Staphylococcus aureus is responsible for a large number of infections in pediatric population; however, information about the behavior of such infections in this population is limited. The aim of the study was to describe the clinical, epidemiological, and molecular characteristics of infections caused by methicillin-susceptible and resistant S. aureus (MSSA–MRSA) in a pediatric population.
MethodA cross-sectional descriptive study in patients from birth to 14 years of age from three high-complexity institutions was conducted (2008–2010). All patients infected with methicillin-resistant S. aureus and a representative sample of patients infected with methicillin-susceptible S. aureus were included. Clinical and epidemiological information was obtained from medical records and molecular characterization included spa typing, pulsed-field gel electrophoresis (PFGE), and multilocus sequence typing (MLST). In addition, staphylococcal cassette chromosome mec (SCCmec) and virulence factor genes were detected.
ResultsA total of 182 patients, 65 with methicillin-susceptible S. aureus infections and 117 with methicillin-resistant S. aureus infections, were included in the study; 41.4% of the patients being under 1 year. The most frequent infections were of the skin and soft tissues. Backgrounds such as having stayed in day care centers and previous use of antibiotics were more common in patients with methicillin-resistant S. aureus infections (p≤0.05). Sixteen clonal complexes were identified and methicillin-susceptible S. aureus strains were more diverse. The most common cassette was staphylococcal cassette chromosomemec IVc (70.8%), which was linked to Panton–Valentine leukocidin (pvl).
ConclusionsIn contrast with other locations, a prevalence of infections in children under 1 year of age in the city could be observed; this emphasizes the importance of epidemiological knowledge at the local level.
O Staphylococcus aureus é responsável por um grande número de infecções na população pediátrica; contudo, as informações sobre o comportamento dessas infecções nessa população são limitadas. O objetivo do estudo foi descrever as características clínicas, epidemiológicas e moleculares de infecções causadas por Staphylococcus aureus suscetíveis e resistentes à meticilina (MSSA-MSRA) em uma população pediátrica.
MétodoUm estudo transversal descritivo foi realizado em pacientes entre 0 e 14 anos de idade de três instituições de alta complexidade (2008-2010). Todos os pacientes infectados com S. aureus resistentes à meticilina e uma amostra representativa de pacientes infectados com S. aureus suscetíveis à meticilina foram incluídos. As informações clínicas e epidemiológicas foram obtidas de prontuários médicos, e a caracterização molecular incluiu tipagem spa, Eletroforese em Gel de Campo Pulsado (PFGE) e Tipagem de sequências multilocus (MLST). Além disso, o Cassete Cromossômico Estafilocócico mec (SCCmec) e genes de fatores de virulência foram detectados.
Resultados182 pacientes, 65 com infecções por S. aureus suscetíveis à meticilina e 117 com infecções por S. aureus resistentes à meticilina, foram incluídos no estudo; 41,4% dos pacientes com menos de um ano de idade. As infecções mais frequentes foram da pele e dos tecidos moles. Os históricos como internações em centros de atendimento e o uso prévio de antibióticos foram mais comuns em pacientes com infecções por S. aureus resistentes à meticilina (p ≤ 0,05). Dezesseis complexos clonais foram identificados, e as cepas de S. aureus suscetíveis à meticilina foram mais diversificadas. O cassete mais comum foi o Cassete Cromossômico Estafilocócicomec IVc (70,8%), relacionado à leucocidina de panton-valentine (pvl).
ConclusõesEm comparação a outros locais, observamos uma prevalência de infecções em crianças com menos de um ano de idade na cidade; o que enfatiza a importância de conhecer a epidemiologia em nível local.
The complex situation of worldwide Staphylococcus aureus resistance to methicillin has led to its current management, representing a priority for the World Health Organization (WHO) and a challenge for human public health in different regions; however, both methicillin-susceptible and resistant S. aureus (MSSA and MRSA) strains possess a virulence and pathogenic capacity that allows them to reach high infection rates.
Some population groups are more susceptible to S. aureus infections; in particular, the pediatric population has less effective immunological function, persistently high bacterial colonization rates, poor hygiene habits, and constant exposure to school environments that favor the acquisition of infection and the dissemination of the microorganism.1,2 The epidemiology of S. aureus infections in this population is diverse, and the frequencies of MRSA infection differ between geographic regions, ranging from 6%3 to 69.9%4; in Colombia, frequencies up to 47.4% have been found.5
In Medellin, although S. aureus is one of the main agents responsible for infections in the pediatric population, both in hospitals and in the community, there is little information on the characteristics of the infections caused by this microorganism in this population. This study aims to describe the clinical, epidemiological, and molecular characteristics of S. aureus infections (MSSA–MRSA) in a pediatric population of the city.
MethodsStudy populationAn observational cross-sectional study was conducted from February 2008 to June 2010, at three tertiary care hospitals from Medellin, the second largest city in Colombia. Patients between birth and 14 years infected with S. aureus (MSSA–MRSA) were recruited prospectively; only the first isolate from each individual was evaluated. All patients with MRSA isolates obtained during the time of the study were included, and considering that the prevalence of MSSA is higher, a sample was defined. The sample size was calculated based on the MSSA prevalence during 2007 within each institution, which numbered 65 isolates. The MSSA isolates included were randomly selected each month, from February 2008 to June 2010, using a table of random numbers according to records of each participating institution.
The research protocol and informed consent (signed by parents or guardians) were approved by the Bioethics Committee for Human Research of the University Research Center at Universidad de Antioquia (approval no. 0841150) and by the bioethics committee of each hospital.
Clinical and epidemiological dataClinical and epidemiological data for each patient were obtained from medical records. Information included clinical and demographic characteristics, antimicrobial use, risk factors, co-morbidities, type of infection, treatment, length of hospital stay, and outcome. According to the Centers for Disease Control and Prevention (CDC) criteria, an infection was considered present on admission (POA) if the date of the event occurred on the day of admission to an inpatient location, two days before admission, or the calendar day after admission. An infection was considered health care-associated (HAI) if the date of event occurred on or after the third calendar day of admission to an inpatient location. Patients with surgical site infections and ventilator-associated event were excluded.6
Identification and antibiotic susceptibilityIdentification of S. aureus was conducted by standard laboratory methods based on colony morphology in sheep blood agar and positive catalase and coagulase tests. Antibiotic susceptibilities of S. aureus isolates were assessed in accordance with Clinical Laboratory Standards Institute guidelines (CLSI, 2009) using the VITEK® 2 system (BioMérieux, Inc., NC, EUA). The antibiotics tested included clindamycin, erythromycin, gentamycin, linezolid, moxifloxacin, oxacillin, rifampicin, tetracycline, tigecycline, trimethoprim-sulfamethoxazole, and vancomycin. The S. aureus strain ATCC 29213 was used for quality control.
