To study the microbiological pattern of late onset neonatal sepsis cultures and to assess the diagnostic performance of serum (1,3)-β-d-glucan level for early diagnosis of invasive fungemia in high-risk infants admitted to a neonatal intensive care unit.
MethodsA prospective multicenter clinical trial conducted on infants at high risk for invasive fungal infections, with suspected late onset sepsis, admitted to a neonatal intensive care unit at Mansoura University Children's Hospital and Mansoura General Hospital between March 2014 and February 2016.
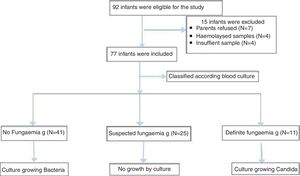
ResultsA total of 77 newborn infants with high risk of invasive fungal infection were classified based on blood culture into three groups: no fungemia (41 neonates with proven bacterial sepsis), suspected fungemia (25 neonates with negative blood culture), and definite fungemia group (11 neonates with culture-proven Candida). The growing organisms were Klebsiella spp. (14/54); Escherichia coli (12/54); Staphylococcus spp. (12/54; coagulase-negative Staphylococcus [9/54]; Staphylococcus aureus [3/54]); Pseudomonas aerouginosa (3/54); and Proteus spp. (2/54). Moreover, 11/54 presented Candida. Serum (1,3)-β-d-glucan concentration was significantly lower in the no fungemia group when compared with the definite fungemia group. The best cut-off value of (1,3)-β-d-glucan was 99pg/mL with sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 63.6%, 95.1%, 77.8%, 90.7%, and 88.5%, respectively.
Conclusion(1,3)-β-d-glucan assay has a limited sensitivity with excellent specificity and negative predictive value, which allow its use as an aid in exclusion of invasive neonatal fungal infection. Accurate diagnosis and therapeutic decisions should be based on combining (1,3)-β-d-glucan assay with other clinical, radiological, and microbiological findings.
Estudar o padrão microbiológico das culturas de sepse neonatal de início tardio e avaliar o desempenho diagnóstico do nível de (1,3)-β-D-glucano no soro para diagnóstico precoce de fungemia invasiva em neonatos de alto risco internados em uma unidade de terapia intensiva neonatal.
MétodosEnsaio clínico multicêntrico prospectivo conduzido em neonatos internados em uma unidade de terapia intensiva neonatal com suspeita de sepse de início tardio que estavam em risco de infecções fúngicas invasivas no hospital universitário infantil de Almançora e no hospital geral de Almançora entre março de 2014 e fevereiro de 2016.
ResultadosForam classificados 77 neonatos recém-nascidos com risco de infecção fúngica invasiva, com base na hemocultura, em: grupo sem fungemia, incluindo 41 neonatos com sepse bacteriana comprovada, grupo com suspeita de fungemia, incluindo 25 neonatos com hemocultura negativa; e grupo com fungemia definida, incluindo 11 neonatos com Candida comprovada por cultura. Os organismos em crescimento foram: {Klebsiella spp 14/54; E. coli 12/54; Staphylococcus spp 12/54 (Staph coagulase negativa 9/54; Staph aureus 3/54); pseudomonous aerouginosa 3/54 e Proteus spp 2/54}, além de 11/54 Candida. A concentração de (1,3)-β-D-glucano no soro foi significativamente inferior no grupo sem fungemia em comparação ao grupo com fungemia definida. O melhor valor de corte da (1,3)-β-D-glucano foi 99 pg/mL com sensibilidade, especificidade, valor preditivo positivo, valor preditivo negativo e precisão de 63,6%, 95,1%, 77,8%, 90,7% e 88,5%, respectivamente.
ConclusãoO ensaio de (1,3)-β-D-glucano possui sensibilidade limitada com especificidade e valor preditivo negativo excelentes que possibilitam seu uso e ajudam na exclusão de infecção fúngica invasiva neonatal. O diagnóstico preciso e as decisões oterapêuticas devem ter como base a combinação di ensaio de (1,3)-β-D-glucano com outros achados clínicos, radiológicos e microbiológicos.
