.10→1.21 | 0.55→0.49 | |
| % Diff. (CI)c | −67 (−76, 33) | −14 (−71, 39) | 55 (−64, 145) | −43 (−67, −55)d | −42 (−59, −48)d | −11 (−21, 2) | |
| Northeast | n pre→posta | 14→10 | 39→12 | 23→26 | 493→469 | 1,006→616 | 848→686 |
| Rate pre→postb | 0.49→0.37 | 0.41→0.17 | 0.09→0.12 | 2.11→1.69 | 0.70→0.50 | 0.26→0.20 | |
| % Diff. (CI)c | −24 (−64, 72) | −59 (−81, −32)d | 33 (−26, 130) | −20 (−15, 9) | −29 (−40, −26)d | −23 (−25, −8)d | |
| Southeast | n pre→posta | 32→8 | 105→42 | 45→16 | 1,753→804 | 4,596→1,727 | 1,415→1,001 |
| Rate pre → postb | 1.77→0.35 | 0.94→0.43 | 0.15→0.03 | 6.36→2.76 | 4.00→1.55 | 0.50→ 0.44 | |
| % Diff. (CI)c | −80 (−89, −47)d | −54 (−70, −39)d | −80 (−79, −34)d | −57 (−59, −51)d | −61 (−62, −57)d | −12 (−32, −20)d | |
| Midwest | n pre→posta | 15→9 | 54→15 | 28→16 | 584→352 | 1493→714 | 749→556 |
| Rate pre→postb | 1.01→0.97 | 0.98→0.35 | 0.17→0.08 | 4.95→2.80 | 2.97→1.49 | 0.58→0.44 | |
| % Diff. (CI)c | −4 (−74, 37) | −64 (−82, −44)d | −27 (−66, 17) | −43 (−49, −34)d | −50 (−54, −45)d | −24 (−32, −15)d | |
| South | n pre→posta | 8→1 | 17→9 | 10 →2 | 355→248 | 759→395 | 429→378 |
| Rate pre→postb | 0.83→0.09 | 0.42→0.21 | 0.11→0.03 | 3.08→2.06 | 1.68→0.91 | 0.33→0.30 | |
| % Dif. (CI)c | −89 (−99, −4)d | −50 (−75, 26) | −73 (−96, −22)d | −33 (−44, −22)d | −46 (−51, −37)d | −9 (−19, 7) | |
| Brazil | n pre→posta | 84→36 | 233→88 | 118→71 | 3,710→2,088 | 8,700→3,822 | 3,919→3,048 |
| Rate pre→postb | 1.36→0.58 | 0.68→0.35 | 0.12→0.10 | 3.96→2.36 | 1.96→1.03 | 0.43→0.36 | |
| % Diff. (CI)c | −57 (−36, −71)d | −49 (−47, −67)d | −17 (−52, −13)d | −40 (−48, −42)d | −47 (−55, −51)d | −16 (−23, −16)d | |
The impact of vaccination was heterogeneous across the country's macro regions. Regarding mortality, the statistically significant decrease among children under 1year was 80% in the Southeast and 89% in the South. As for children aged 1–4 years, the decrease was 54% in the Southeast, 59% in the Northeast, and 64% in the Midwest, with vaccine coverage of 87%, 79%, and 86%, respectively. In the age group of 5–14 years, there was a statistically significant decrease only in the South (73%) and Southeast (80 %) regions.
The reduction in the HR ranged from 33% in the South to 57% in the Southeast in children under 1year. In children aged 1–4 years, a significant reduction was observed in all regions, ranging from 29% in the Northeast to 61% in the Southeast, with average vaccine coverage of 78% and 87%, respectively. In the Northeast there was a 23 % reduction in HR in children aged 5–14 years, whereas in the Southeast the decrease was 12% in this age group. The Southeast region showed the highest consistency and magnitude of reduction in mortality and hospitalization rates in the analyzed age groups. The South region had the lowest number of deaths and hospitalizations, probably associated with its vaccination coverage rate of almost 86%. At the same time, the low number of events contributed to the increase in the variation of estimates for this region due to statistical reasons.
At the national level, none of the age groups showed a value of zero in the 95% confidence interval (95% CI). Therefore, there was a significant reduction in the percentage difference after vaccination when compared to the pre-vaccination period for MR and HR.
DiscussionThis study showed a significant reduction in varicella morbidity and mortality in Brazil after the introduction of the tetra viral vaccine into the PNI. It is noteworthy that, overall, there was a more significant decrease in the MR compared to HR from varicella. This may be due to factors such as improved hospital management of VZV infection complications and the reduction of infection in groups at higher risk of death such as infants, immune deficient individuals, and adolescents.