Polymerase chain reaction (PCR) confirmation of S. aureus and methicillin resistanceThe presence of the species-specific nuc and femA genes as well as the mecA gene were verified by PCR, as previously described.7,8
Molecular typing: spa typing (spa), multilocus sequence typing (MLST) and pulsed field gel electrophoresis (PFGE)In all isolates, the polymorphic X region of the protein A gene (spa) was amplified and sequenced as previously described.9 Corresponding spa types were assigned using eGenomics software9,10 and Ridom spa-types were subsequently assigned using the spa typing website (http://www.spaserver.ridom.de/) developed by Ridom GmbH and curated by SeqNet.org (http://www.SeqNet.org/).11 MLST was performed on a subset of ten isolates representing the more frequent spa types, using the methodology described by Enright et al.12 Allele numbers and sequence types (ST) were assigned using the database maintained at http://saureus.mlst.net/while clonal complexes (CC) were inferred using eBURST analysis.13 Clonal complexes for all remaining strains were inferred by spa repeat pattern analysis,10 or by referring to the Ridom spa server website.
PFGE, following SmaI digestion, was performed according to a protocol described elsewhere.14 DNA fragment patterns were normalized using S. aureus strain NCTC 8325. Cluster analysis was performed using the Dice coefficient in BioNumerics software (BioNumerics®, software version 6.0, Belgium). Dendrograms were generated by the unweighted pair group method using average linkages (UPGMA). Similarity cut-offs of 80% and 95% were used to define types and subtypes, respectively.14
SCCmec typingFor MRSA isolates, SCCmec types and subtypes were determined using sets of multiplex PCR reactions, as previously described.15,16 MRSA strains were included as positive controls for SCCmec types and subtypes.
Detection of staphylococcal virulence factorsAll isolates were screened for the genes encoding staphylococcal enterotoxins (sea, seb, sec, sed, see), toxic shock syndrome toxin 1 (tst), and exfoliative toxins a and b (eta, etb), using the protocols and primers described by Mehrotra et al.8 The lukS/F-PV genes, encoding Panton-Valentine leukocidin (PVL); and the arcA gene, associated arginine catabolic mobile element (ACME), were also assessed by PCR.17,18
Statistical analysesComparisons of clinical, epidemiological, and molecular characteristics were conducted between MSSA and MRSA infected patients, and among the different groups of infections obtained after applying CDC criteria.
Categorical variables were compared using the chi-squared test or Fisher's exact test; Student's t- and Mann–Whitney U tests were used for continuous variables. p values ≤0.05 were considered statistically significant. Statistical analyses were carried out using the software package in SPSS® (IBM SPSS Statistics for Windows, version 21.0, NY, USA).
ResultsClinical and epidemiological dataThere were 182 pediatric patients with S. aureus infections included; of these, 65 had MSSA infections and 117 had MRSA infections; 119 patients came from hospital A, 51 from hospital B, and 12 from hospital C.
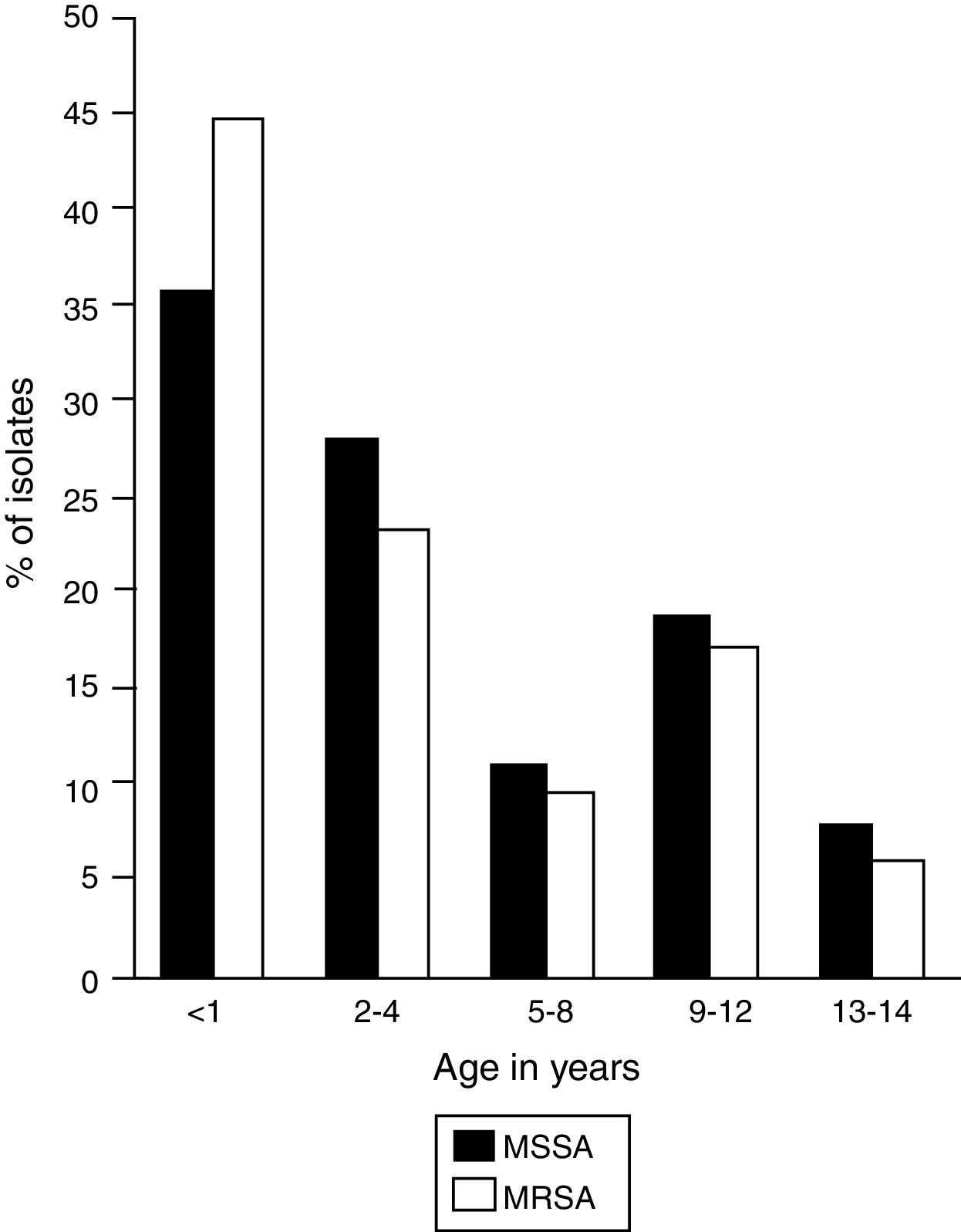
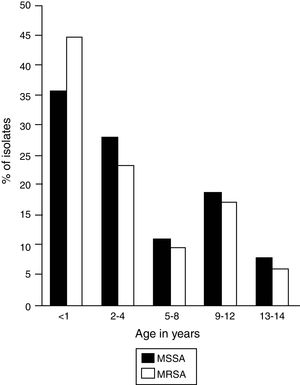
In the selected study population, the ratio of boys and girls was 2:1 and the median age was 2 years; however, 41.2% (n=75) of the infections occurred in patients between birth and 1 year of age, in which a high frequency of MRSA and MSSA infections was observed (Fig. 1).
