To evaluate the overall health-related quality of life in patients with bronchiolitis obliterans.
MethodsParticipants with a diagnosis of post-infectious bronchiolitis obliterans, who were being followed-up at two specialized outpatient clinics of Pediatric Pulmonology in Porto Alegre, Brazil, and controls aged between 8 and 17 years, of both genders, were included in the study. Controls were paired by gender, age, and socioeconomic level in relation to the group of participants with post-infectious bronchiolitis obliterans. The version of the Pediatric Quality of Life Inventory (PedsQ) tool validated for Brazil was applied for the assessment of Health-related Quality of Life, through an interview. The comparison of the Health-related Quality of Life means between the groups was performed using Student's t-test for independent samples and the chi-squared test, for categorical variables.
Results34 patients diagnosed with post-infectious bronchiolitis obliterans and 34 controls participated in the study. The mean age of the children included in the study was 11.2±2.5 years, and 49 (72%) of them were males. The groups showed no significant differences in relation to these variables. The quality of life score was significantly and clinically lower in the post-infectious bronchiolitis obliterans group when compared with controls in the health (72.36±15.6, 81.06±16.4, p=0.031) and school domains (62.34±20.7, 72.94±21.3, p=0.043), as well as in the total score (69.53±14.9, 78.02±14.8, p=0.024), respectively.
ConclusionPatients with post-infectious bronchiolitis obliterans presented lower health-related quality of life scores when compared with healthy individuals in the total score and in the health and school domains.
Avaliar a qualidade de vida relacionada à saúde geral em participantes com bronquiolite obliterante.
MétodosForam incluídos no estudo participantes com diagnóstico de bronquiolite obliterante pós-infecciosa que estavam em acompanhamento em dois ambulatórios especializados de pneumologia pediátrica em Porto Alegre, Brasil e controles, com idades entre 8 e 17 anos, de ambos os sexos. Os controles foram pareados por sexo, idade e nível socioeconômico em relação ao grupo de participantes com bronquiolite obliterante pós-infecciosa. Para avaliação da Qualidade de Vida Relacionada à Saúde geral foi aplicado a versão validada para o Brasil do instrumento PedsQL (Pediatric Quality of Life Inventory), por meio de entrevista. A comparação entre as médias da Qualidade de Vida Relacionada à Saúde entre os grupos foi realizada mediante o teste t para amostras independentes e para as variáveis categóricas por teste Qui-quadrado.
ResultadosParticiparam do estudo 34 pacientes com diagnóstico de bronquiolite obliterante pós-infecciosa e 34 controles. A média da idade das crianças incluídas foi 11,2 ± 2,5 anos e 49 (72%) deles eram do sexo masculino. Os grupos não apresentaram diferenças significativas em relação a essas variáveis. O escore de qualidade de vida foi significativamente e clinicamente menor no grupo bronquiolite obliterante pós-infecciosa em comparação com o controle nos domínios saúde: (72,36 ± 15,6; 81,06 ± 16,4; p = 0,031); escolar: (62,34 ± 20,7; 72,94 ± 21,3; p = 0,043) e no escore total (69,53 ± 14,9; 78,02 ± 14,8, p = 0,024), respectivamente.
ConclusãoConcluímos que os pacientes com bronquiolite obliterante pós-infecciosa apresentam escores de qualidade de vida relacionados à saúde menor que indivíduos saudáveis no escore total e nos domínios saúde e escolares.