Neonatal sepsis (NS), together with pneumonia and meningitis, account for 1.4 million annual neonatal deaths worldwide.1 Newborn infants are at high risk for infection due to underdevelopment of their immune barriers, including fragile skin and relative immune tolerance.2 Fungal sepsis is a type of late onset sepsis (LOS) and should be considered in neonates with prolonged hospitalization and in those on prolonged antibiotic treatment.3 The incidence of fungal sepsis ranges from 0.4 to 2 cases per 1000 live births, and from 3.8% to 12.9% among very low birth weight infants.4 The most commonly reported risk factors for invasive fungal infection (IFIs) are prematurity, low birth weight, major congenital malformations, exposure to broad spectrum antibiotics, central venous catheters, delayed enteral feeds, prolonged parenteral nutrition, endotracheal intubation, surgery, postnatal steroids, and longer neonatal intensive care unit (NICU) stay.5,6 IFI manifestations are nonspecific, and blood culture is considered the gold standard for its diagnosis.7 Unfortunately, preliminary results of blood cultures are usually obtained after 48h or more. Moreover, diagnosis of IFI in neonatal infants is difficult, due to the high rate of false negative in cultures8; blood cultures are negative in approximately 50% of cases of autopsy-proven disseminated candidiasis.9 Therefore, new tools are required for early diagnosis of IFIs in neonates. 1,3-β-d-Glucan (BG) is a component of the outer cell wall of fungi, including Candida spp., Aspergillus spp., and Pneumocystis jiroveci, and it is released into the blood stream during IFI.10 To date, only three small trials were conducted in neonates; they concluded that BG is a useful adjunctive diagnostic method of IFI.11–13
The authors hypothesized that early assessment of serum BG in neonates with suspected fungal sepsis is a good substitute to fungal blood culture. Accordingly, this study aimed to assess the microbiological pattern of neonatal LOS and the diagnostic performance of BG for early diagnosis of IFI in high-risk infants admitted to NICUs.
Subjects and methodsThis was a prospective multi-center cohort study conducted in NICUs of the Mansoura University Children's Hospital and of the Mansoura General Hospital from March 2014 to February 2016.
EthicsThe Research Ethics Committee of Mansoura Faculty of Medicine approved the study, and written informed consents were obtained from the parents of all neonates included in the study.
Included subjectsNeonates with clinically suspected LOS (sepsis after 72h from birth) who were at high risk of IFI were included. Clinical manifestations suggestive of neonatal sepsis were defined in accordance with the Brazilian National Health Surveillance Agency (ANVISA) criteria, in which neonatal sepsis was defined as a systemic response, without any other recognized cause than infection, associated with at least two or more of the following signs and symptoms: thermal instability, apnea, worsening of respiratory discomfort, hemodynamic instability, bradycardia, feeding intolerance, glucose intolerance, hypoactivity, and lethargy.14
Patients were considered at high risk for IFI if they had three or more of the following criteria: Low birth weight (<2500g), hospitalization for >3 weeks, prolonged mechanical ventilation (>1 week), systemic antibiotic exposure >72h, postoperative patients, abdominal wall defects, central venous catheter, arterial catheter >72h, total parenteral nutrition administration, and persistent severe thrombocytopenia despite second line antibiotics administration.13