Frequency of infection by age group. MSSA, methicillin-susceptible Staphylococcus aureus (n=65); MRSA, methicillin-resistant Staphylococcus aureus (n=117). The figure shows the age distribution of patients infected by MSSA and MRSA strains. A higher frequency of infections is observed in children under 1 year.
According to the CDC criteria, 62.7% (n=101) of the infections were POA, and 37.3% (n=60) corresponded to HAI (p=0.391). Infection type showed statistically significant differences by hospital. In hospitals A and B there was a predominance of POA infections; in hospital C there was a predominance of HAI (p=0.029). Twenty-one (11.5%) surgical site infections were identified, which were not included in this classification.
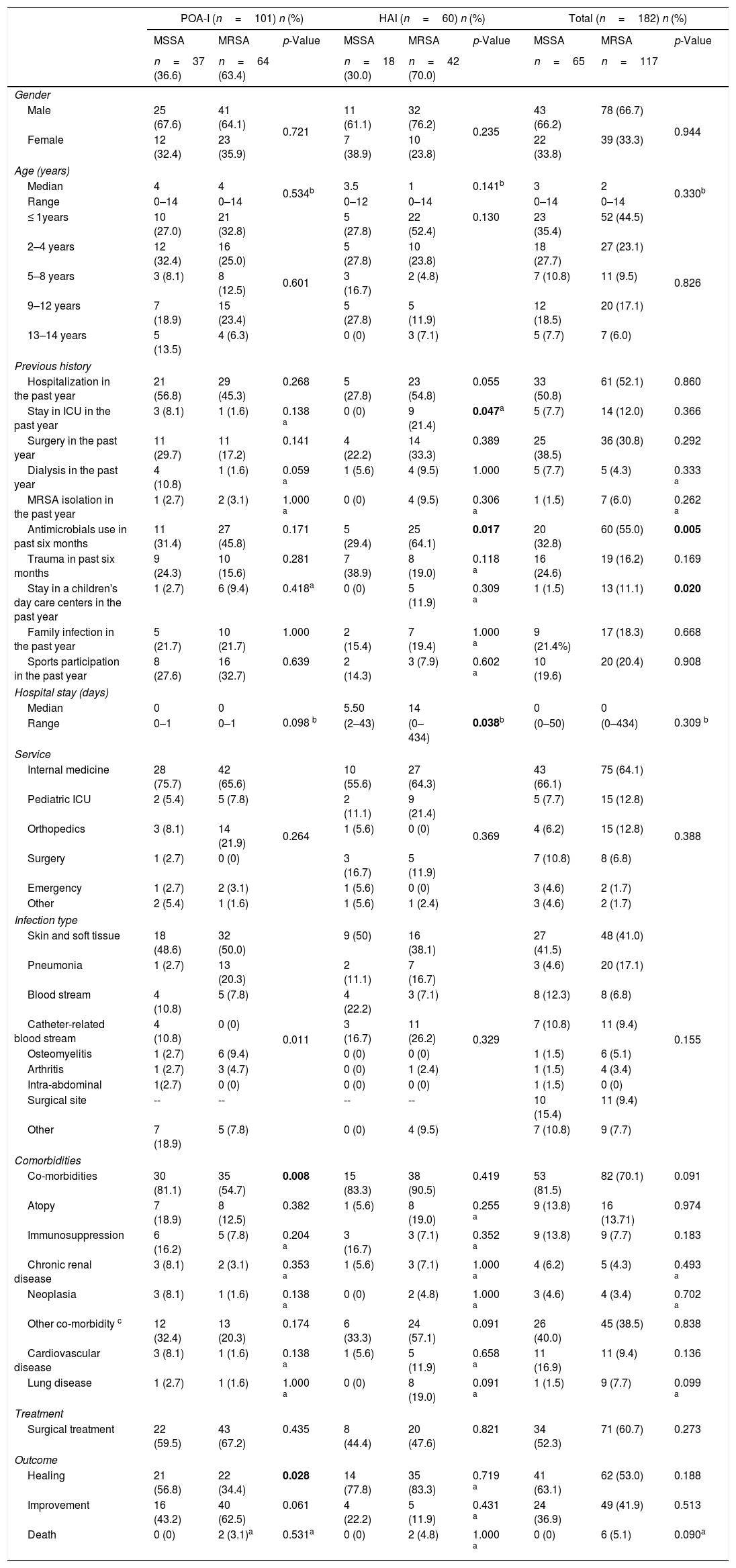
The clinical–epidemiological characteristics of the patients evaluated in the study are described in Table 1. In general, a previous history of hospitalization was the most frequent antecedent among patients, with 51.65% (n=94); furthermore, having stayed in day care centers (MRSA 11.1%, n=13 vs. MSSA 1.5%, n=1; p=0.020) and previous use of antibiotics (MRSA 55%, n=60 vs. MSSA 32.8%, n=20; p=0.005) was the most frequent antecedent in patients with MRSA infections.