Post-infectious bronchiolitis obliterans (PIBO) is a chronic obstructive pulmonary disease that is uncommon in childhood, but leads to a significant impairment of lung function at rest.1,2 Recently, two studies also demonstrated a decrease in cardiometabolic capacity during maximal exercise test in children with PIBO,3,4 which may indicate impairment in daily life activities and in subjective outcomes in health-related quality of life.5
Currently, the use of tools that evaluate the individual's different domains has been promoted in clinical practice, since these tools allow the evaluation of the disease impact through a subjective view of the patient.6,7 Studies indicate that structured questionnaires answered by the patient can capture the individual's perspective in a more neutral manner, despite the interpretation bias. Moreover, the use of a subjective and multidimensional tool in clinical practice allows the consideration that changes in health can lead to changes in well-being in the physical, psychological, and social aspects of the patient's life, and vice versa. Factors that are specific to the individuals and that interact with them can modify the health status. Among these, the health-related quality of life (HRQoL) assessment has been recognized as an important method for evaluating patients’ physical and psychosocial health.6 Studies investigating HRQoL in children with asthma, otitis, and cystic fibrosis suggested that children with chronic diseases have lower HRQoL scores than healthy controls, and that specific interventions can improve this outcome.8
A significant number of guidelines for patients with chronic pulmonary disease recommend the use of tools that assess HRQoL in clinical practice.9 HRQoL assessment has also been highly appreciated as a clinical outcome in studies that evaluate the effectiveness of interventions; from health planning to studies that monitor the health of populations.
Although in recent years there has been an improvement in the diagnostic and therapeutic techniques of PIBO patients, more multidimensional and subjective methods that can describe the consequences of the disease and the treatment in the different domains considered are still necessary. Based on this perspective, the present study aimed to evaluate the HRQoL of patients with a diagnosis of PIBO.
MethodsThe writing of the article followed the STROBE guidelines.10
Study designThis was a cross-sectional study.
ContextThe research is part of an umbrella project entitled “Cardiorespiratory Evaluation of Children with Post-Viral Bronchiolitis Obliterans.” The patients included in the present study were periodically followed-up at pediatric pulmonology outpatient clinics of the Brazilian Unified Health System (Sistema Único de Saúde [SUS]), referral services for the treatment of this disease in the city of Porto Alegre, state of Rio Grande do Sul, Brazil. The sample was recruited in the outpatient clinics, whereas the questionnaires and pulmonary function tests were performed in a third institution, a referral service for this type of examination.
ParticipantsInclusion criteriaThe study included a convenience sample of children and adolescents with a previous diagnosis of PIBO aged 8–16 years.
The diagnosis was based on the association of previously defined clinical, radiological, and functional criteria11: 1 – history of acute pulmonary infection in a previously healthy child younger than 2 years; 2 – persistent respiratory signs and symptoms four weeks after the initial event; 3 – high-resolution computed tomography showing alterations suggestive of bronchiolitis obliterans (BO), such as mosaic pattern, bronchiectasis, or atelectasis; 4 – airflow limitation by pulmonary function tests; 5 – exclusion of other chronic lung diseases that occur with persistent respiratory symptoms such as: severe asthma, cystic fibrosis, alpha-1-antitrypsin deficiency, and immunodeficiencies, among others.
Children with other chronic conditions that could compromise their quality of life were excluded. Healthy children were thus classified as they did not have any chronic diseases and did not use medication continuously. The children were selected in a public school due to social class similarities. The individuals in the control group were gender- and age-matched with the patient group.
Outcome variableThe present study adopted HRQoL as the outcome variable.
Data source and measurementsAll evaluations of the present study, pulmonary function tests, and questionnaires were carried out at Hospital de Clínicas de Porto Alegre (HCPA).
Pulmonary function testThe technical procedures and the acceptability and reproducibility criteria for the performance of pulmonary function tests followed the guidelines of the American Thoracic Society.3 Spirometry was performed in a Master-Screen Jaeger equipment (CareFusion, Germany) and the following parameters were evaluated: forced vital capacity; %FVC; forced expiratory volume in the first second, %FEV1; ratio between FEV1 and FVC, FEV1/FVC; forced expiratory flow between 25% and 75% of FVC; and FEF25–75%.
Data are shown as a percentage of the predicted value based on the Knudson reference values10 for spirometry. All examinations were performed by the same technician and in the morning.
Health-related Quality of Life (HRQoL)The generic questionnaire PedsQLTM 4.0, validated in Brazil, was applied to assess HRQoL.12,13 The tool includes 23 items that comprise the following domains: 1 – physical domain (eight items); 2 – emotional domain, (five items); 3 – social domain (five items); and 4 – school domain (five items). The PedsQLTM 4.0 generic questionnaire consists of parallel self-assessment forms for children.