Excluded subjectsNeonates who received systemic antifungal drugs (prophylactic or therapeutic), intravenous immunoglobulin, albumin, plasma protein, and amoxicillin-clavulanic acid antibiotic were excluded from the study.
All recruited infants underwent LOS investigations: complete blood count, total and differential; quantitative C-reactive protein (CRP); and full septic workup, including blood, urine, CSF cultures, as well as serum BG level. Other laboratory and radiological investigations were performed according to the decision of the attending physician.
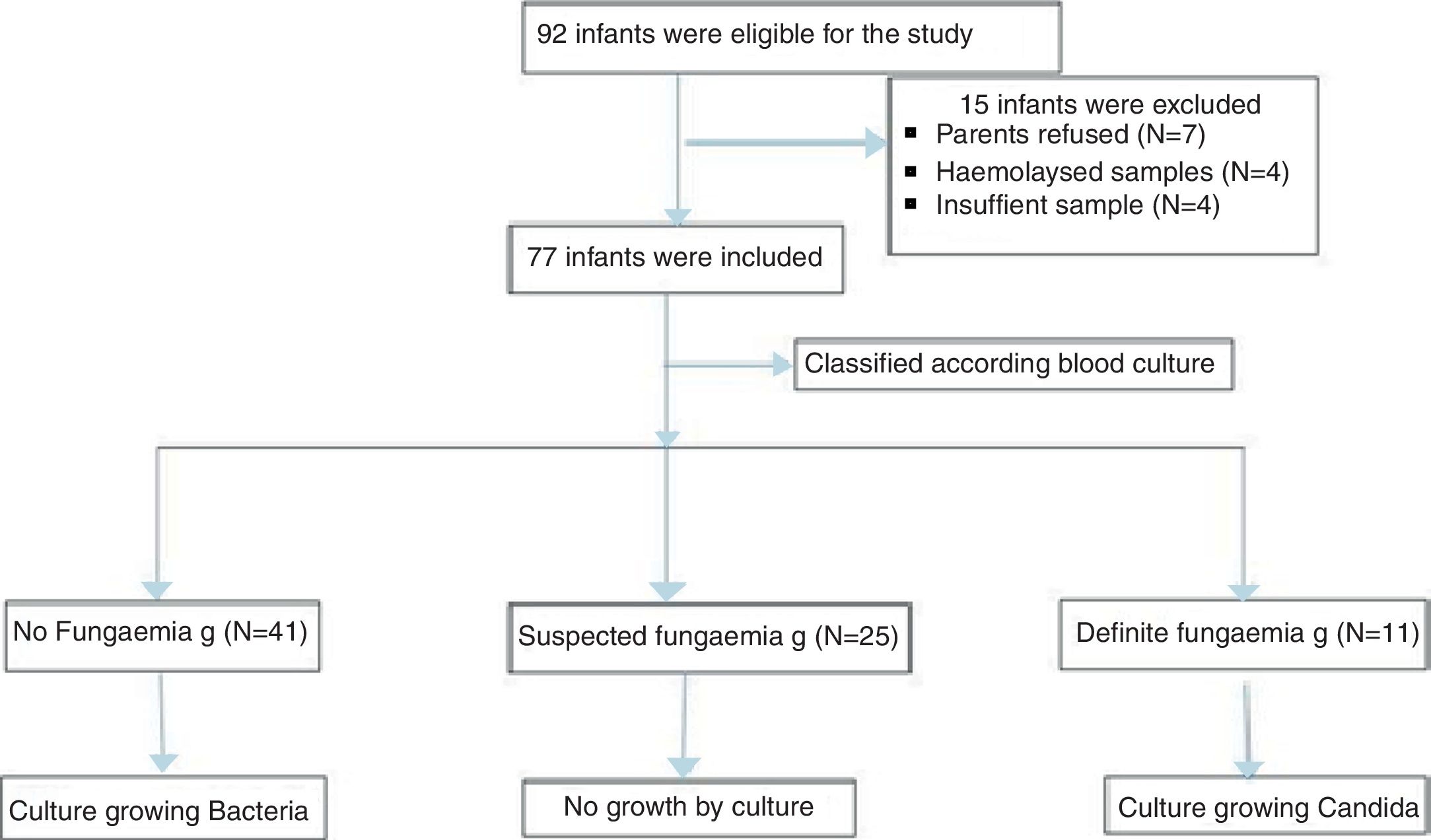
Neonates included were categorized according to their blood culture results into three groups. The no fungemia group included 41 neonates with blood culture-proven bacterial sepsis. The definite fungemia group included 11 neonates with blood culture-proven fungemia. Finally, the suspected fungemia group included 25 neonates with negative blood culture for bacteria and fungi, but at high risk of IFI with abnormal white blood cells (WBCs), low platelets, or elevated CRP.
Laboratory workupAll infants included in the study underwent full septic workup, as well as BG assay, within 24h of recruitment. A total of 3mL of blood were collected; 2mL were used for blood culture using blood culture vials (BD BactecPedsPlus™/F culture vials; Becton Dickinson, Maryland, USA) and the other 2mL were used for complete blood count (CBC), CRP, and BG analysis.
MethodsBlood cultureAn automated continuous-monitoring blood culture system, BD BACTEC™ FX 40 (Becton Dickinson Maryland, USA) was used. Bottles were incubated for five days before being discarded as negative; however, they were sub-cultured according to the laboratory operating procedure if they were flagged as positive before this time. Bacterial isolates were identified through colonies morphology, haemolysis on blood agar, Gram-stained smears, catalase test, coagulase test, and biochemical reactions.
CRP was quantified using latex-enhanced turbid metric assay. The turbidity is measured at 630nm using HEALES analyzer (Shenzhen Huisong Technology Department Co., Ltd. – China).
BG was detected with the Dynamiker Fungus (Dynamiker Biotechnology, Tianjin, China) in clinical pathology laboratory of Mansoura faculty of medicine. This colorimetric assay based upon a modification of the limulus amebocyte lysate pathway. The absorbance is measured at 405nm kinetically every 60s for 40min at 37°C with TECAN reader (TECAN, IInfinite F 50, Life Sciences, Mannedorf, Switzerland). The concentration of BG is interpreted according to standard curve: values >95, 70–95, and <70pg/mL were considered positive, equivocal, and negative, respectively, and abnormal results were omitted.
Data analysisStatistical analysis was done using SPSS software (IBM SPSS Statistics for Windows, version 20.0. NY, USA). The Kolmogorov-Smirnov test was used to check for normal data distribution. Categorical data were described using number and percentage. Continuous data were described using median, range, mean, and standard deviation. The Chi-squared test was used for categorical variables, one-way ANOVA for Gaussian data, and Kruskal–Walls test for non-Gaussian data to compare between three or more groups. The sensitivity and specificity of the BG assay were determined; the positive (PPV) and negative predictive values (NPV) of the test were calculated, and the ROC curve was constructed.