Clinical and epidemiological characteristics of patients with Staphylococcus aureus (MSSA–MRSA) infections.
| POA-I (n=101) n (%) | HAI (n=60) n (%) | Total (n=182) n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MSSA | MRSA | p-Value | MSSA | MRSA | p-Value | MSSA | MRSA | p-Value | |
| n=37 (36.6) | n=64 (63.4) | n=18 (30.0) | n=42 (70.0) | n=65 | n=117 | ||||
| Gender | |||||||||
| Male | 25 (67.6) | 41 (64.1) | 0.721 | 11 (61.1) | 32 (76.2) | 0.235 | 43 (66.2) | 78 (66.7) | 0.944 |
| Female | 12 (32.4) | 23 (35.9) | 7 (38.9) | 10 (23.8) | 22 (33.8) | 39 (33.3) | |||
| Age (years) | |||||||||
| Median | 4 | 4 | 0.534b | 3.5 | 1 | 0.141b | 3 | 2 | 0.330b |
| Range | 0–14 | 0–14 | 0–12 | 0–14 | 0–14 | 0–14 | |||
| ≤ 1years | 10 (27.0) | 21 (32.8) | 0.601 | 5 (27.8) | 22 (52.4) | 0.130 | 23 (35.4) | 52 (44.5) | 0.826 |
| 2–4 years | 12 (32.4) | 16 (25.0) | 5 (27.8) | 10 (23.8) | 18 (27.7) | 27 (23.1) | |||
| 5–8 years | 3 (8.1) | 8 (12.5) | 3 (16.7) | 2 (4.8) | 7 (10.8) | 11 (9.5) | |||
| 9–12 years | 7 (18.9) | 15 (23.4) | 5 (27.8) | 5 (11.9) | 12 (18.5) | 20 (17.1) | |||
| 13–14 years | 5 (13.5) | 4 (6.3) | 0 (0) | 3 (7.1) | 5 (7.7) | 7 (6.0) | |||
| Previous history | |||||||||
| Hospitalization in the past year | 21 (56.8) | 29 (45.3) | 0.268 | 5 (27.8) | 23 (54.8) | 0.055 | 33 (50.8) | 61 (52.1) | 0.860 |
| Stay in ICU in the past year | 3 (8.1) | 1 (1.6) | 0.138 a | 0 (0) | 9 (21.4) | 0.047a | 5 (7.7) | 14 (12.0) | 0.366 |
| Surgery in the past year | 11 (29.7) | 11 (17.2) | 0.141 | 4 (22.2) | 14 (33.3) | 0.389 | 25 (38.5) | 36 (30.8) | 0.292 |
| Dialysis in the past year | 4 (10.8) | 1 (1.6) | 0.059 a | 1 (5.6) | 4 (9.5) | 1.000 | 5 (7.7) | 5 (4.3) | 0.333 a |
| MRSA isolation in the past year | 1 (2.7) | 2 (3.1) | 1.000 a | 0 (0) | 4 (9.5) | 0.306 a | 1 (1.5) | 7 (6.0) | 0.262 a |
| Antimicrobials use in past six months | 11 (31.4) | 27 (45.8) | 0.171 | 5 (29.4) | 25 (64.1) | 0.017 | 20 (32.8) | 60 (55.0) | 0.005 |
| Trauma in past six months | 9 (24.3) | 10 (15.6) | 0.281 | 7 (38.9) | 8 (19.0) | 0.118 a | 16 (24.6) | 19 (16.2) | 0.169 |
| Stay in a children's day care centers in the past year | 1 (2.7) | 6 (9.4) | 0.418a | 0 (0) | 5 (11.9) | 0.309 a | 1 (1.5) | 13 (11.1) | 0.020 |
| Family infection in the past year | 5 (21.7) | 10 (21.7) | 1.000 | 2 (15.4) | 7 (19.4) | 1.000 a | 9 (21.4%) | 17 (18.3) | 0.668 |
| Sports participation in the past year | 8 (27.6) | 16 (32.7) | 0.639 | 2 (14.3) | 3 (7.9) | 0.602 a | 10 (19.6) | 20 (20.4) | 0.908 |
| Hospital stay (days) | |||||||||
| Median | 0 | 0 | 0.098 b | 5.50 | 14 | 0.038b | 0 | 0 | 0.309 b |
| Range | 0–1 | 0–1 | (2–43) | (0–434) | (0–50) | (0–434) | |||
| Service | |||||||||
| Internal medicine | 28 (75.7) | 42 (65.6) | 0.264 | 10 (55.6) | 27 (64.3) | 0.369 | 43 (66.1) | 75 (64.1) | 0.388 |
| Pediatric ICU | 2 (5.4) | 5 (7.8) | 2 (11.1) | 9 (21.4) | 5 (7.7) | 15 (12.8) | |||
| Orthopedics | 3 (8.1) | 14 (21.9) | 1 (5.6) | 0 (0) | 4 (6.2) | 15 (12.8) | |||
| Surgery | 1 (2.7) | 0 (0) | 3 (16.7) | 5 (11.9) | 7 (10.8) | 8 (6.8) | |||
| Emergency | 1 (2.7) | 2 (3.1) | 1 (5.6) | 0 (0) | 3 (4.6) | 2 (1.7) | |||
| Other | 2 (5.4) | 1 (1.6) | 1 (5.6) | 1 (2.4) | 3 (4.6) | 2 (1.7) | |||
| Infection type | |||||||||
| Skin and soft tissue | 18 (48.6) | 32 (50.0) | 0.011 | 9 (50) | 16 (38.1) | 0.329 | 27 (41.5) | 48 (41.0) | 0.155 |
| Pneumonia | 1 (2.7) | 13 (20.3) | 2 (11.1) | 7 (16.7) | 3 (4.6) | 20 (17.1) | |||
| Blood stream | 4 (10.8) | 5 (7.8) | 4 (22.2) | 3 (7.1) | 8 (12.3) | 8 (6.8) | |||
| Catheter-related blood stream | 4 (10.8) | 0 (0) | 3 (16.7) | 11 (26.2) | 7 (10.8) | 11 (9.4) | |||
| Osteomyelitis | 1 (2.7) | 6 (9.4) | 0 (0) | 0 (0) | 1 (1.5) | 6 (5.1) | |||
| Arthritis | 1 (2.7) | 3 (4.7) | 0 (0) | 1 (2.4) | 1 (1.5) | 4 (3.4) | |||