The evaluation of the children used in the present research includes the age groups of 8–12 and 13–18 years. The items refer to the evaluation of the previous month, and the child/adolescent respondents use a five-level response scale, in which 0=never a problem; 1=almost never a problem; 2=sometimes it is a problem; 3=is often a problem; and 4=almost always a problem. The PedsQLTM was applied by the interviewer to children and adolescents. The items were inversely scored and linearly transposed into a 0–100 scale, in which 0=100, 1=75, 2=50, 3=25, 4=0; thus, the higher the score, the better the HRQoL.
BiasTo avoid possible measurement biases, all measurements were performed by the same evaluator, following the established standards for performing the examinations and applying the questionnaires. The children answered the questionnaire before the medical appointment, in the presence of the interviewer only.
Sample sizeStarting from a significant minimum difference of four points between the groups in the questionnaire, a standard deviation of 4, considering an alpha value of 5% and a beta of 20%, the minimum number of participants in each group was defined as 22.
Statistical analysisThe distribution of variables was assessed using the Kolmogorov–Smirnov test. Continuous variables were shown as means and standard deviations, whereas the categorical variables were shown as absolute and relative frequencies. The comparison between the measures of quality of life and age was performed using Student's t-test for independent samples and the chi-squared test for the gender variable. The minimum significant difference considered in the PedsQL life questionnaire is 4 points. The level of significance was set at 5%. Data analysis was performed using SPSS (SPSS Statistics for Windows, Version 17.0, Chicago, USA).
Ethical aspectsThe use of the research tool was previously authorized by the author of the PedsQL questionnaire. The project was approved by the Research Ethics Committee (REC) of the HCPA, in accordance with the current international and national standards and guidelines under No. 09-492. The participants’ parents or guardians signed the informed consent form and the children also expressed their willingness to participate in the research as suggested by the REC.
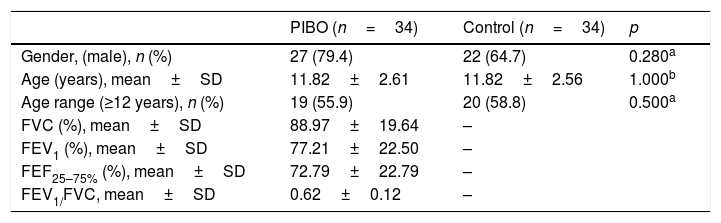
ResultsA total of 34 patients diagnosed with PIBO and 34 controls participated in the study. The mean age of the children included in the study was 11.2±2.5 years; 39 (57.3%) were schoolchildren aged >12 years and 49 (72%) of them were males. The groups did not show any significant differences in relation to these variables. Regarding pulmonary function, PIBO patients had a mean percentage of the predicted value for forced vital capacity, forced expiratory volume in the first second, mean forced expiratory flow, and FEV1/FVC of 88.97%, 77.21% 72.79% and 0.62, respectively, demonstrating pulmonary involvement (Table 1). The mean level of schooling of the parents of children with PIBO was 6.2 years.
Sample characteristics.
| PIBO (n=34) | Control (n=34) | p | |
|---|---|---|---|
| Gender, (male), n (%) | 27 (79.4) | 22 (64.7) | 0.280a |
| Age (years), mean±SD | 11.82±2.61 | 11.82±2.56 | 1.000b |
| Age range (≥12 years), n (%) | 19 (55.9) | 20 (58.8) | 0.500a |
| FVC (%), mean±SD | 88.97±19.64 | – | |
| FEV1 (%), mean±SD | 77.21±22.50 | – | |
| FEF25–75% (%), mean±SD | 72.79±22.79 | – | |
| FEV1/FVC, mean±SD | 0.62±0.12 | – |
PIBO, post-infectious bronchiolitis obliterans; SD, standard deviation; FVC, percentage of the predicted for forced vital capacity; FEV1, percentage of the predicted for forced expiratory volume in the first second; FEF25–75%, percentage of the predicted for the mean forced expiratory flow.