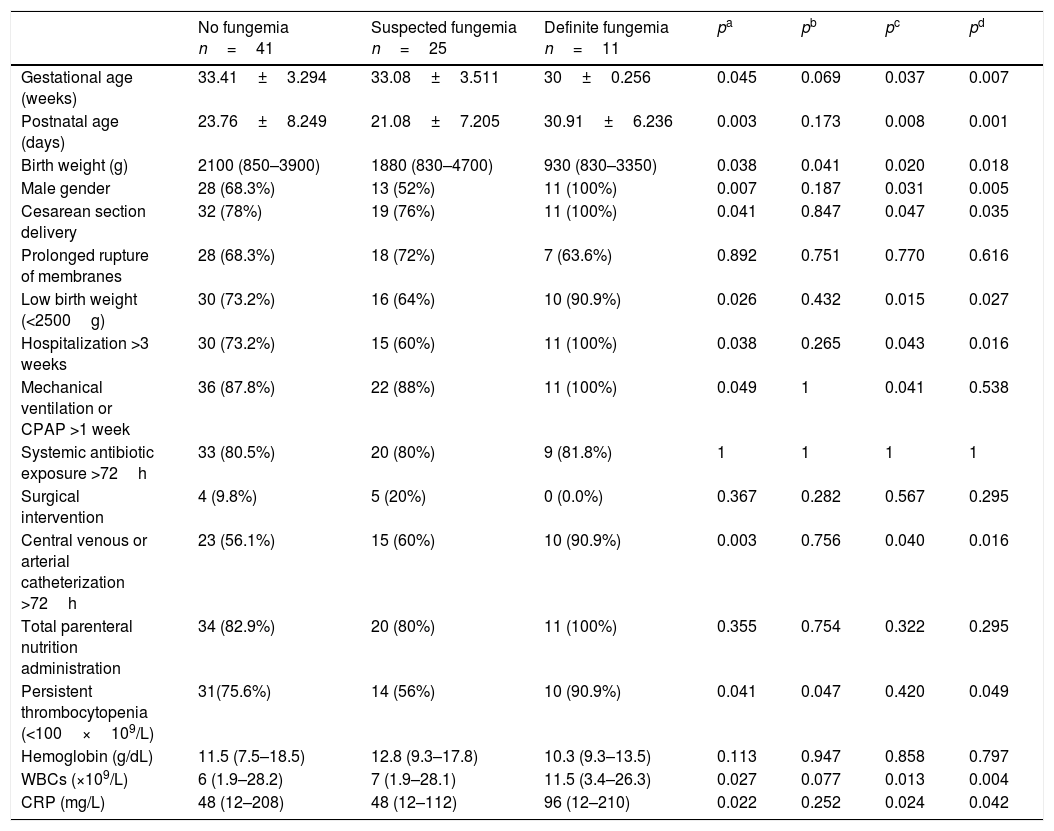
ResultsDuring the study period, a total of 77 neonates with LOS at a high risk of IFI were included; the stratification of patients into groups is demonstrated in the study flow chart (Fig. 1). Baseline characteristics of studied groups revealed that gestational age and birth weight were significantly lower in definite fungemia patients when compared with those in the no fungemia and suspected fungemia groups. Postnatal age, Cesarean section delivery, and male gender were significantly higher in definite fungemia patients when compared with no fungemia and suspected fungemia patients (Table 1). Low birth weight, hospitalization >3 weeks, central device, persistent thrombocytopenia, WBC, and CRP were significantly higher in the definite fungemia group when compared with the other two groups, while mechanical ventilation or continuous positive airway pressure (CPAP) >1 week was significantly higher in the definite fungemia group when compared with the no fungemia group only (Table 1). Gram-negative bacteria were more predominant than Gram-positive bacteria, with a frequency of 32/54 and 11/54, respectively. The growing organisms were Klebsiella spp. (14/54); Escherichia coli (12/54); Staphylococcus spp. (12/54; coagulase-negative Staphylococcus [9/54]; Staphylococcus aureus [3/54]); Pseudomonas aerouginosa (3/54); and Proteus spp. (2/54). Moreover, 11/54 presented Candida. Two neonatal blood cultures revealed mixed growth of Candida with bacteria.
Demographic, clinical, and laboratory characteristics of the studied groups.