| Intra-abdominal | 1(2.7) | 0 (0) | 0 (0) | 0 (0) | 1 (1.5) | 0 (0) | |||
| Surgical site | -- | -- | -- | -- | 10 (15.4) | 11 (9.4) | |||
| Other | 7 (18.9) | 5 (7.8) | 0 (0) | 4 (9.5) | 7 (10.8) | 9 (7.7) | |||
| Comorbidities | |||||||||
| Co-morbidities | 30 (81.1) | 35 (54.7) | 0.008 | 15 (83.3) | 38 (90.5) | 0.419 | 53 (81.5) | 82 (70.1) | 0.091 |
| Atopy | 7 (18.9) | 8 (12.5) | 0.382 | 1 (5.6) | 8 (19.0) | 0.255 a | 9 (13.8) | 16 (13.71) | 0.974 |
| Immunosuppression | 6 (16.2) | 5 (7.8) | 0.204 a | 3 (16.7) | 3 (7.1) | 0.352 a | 9 (13.8) | 9 (7.7) | 0.183 |
| Chronic renal disease | 3 (8.1) | 2 (3.1) | 0.353 a | 1 (5.6) | 3 (7.1) | 1.000 a | 4 (6.2) | 5 (4.3) | 0.493 a |
| Neoplasia | 3 (8.1) | 1 (1.6) | 0.138 a | 0 (0) | 2 (4.8) | 1.000 a | 3 (4.6) | 4 (3.4) | 0.702 a |
| Other co-morbidity c | 12 (32.4) | 13 (20.3) | 0.174 | 6 (33.3) | 24 (57.1) | 0.091 | 26 (40.0) | 45 (38.5) | 0.838 |
| Cardiovascular disease | 3 (8.1) | 1 (1.6) | 0.138 a | 1 (5.6) | 5 (11.9) | 0.658 a | 11 (16.9) | 11 (9.4) | 0.136 |
| Lung disease | 1 (2.7) | 1 (1.6) | 1.000 a | 0 (0) | 8 (19.0) | 0.091 a | 1 (1.5) | 9 (7.7) | 0.099 a |
| Treatment | |||||||||
| Surgical treatment | 22 (59.5) | 43 (67.2) | 0.435 | 8 (44.4) | 20 (47.6) | 0.821 | 34 (52.3) | 71 (60.7) | 0.273 |
| Outcome | |||||||||
| Healing | 21 (56.8) | 22 (34.4) | 0.028 | 14 (77.8) | 35 (83.3) | 0.719 a | 41 (63.1) | 62 (53.0) | 0.188 |
| Improvement | 16 (43.2) | 40 (62.5) | 0.061 | 4 (22.2) | 5 (11.9) | 0.431 a | 24 (36.9) | 49 (41.9) | 0.513 |
| Death | 0 (0) | 2 (3.1)a | 0.531a | 0 (0) | 2 (4.8) | 1.000 a | 0 (0) | 6 (5.1) | 0.090a |
POA-I, present on admission infection; HAI, health care-associated infection; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; ICU, intensive care unit.
Significant differences (p-values ≤0.05) are shown in bold.
Other co-morbidities: cardiovascular disease, chronic lung disease, nervous system diseases, skin diseases, malnutrition, cholestatic syndrome, subglottic stenosis, snake bite, Rh-ABO incompatibility, short bowel syndrome, nesidioblastosis, laryngeal and lung papillomatosis, hemophilia B, chronic osteomyelitis, congenital malformations, nephropathy, and others.
In HAI, a previous history of intensive care unit (ICU) stay (MRSA 21.4%, n=9 vs. MSSA 0%; p=0.047) and prior antibiotic use in the last six months (MRSA 64.1%, n=25 vs. MSSA 29.4%, n=5; p=0.017) were more frequent in patients with MRSA infections.
However, the hospital stay was longer in patients with HAI, with a significant difference in favor of patients with MRSA infections (MRSA 0–434 days, Me=14 vs. MSSA 2–43 days, Me=5.5; p=0.038).
The most frequent sites of infection were skin and soft tissue, with 41.21% (n=75), and the most common medical service was internal medicine, with 64.84% (n=110).
The frequency of co-morbidities in the population was 74.1% (n=135), more frequent in patients with POA infections caused by MSSA (MSSA 81.1%, n=30 vs. MRSA 54.7%, n=35; p=0.008).
Of the population, 57.6% required surgical treatment and healing was the most frequent outcome; especially in POA infections caused by MSSA (MSSA 56.8%, n=21 vs. MRSA 34.4%, n=22; p=0.028). Six of the patients included in the study died; however, this was the crude mortality and not attributed to S. aureus infection.
Resistance profileThe MSSA isolates had six resistance profiles; 61.5% (n=40) were susceptible to all antibiotics evaluated, 16.9% (n=11) were resistant to tetracycline, and 9.2% (n=6) to erythromycin and clindamycin. The MRSA isolates had eight resistance profiles; 44.4% (n=52) were resistant only to oxacillin, followed by 28.2% (n=33) with resistance to oxacillin and tetracycline. All of the isolates were susceptible to vancomycin, linezolid, and tigecycline.