Although the MMRV vaccine was only provided for children at 15 months of age, there was a reduction in hospitalization and mortality rates in all studied age groups. The most significant reduction in hospitalization was generally in the age group of 1–4 years, which is in agreement with the expectations, as this is the vaccination target population.8 The short post-vaccination period studied may be underestimating the indirect effect provided by the vaccination in the long-term. However, doubts remain as to whether a single dose of the vaccine is enough to generate group immunity.9
The data also show the maintenance of a higher absolute number of severe cases in infants <1year when compared to other age groups, probably due to the low concentration of maternal antibodies against the VZV and a still-maturing immune system.10,11 However, there was a significant reduction in mortality and hospitalizations in this group, a probable consequence of indirect (herd) protection. Nevertheless, it is important to emphasize that the cohort of immunized children tends to increase progressively over the years, which will lead to a lower virus circulation, enhancing the herd immunity effect.12
In the age group between 5–14 years, there was a less marked decrease in the rates. This may be due, in large part, to the fact that they are not the directly immunized population, due to their greater time spent in closed environments (day care centers and schools) and the tendency of more severe cases of the disease to occur as they become older.13,14
Two studies conducted in the United States between two and four years after the introduction of the VZV vaccine in the country found little significant results regarding the decrease in the incidence of hospitalizations. The data released by Rhein et al., related to the first two years after the vaccination, showed no decrease in hospitalization rates. However, the second study, analyzing the four years after the vaccine introduction, showed a 23.5% decrease in hospitalizations from varicella in the country. The gradual improvement was attributed to the increase in the country’s immunization coverage, from 43.2% to 59% over the studied years. However, in 2004, Davis et al. highlighted methodological errors in the abovementioned studies. In a new analysis, they observed a 74% decrease in HR over the nine assessed years.15–17
In an Australian national survey that compared the previous three years with the five years after the national introduction of the vaccine against VZV, a 57.3% decrease in varicella-related HR was observed in the general population, with a steeper decline (from 83.3 to 29.3/100,000/year) in children aged 1–4 years, with vaccination coverage ranging from 82% to 90% during the study period.18
A systematic review published in 2019, which included studies from Latin American and Caribbean countries, showed, among other data, a significant reduction in HR of 87% in children under 5 years old after the vaccine’s introduction in Costa Rica, as well as in Uruguay (81%) and Puerto Rico (76%). In Costa Rica there was also an increase, from 23 to 24, in the absolute number of deaths between the pre- and post vaccination periods.19
After the pioneering introduction of varicella vaccination in the city of Florianópolis, state of Santa Catarina, Brazil, in 2002, a reduction in the incidence of varicella in children aged 1–4 years was observed, with no evidence of an associated indirect effect.20 The present study observed that in the first three years after the implementation of the universal vaccination in children aged 15 months, there was a 16% decrease in HR in children older than 5 years and in Brazilian adolescents, a probable consequence of the indirect effect of vaccination coverage, which reached an average of 82% in the studied period.21
It was also observed, in the age groups <1year and 5–14 years, an increase in HR between the years 2015 and 2016; this increased incidence is due to the decline in vaccination coverage in 2015, reflecting the lack of vaccines in some national regions in the aforementioned years and possibly from the increasing antivaccine movement.22,23
The present study shows that the impact of vaccination was heterogeneous and, therefore, the national average, described by Scotta et al. in 2018, is not enough to elucidate the evolution of morbidity and mortality in the analyzed period. The five regions showed different epidemiological patterns of decrease. For instance, the Southeast and South regions showed a greater reduction in mortality in the population aged less than 1year, while the North region showed a significant decrease in HR when compared even with the South region. This could be explained by the different socioeconomic characteristics of the studied regions, such as the human development index, access to private health services, and vaccination coverage of the regions.24,25
Another important divergence found between the present study and the 2018 study is the pre-vaccination period studied. The eleven-year period used by Scotta et al. is long enough to reflect non-vaccine-related causes of hospitalization, such as improved access to the public health system. The same effect is dramatically reduced with the three-year pre-vaccination period used in the present study. Moreover, the study included 2013 in the post-vaccination period analysis despite its atypical characteristics, such as low vaccination coverage (<50%), which was probably insufficient to produce a significant effect.
Additionally, the study by Scotta et al. used a time series with monthly VZV hospitalization rate data, and the difference between the mean pre- and post-vaccination rates was calculated using Student’s t-test for independent samples, although this assumption is unreliable, since the circulation of the virus in the previous months was the main determinant of the infection risk in the period. The present study used hierarchical (multilevel) Poisson regression to account for this temporal dependence through an interclass correlation within the repeated measures in the same federative unit each year, and within the units of the same macro region of the country. Poisson regression allows making robust estimates of the standard error.
The present study has some limitations because it is a retrospective ecological study, based on secondary data, making it difficult to assess the quality of the data obtained. Additionally, the diagnosis of varicella is predominantly clinical, so it is possible that the disease is underdiagnosed and underreported, mainly in regions of the country where access to health services is still precarious. It is also emphasized that the short post-intervention period available for analysis reduces the statistical analysis power. Further studies with longer post-vaccination periods may more accurately show the effectiveness of vaccination in reducing hospitalizations and mortality from varicella in the medium and long term.
It is vital to point out that time series with before-and-after comparisons presuppose homogeneity between the groups compared in the two periods. The reduction in the rates demonstrated here is unlikely to result exclusively from increased access to health services and improved socioeconomic status of the studied populations.20 Finally, it is noteworthy that effective treatment for varicella has not changed significantly after the introduction of the VZV vaccine and, therefore, it would not explain the magnitude of the reduction in mortality and hospitalizations from the disease observed in the present study.4
In conclusion, the present study demonstrated a significant reduction in mortality and hospitalization rates in children and adolescents up to 14 years of age after the introduction of varicella vaccine into the PNI in 2013. However, the figures are still below those observed in countries with longer vaccination adherence time and vaccination programs that include a second dose of the vaccine. Epidemiological follow-up of advances in varicella cases and of Brazilian vaccination coverage is essential to assess the creation of public health policies aimed at maintaining effective vaccination coverage. A new evaluation of vaccine effectiveness after the introduction of the second dose of varicella vaccine in 2018 is also needed, which aims at correcting the failure of the first vaccine dose, preventing outbreaks in schoolchildren, and increasing herd protection.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ribeiro MZ, Kupek E, Ribeiro PV, Pinheiro CE. Impact of the tetra viral vaccine introduction on varicella morbidity and mortality in the Brazilian macro regions. J Pediatr (Rio J). 2020;96:702–9.