| No fungemia n=41 | Suspected fungemia n=25 | Definite fungemia n=11 | pa | pb | pc | pd | |
|---|---|---|---|---|---|---|---|
| Gestational age (weeks) | 33.41±3.294 | 33.08±3.511 | 30±0.256 | 0.045 | 0.069 | 0.037 | 0.007 |
| Postnatal age (days) | 23.76±8.249 | 21.08±7.205 | 30.91±6.236 | 0.003 | 0.173 | 0.008 | 0.001 |
| Birth weight (g) | 2100 (850–3900) | 1880 (830–4700) | 930 (830–3350) | 0.038 | 0.041 | 0.020 | 0.018 |
| Male gender | 28 (68.3%) | 13 (52%) | 11 (100%) | 0.007 | 0.187 | 0.031 | 0.005 |
| Cesarean section delivery | 32 (78%) | 19 (76%) | 11 (100%) | 0.041 | 0.847 | 0.047 | 0.035 |
| Prolonged rupture of membranes | 28 (68.3%) | 18 (72%) | 7 (63.6%) | 0.892 | 0.751 | 0.770 | 0.616 |
| Low birth weight (<2500g) | 30 (73.2%) | 16 (64%) | 10 (90.9%) | 0.026 | 0.432 | 0.015 | 0.027 |
| Hospitalization >3 weeks | 30 (73.2%) | 15 (60%) | 11 (100%) | 0.038 | 0.265 | 0.043 | 0.016 |
| Mechanical ventilation or CPAP >1 week | 36 (87.8%) | 22 (88%) | 11 (100%) | 0.049 | 1 | 0.041 | 0.538 |
| Systemic antibiotic exposure >72h | 33 (80.5%) | 20 (80%) | 9 (81.8%) | 1 | 1 | 1 | 1 |
| Surgical intervention | 4 (9.8%) | 5 (20%) | 0 (0.0%) | 0.367 | 0.282 | 0.567 | 0.295 |
| Central venous or arterial catheterization >72h | 23 (56.1%) | 15 (60%) | 10 (90.9%) | 0.003 | 0.756 | 0.040 | 0.016 |
| Total parenteral nutrition administration | 34 (82.9%) | 20 (80%) | 11 (100%) | 0.355 | 0.754 | 0.322 | 0.295 |
| Persistent thrombocytopenia (<100×109/L) | 31(75.6%) | 14 (56%) | 10 (90.9%) | 0.041 | 0.047 | 0.420 | 0.049 |