Molecular typingAmong all the isolates, 16 clonal complexes (CC) were identified. MSSA strains were the more diverse, with a higher number of CCs; the most common were CC8 (29.2%, n=19), CC45 (16.9%, n=11), and CC1 (10.8%, n=7), whereas in the MRSA strains the most common CCs were CC8 (70.9%, n=83) and CC5 (22.2%, n=26).
The presence of mecA gene was confirmed in the 117 selected MRSA strains. Of these, it was possible to identify the SCCmec type in 113 strains; four isolates were nontypeable. Of the MSRA isolates, 70.8% harbored SCCmec IVc (n=80), followed by SCCmec I (20.3%, n=23), SCCmec IVa (5.3%, n=6), and SCCmec V (2.7%, n=3). An isolate was identified with SCCmec IV, but its subtype was not identifiable (0.9%; n=1).
Among patients with POA infections, 87.1% (n=54) of MRSA isolates harbored SCCmec IVc, followed by SCCmec I (8.1%, n=5) and SCCmec IVa (4.8%, n=3). Meanwhile, MRSA isolates from HAI patients harbored SCCmec type IVc in 56.1% (n=23), followed by SCCmec type I (26.8%; n=11), IVa (7.3%; n=3), V (7.3%, n=3), and IV (2.43%, n=1). SCCmec IVc was the most frequent type in both types of infection.
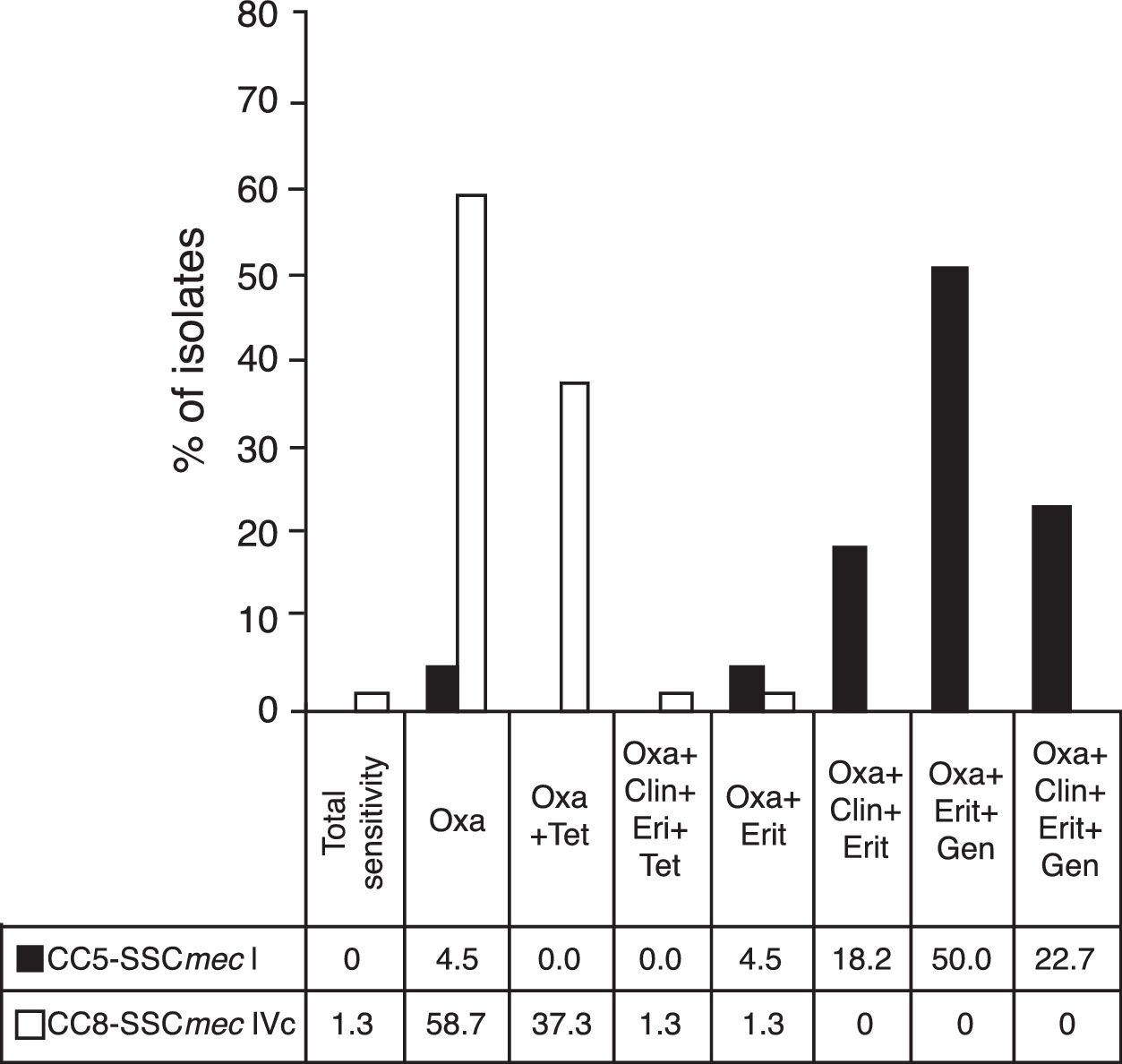
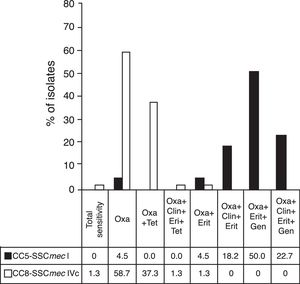
The resistance profiles of the most important MRSA clones are shown in Fig. 2.
Resistance profiles of the main MRSA clones. MRSA, methicillin-resistant Staphylococcus aureus. Figure shows the profile of antimicrobial resistance of the main MRSA clones found in the study. Antibiotics assessed: oxacillin (Oxa), tetracycline (Tet), clindamycin (Clin), erythromycin (Eri), gentamycin (Gen). Dark column: isolates belonging to CC5-SCCmec-I (n=22); clear column: isolates belonging to CC8-SCCmec-IVc (n=75).