| Hemoglobin (g/dL) | 11.5 (7.5–18.5) | 12.8 (9.3–17.8) | 10.3 (9.3–13.5) | 0.113 | 0.947 | 0.858 | 0.797 |
| WBCs (×109/L) | 6 (1.9–28.2) | 7 (1.9–28.1) | 11.5 (3.4–26.3) | 0.027 | 0.077 | 0.013 | 0.004 |
| CRP (mg/L) | 48 (12–208) | 48 (12–112) | 96 (12–210) | 0.022 | 0.252 | 0.024 | 0.042 |
Data are expressed as number (percentage), mean±SD or median (range).
CPAP, continuous positive airway pressure; WBCs, white blood cells; CRP, C-reactive protein.
The results of the BG assay were negative in 34, equivocal in 23, and positive in 20 cases. The no fungemia group was significantly associated with negative BG results when compared with the definite fungemia group (p=0.005); in turn, the definite fungemia group was significantly associated with positive BG results when compared with the no fungemia group (p=0.001).
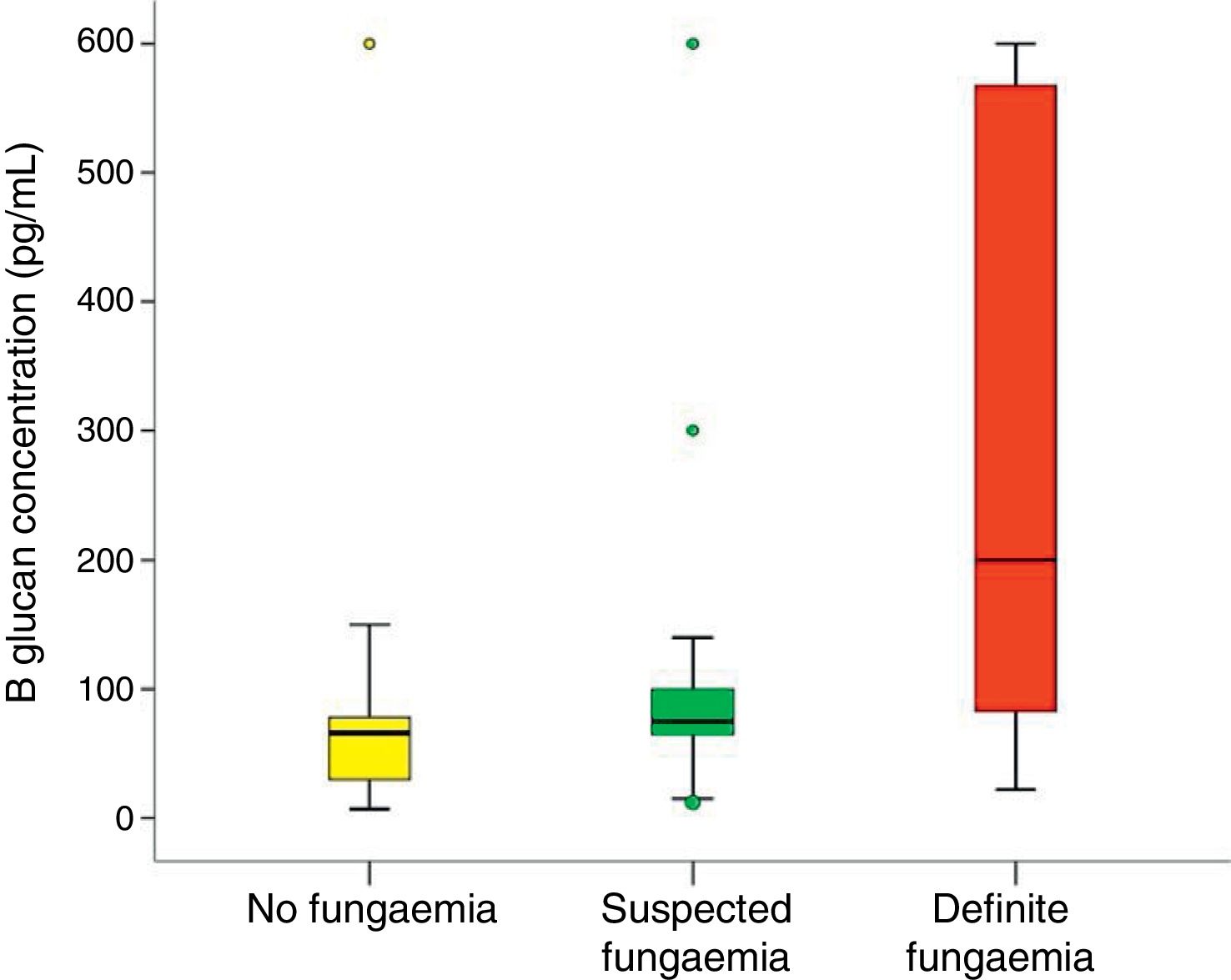
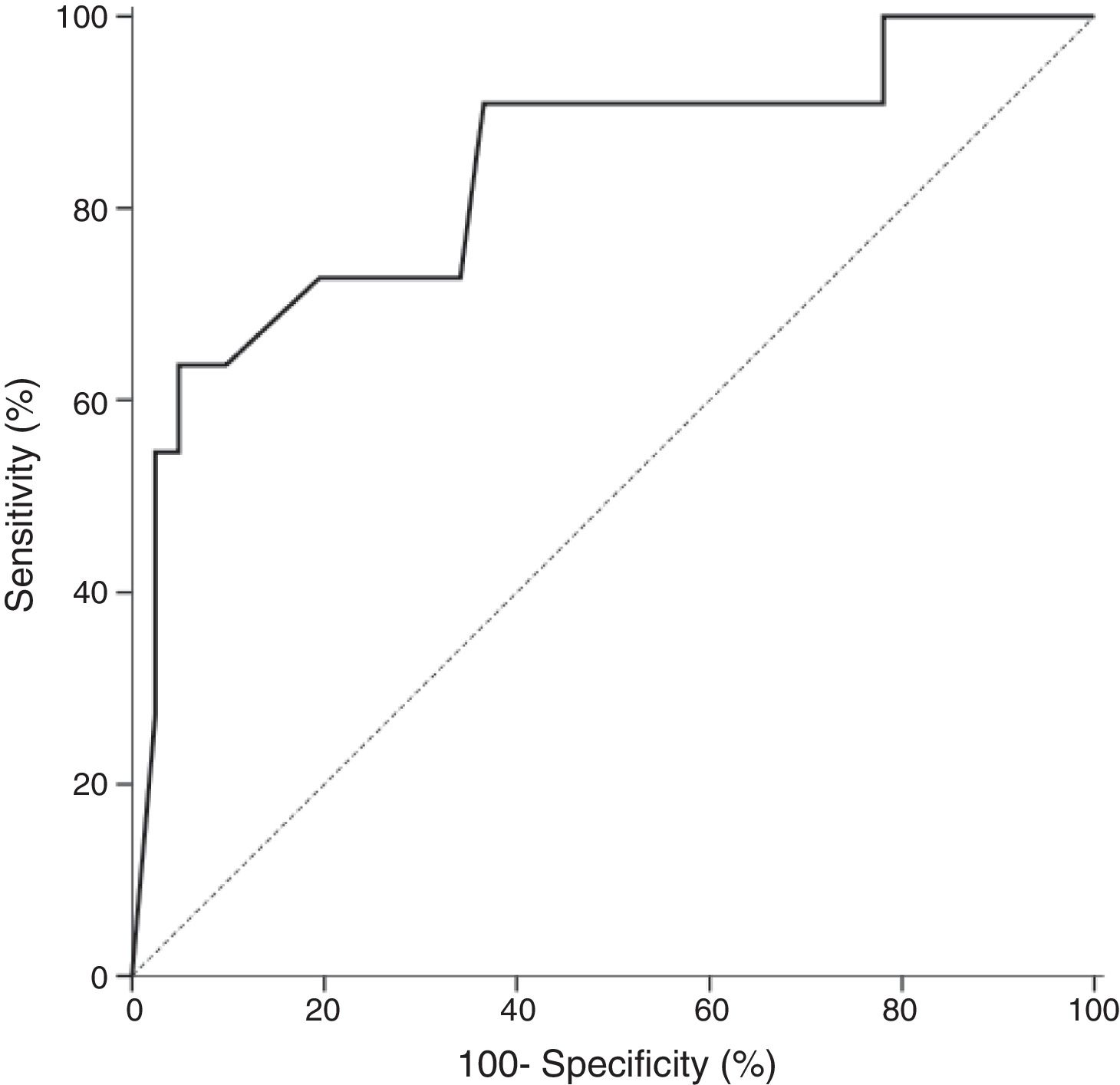
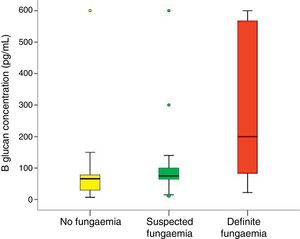
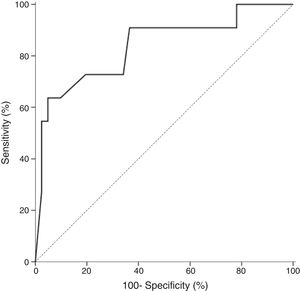
In all studied neonates, the median and ranges of BG concentrations are demonstrated in Fig. 2. BG concentration was significantly higher in the definite fungemia group when compared with the no fungemia and suspected fungemia groups (p=0.001 and p=0.011, respectively; Fig. 2). The sensitivity and specificity of BG assay at the 99pg/mL level were 63.6% and 95.1%, respectively. The PPV and NPV were 77.8% and 90.7%, respectively with accuracy 88.5%. The area under the receiver operating curve (ROC) was 0.837 (95% CI [0.689–0.985]; p=0.001; Fig. 3).