Forty different spa types were identified in MSSA and MRSA strains. The most common types were t1610 (17.6%, n=32), t008 (17%, n=31), t149 (11%, n=20), and t024 (9.9%; n=18). In MSSA isolates belonging to CC8, types t1635 and t008, each with 9.2% (n=6) were the most frequent and t922, with 6.2% (n=4), belonging to CC1, were highlighted. Most important strains of MRSA were those belonging to CC8, and the most frequent were CC8-SCCmec IVc-t1610 (27.43%, n=31), CC8-SCCmec IVc-t008 (19.47%, n=22), CC5-SCCmec I-t149 (16.81%, n=19), and CC8-SCCmec IVc-t024 (15.04%, n=17).
During the study period, a change was observed in the most important clonal complexes, both in MSSA and MRSA isolates. CC5 almost completely disappeared, while CC8 and CC45 remained constant. In addition, the increase of other CCs was evident, although none of them were specifically predominant. During the three years of the study, the clone belonging to CC8-SCCmec IVc of MRSA was increasing, representing the most prevalent, while the CC5-SCCmec I disappeared in the third year.
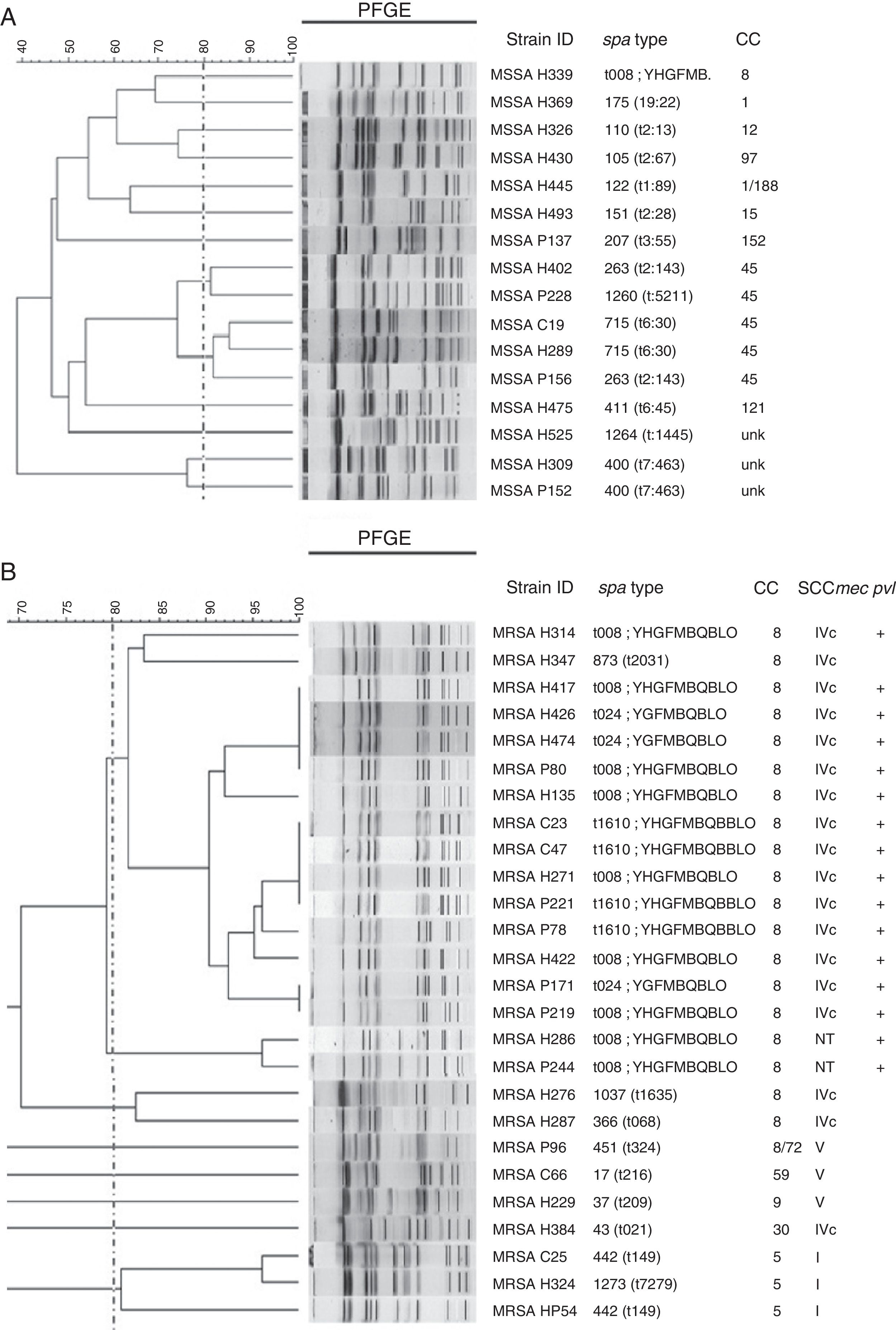
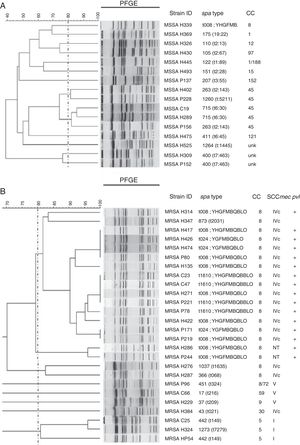
Sixteen isolates of MSSA were analyzed using PFGE, and a great diversity was found in the results (Fig. 3A). Analysis of MRSA by PFGE was performed on 26 isolates and four related groups were identified. The largest group had 15 isolates, which belonged to CC8, and harbored SCCmec IVc; 14 of them were positive for pvl and had different spa types (t008, 7/15; t1610, 4/15; t024, 3/15; t2031, 1/15; Fig. 3B).
Genetic relatedness in MSSA and MRSA isolates. UPGMA dendrogram showing genetic relatedness in a sample of methicillin-susceptible Staphylococcus aureus (MSSA) (A) and methicillin-resistant Staphylococcus aureus (MRSA) (B) isolates. Dotted line indicates the cut-off point, or the Dice coefficient of 80%; this is used to define PFGE clones. Clusters above this percentage are considered to be genetically related. Isolates identified with the letter H belong to hospital A, isolates identified with the letter P belong to hospital B, and isolates identified with the letter C belong to hospital C.