DiscussionIn spite of the development of new antifungal drugs, mortality and life-threatening complications of IFIs are still frequently reported in critically ill patients.15 The diagnosis of nosocomial IFI is negatively affected by suboptimal culture results. Therefore, new tools are required for early diagnosis of IFIs in neonates.
In the present study, the microbiological pattern of blood stream neonatal LOS was prospectively tested, and the diagnostic performance of serum BG assay in early diagnosis of IFI in high-risk infants admitted to a NICU was assessed.
In the present study, the most prevalent isolates were Gram-negative bacteria (57.2%), particularly Klebsiella spp. accounting for 25.9% of culture positive cases. Similarly, multiple studies reported Gram-negative pathogens as the predominant bacterial isolate in neonatal sepsis, with Klebsiella pneumoniae as the most frequently detected organism.16,17 Moreover, Al-Shamahy et al. conducted a study in Sanaa (Yemen), and reported a very high rate of Gram-negative bacteria, comprising 97.8% of the total isolates; Klebsiella spp. was the most predominant (36.7%) pathogen.18 In contrast, Hornik et al. found that Gram-positive organisms accounted for 45–75% of causes of LOS in neonates.19
Fungi represented 20.3% of culture positive cases in the present study. Similarly, a multicenter study conducted by Cotton et al. demonstrated that the rate of IFIs was 2.4–20.4% in extremely low birth weight infants.20 However, a large retrospective study demonstrated a very low incidence of IFIs (0.06%) in neonates >1500g birth weight.21 The high incidence of IFIs in the present study can be explained by the fact that infants who had three or more risk factors for fungal infection were included, as well as by the lack of compliance to strict hygiene in developing countries. In the present study, Candida albicans was the only isolated fungal growth, which is in agreement with the results of Hope et al., who stated that C. albicans is the most frequent Candida species causing invasive candidiasis in neonates.22
Regarding the BG assay in the present study, in the no fungemia group, 23 were found negative, 14 were equivocal, and four were positive. In turn, in the definite fungemia group, seven were found positive, three were equivocal, and one was negative. False-positive BG reactions are suspected to occur in patients treated with intravenous immunoglobulins, albumin, coagulation factors, and plasma protein fraction manufactured by certain fungal vendors, or antibiotics derived from fungal sources as amoxicillin-clavulanic acid, which were all excluded in our study. Patients’ exposure to gauze or materials containing glucans during surgery, mucosal damage from chemotherapy or radiotherapy, cross-experimental contamination with BG due to excess manipulation of a sample, certain streptococci, and P. aeruginosa may also cause false positive reactivity.23,24 Moreover, Zheng et al. reported that postnatal corticosteroids therapy can cause false positive BG result.25 In the present study, two neonates from the no fungemia group had history of recent surgery, with possible exposure to materials that contained glucans. In addition, P. aeruginosa was isolated from three neonates within the no fungemia group, which may explain their false positive reactivity. In the suspected fungemia group, nine of the neonates presented positive BG reaction, which might be explained by the low sensitivity of blood culture in the diagnosis of invasive candidiasis, as only 50% of invasive candidiasis are blood-culture positive.9 Furthermore, fungi other than Candida, such as Aspergillus, may be the cause of positive BG in these patients, since blood cultures are almost always negative in disseminated aspergillosis.26 Finally, the false negative result of BG in one patient from the definite fungemia group may be explained by IFI at an early stage.
The sensitivity, specificity, PPV, NPP, and accuracy of the BG assay in the present study at the cut-off value of 95pg/mL recommended by the manufacturer (after considering the equivocal results to be negative) were 63.6%, 90.2%, 63.6%, 90.2%, and 84.6%, respectively, in comparison to 90%, 56.1%, 35.15, 95.8%, and 63.5%, respectively, at a cut-off value ≥70pg/mL (after considering equivocal results to be positive). At the best obtained cut-off value (99pg/mL), these levels were 63.6%, 95.1%, 77.8%, 90.7%, and 88.5%, respectively. In the present study, the specificity of BG assay used for early diagnosis of IFIs was excellent (95.1%), while its sensitivity (63.6%) is limited at an optimal cut-off value of 99pg/mL. The excellent specificity and NPV suggest the good ability of BG assay to exclude IFI in suspected neonates, while its ability to diagnose the disease is limited. Thus, BG assay is better when combined with other clinical, radiological, and microbiological findings to improve its sensitivity. The primary value of the BG assay is to exclude the presence of IFIs based on a negative result, which would eliminate the need for toxic and expensive antifungal therapy.
In a previous study in a similar population of neonates with clinically suspected LOS who were at high risk of fungemia, the BG assay at the 60pg/mL level showed sensitivity, specificity, PPV, NPV values of 73.2%, 71.0%, 76.9%, and 66.7%, respectively while at the level of 80pg/mL, these values were 70.7%, 77.4%, 80.6%, and 66.7%, respectively.13 In turn, in a retrospective study carried out by Goudjil et al., it was reported that BG levels increased in neonatal IFI with an optimal cut-off value for BG positivity >125pg/mL; at this cut-off value, Sensitivity and specificity were 84% and 75%, respectively.11 Moreover, a study conducted by Zhao et al. found that BG sensitivity in diagnosing IFD at cut-off levels ≥10pg/mL, ≥200pg/mL, and ≥400pg/mL was 68.3%, 49.2%, and 33.3%, respectively while its specificity was 75.6%, 91.3%, and 96.3%, respectively. However, serum BG was measured in their study by a different technique than that of the present study, with BG values <10pg/mL interpreted as negative and values >20pg/mL as positive.12 Furthermore, Liu et al. evaluated the BG assay for diagnosis of candidemia in pediatric patients; they reported results similar to those of the present study, with sensitivity, specificity, PPV, NPV values of 68%, 91%, 66%, and 91%, respectively. They concluded that plasma BG assay has a moderate efficacy to aid the diagnosis of candidemia in pediatric patients, while it is useful to rule out candidemia due to its high NPV.27
In a meta-analysis including 31 studies, it was concluded that BG has a sensitivity of 80% and a specificity of 82% in the diagnosis of IFI. However, most of the studies included in that meta-analysis were conducted in adults.28 In a more recent meta-analysis by Hou et al.,29 in which a total of 1068 patients of different age groups from 11 studies were analyzed, the pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and area under the summary ROC curve, with 95% confidence intervals, were 0.75 (0.63,0.84), 0.87 (0.81,0.92), 5.85 (3.96,8.63), 0.30 (0.20,0.45), 19.53 (11.16,34.18), and 0.89 (0.86,0.91), respectively.29
It is evident that the cut-off value of serum BG can vary according to the kits and techniques used in the assay; in the present study, the best obtained cut-off value for diagnosing IFI was 99pg/mL with the Dynamiker Fungus technique. Meanwhile, according to a systematic review and meta-analysis conducted by He et al. to evaluate the diagnostic accuracy of serum BG for IFI, the best diagnostic accuracy in the Fungitell assay was observed using a cut-off of 60pg/mL; in the Fungitec G-Test assay (Seikagaku Corporation, Tokyo, Japan), 20pg/mL; and in the Wako assay (Wako Chemicals, VA, USA), 11pg/mL.30
The limitations of the present study include a small sample size, heterogeneous cohort of newborn infants, the fact that two different centers manipulated the samples, and use of blood culture as a gold standard for diagnosis of IFI, which is only positive in 50% of cases. Furthermore, there is the limitation of BG itself as an expensive test, especially for developing countries, and it does not identify species.
It is difficult to measure the actual performance of BG test, due to the poor sensitivity of blood culture. The available data indicates that the BG test has a limited sensitivity and excellent specificity that allow its use as an aid in diagnosis or exclusion of fungal neonatal sepsis. The adjustment of BG cut-off value to 99pg/mL appears suitable when there are no other causes of false reactivity. The NPV at this level could exclude the presence of fungemia by 90.7% based on a negative result. The authors recommend that accurate diagnosis and therapeutic decisions should be based on combining BG assay with other clinical, radiological, and microbiological findings, due to its limited sensitivity.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Shabaan AE, Elbaz LM, El-Emshaty WM, Shouman B. Role of serum (1,3)-β-d-glucan assay in early diagnosis of invasive fungal infections in a neonatal intensive care unit. J Pediatr (Rio J). 2018;94:559–65.