Eight virulence factor genes were detected, both in MSSA and MRSA strains; however, higher gene prevalence was observed in MSSA compared to MRSA isolates. Statistically significant differences were observed between MSSA and MRSA strains regarding the pvl genes (MRSA 76.9%, n=90 vs. MSSA 32.3%, n=21; p=0.000), sed (MSSA 33.8%, n=8 vs. MRSA 10.3%, n=12; p=0.000), and see (MSSA 12.3%, n=8 vs. MRSA 2.6%, n=3; p=0.018).
The presence of pvl showed statistically significant differences between isolates with SCCmec IVc and other types of SCCmec (SCCmec IVc 95%, n=76 vs. other SCCmec 30.3%, n=10; p=0.000); It suggests that the pvl gene is more frequently associated with isolates with SCCmec IVc than SCCmec I. Genes such as sed and tst were associated with SCCmec IVa (sed, p=0.001; tst, p=0.012), while the eta gene was associated with SCCmec V (p=0.027). No etb and arcA (ACME) genes were found in any of the isolates.
DiscussionInfections caused by S. aureus (MSSA–MRSA) in child populations continue to be a major concern, both in the community and the hospital environment. In general, studies in the pediatric population are few, and some have limitations: many of them focus on certain infection types and most do not present information on infections caused by MSSA strains, which remain relevant and have not been displaced by MRSA strains.
In this study, a significant number of infections occurred on patients between birth and 1 year of age (41.2%), who mainly had skin and soft tissue infections caused by MRSA. Interestingly, few studies on S. aureus infections in children population agree with these findings, as reported in China by Wu et al. in 2010; they found that 37.6% of skin and soft tissue infections occurred in children younger than 1 year.19 Likewise, morbidity and mortality reports by the CDC in the United States have described an important prevalence of skin and soft tissue infections in neonatal patients.20 However, other publications differ on infection types; authors such as Ilczyszyn et al. in Poland21 and Qiao et al. in China22 have shown that S. aureus infections in children between birth and 1 year are mainly of the invasive type (bacteremia and pneumonia).22
Likewise, results of other studies contrast with the present study regarding age; studies in Brazil23 and Argentina,24 for example, have shown a high prevalence of S. aureus infections in patients between 2 and 5 years of age, and other studies carried out in Colombia, in cities such as Bucaramanga25 and Cartagena,5 describe a high prevalence of S. aureus infections in children aged between 4 and 5 years and between 10 and 17 years, respectively.
Infections in different age groups are determined by the particularities of each population and by the risk factors to which infants are exposed; furthermore, the infant population has a less adapted immune system, which allows greater colonization, more readily developed clinical presentations, and more complications.1,2
In this regard, a previous study, carried out in Medellin during 2011, revealed colonization frequencies by S. aureus (MSSA and MRSA) of 45.9% in children under 2 years.26 This is an important aspect taking into account the relationship between colonization and the development of S. aureus infections, since it is possible that the frequencies of infection found in pediatric patients are related, among other aspects, to the frequencies of colonization reported in the city.
In particular, in this study a relationship between the antecedent of having stayed in day care centers and MRSA infections was found, which could be due to the frequency of colonization previously reported in children from Medellín day care centers. Rodríguez et al. were able to establish that the colonizing strains were closely related to the strains causing infection in the city.26 Day care centers have been described as an important reservoir of the microorganism, becoming favorable places for its dissemination. These environments allow the swapping objects between children who have poor hygiene habits, which therefore makes dissemination more likely.2
According to a previous study published on S. aureus infections in the adult population from Medellín,27 this study found that S. aureus strains harboring SCCmec IVc, usually associated with the community environment, predominated as etiological agents of HAIs, displacing the traditional strains with the SCCmec type I that dominated in hospitals. Further, frequencies of SCCmec IVc were higher in the infant population compared to those reported in the adult population (58.7% adult vs. 70.8% pediatric).27
This discovery shows the success of SCCmec IVc in the child population, which is a cause of great concern because it facilitates the maintaining of virulence factors like Panton-Valentine leukocidin (PVL) and its dissemination may be greater in this population. As described, SCCmec IV is smaller; therefore, the biological cost of resistance decreases, favoring its propagation over other strains. Spread occurs mainly in strains belonging to specific clonal complexes such as CC8, which could be evidenced in the PFGE results, as was reported previously.28
Nonetheless, the spa types predominant in this study; t1610, t008, t149, and t024 have been previously identified, mainly in MRSA strains infecting pediatric and adult patients, not only in Colombia and Latin American but also in many countries around the world.29,30 These findings show the wide circulation among the general populations, and suggest the pathogenic capacity and dissemination ability of these strains.
With regard to the resistance profiles, a difference was observed between this study and others, which may be due to differences in use of antimicrobials, either in the clinical setting or elsewhere. Heterogeneity confirms the importance of knowing the behavior of these infections in each region and institution. Further, the differences between the resistance profiles of the main MRSA clones reported in the study allow a clinical approach to the recognition of the predominant clones, without the need for molecular typing.
In the present study a predominance of POA infections were observed; however, health-care associated infections continue being very important in the pediatric population. At the same time, MSSA strains constitute an important source of infections; in this case, they harbored a variety of virulence factors genes in contrast with the MRSA strains, an aspect previously described.
The results of the present study represent valuable information for the knowledge of local epidemiology and the control of pediatric infections in the city. In addition, they demonstrate that the epidemiology of this microorganism is diverse and that, due to the particularities of each region, the data cannot be extrapolated. In general, the study demonstrated a higher prevalence of SCCmec IVc in children than adults, which could indicate a greater spread of the chromosomal cassette in this population group and may require additional studies.
Although the study group does not constitute a complete cohort, the patients and the evaluated isolates are the result of carefully designed sampling.
FundingSustainability strategy consolidation process CODI (Comitê de Desenvolvimento e Pesquisa). University of Antioquia, research Group on Basic and Applied Microbiology. MICROBA (Grupo de Pesquisa em Microbiologia Básica e Aplicada) 2016–2017.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Salazar-Ospina L, Jiménez JN. High frequency of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in children under 1 year old with skin and soft tissue infections. J Pediatr (Rio J). 2018;94:380–9.