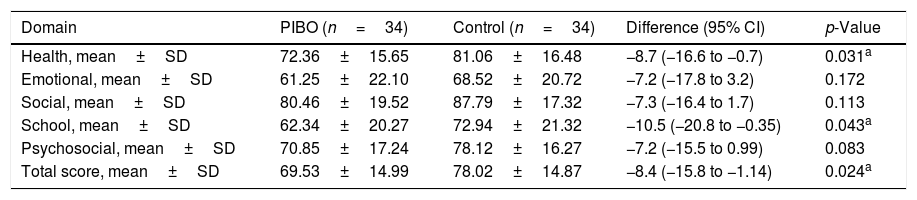
The quality of life score was significantly and clinically lower in the PIBO group compared to the control in the health (72.36±15.6 vs. 81.06±16.4, respectively; p=0.031) and school domains: (62.34±20.7 vs. 72.94±21.3, respectively; p=0.043) and in the total score (69.53±14.9 vs. 78.02±14.8, respectively; p=0.024; Table 2).
Quality of life score by domain.
| Domain | PIBO (n=34) | Control (n=34) | Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Health, mean±SD | 72.36±15.65 | 81.06±16.48 | −8.7 (−16.6 to −0.7) | 0.031a |
| Emotional, mean±SD | 61.25±22.10 | 68.52±20.72 | −7.2 (−17.8 to 3.2) | 0.172 |
| Social, mean±SD | 80.46±19.52 | 87.79±17.32 | −7.3 (−16.4 to 1.7) | 0.113 |
| School, mean±SD | 62.34±20.27 | 72.94±21.32 | −10.5 (−20.8 to −0.35) | 0.043a |
| Psychosocial, mean±SD | 70.85±17.24 | 78.12±16.27 | −7.2 (−15.5 to 0.99) | 0.083 |
| Total score, mean±SD | 69.53±14.99 | 78.02±14.87 | −8.4 (−15.8 to −1.14) | 0.024a |
PIBO, post-infectious bronchiolitis obliterans; SD, standard deviation; 95% CI, 95% confidence interval for the difference.
Student's t-test for independent samples.
The present study demonstrated that patients with PIBO present lower HRQoL scores than healthy individuals, in the total score and in the health and school domains.
There is a scarcity of studies investigating quality of life impairment in PIBO patients. However, studies with other chronic obstructive pulmonary diseases, such as cystic fibrosis and asthma, have already demonstrated the HRQoL impairment in children.14 A recent meta-analysis15 assessed the quality of life of pediatric patients with asthma compared with healthy controls, using 15 studies published between 1994 and 2013.
The study identified a decrease in the quality of life scores in children with asthma when compared to controls; the patients’ mean values were comparable to the present results in patients with PIBO.15 The similarity of HRQoL scores between patients with PIBO and asthma, in spite of the differences in disease severity, may be due to the sample of assessed children, which has received interdisciplinary care since the disease diagnosis, a factor that may have contributed to a greater control of the psychosocial factors related to the disease.
A multicenter study carried out in Argentina showed a significant difference in values related to the quality of life in the school domain of patients with confirmed chronic diseases when compared with healthy children, most of which were asthmatic.16 The impact of the disease on the school and health domains has already been demonstrated even in patients with acute infectious events.17 These findings promote the importance of the subjective viewpoint of these patients in different health domains in relation to disease impact. The ongoing study of HRQoL allows professionals from different areas to identify when it is necessary to intervene more specifically in these aspects of clinical practice.
The minimum significant difference for PedsQL in children with health conditions is of four points for each domain.18 The cutoff points for healthy patients with lower, moderate, and higher impairment have also been established. When analyzing the present results with the cutoff points described, it can be inferred that in the studied sample, the HRQoL impairment was moderate and that the difference between the means of the groups was clinically significant.
This study has some limitations; first, the cross-sectional design of the study does not allow conclusions to be drawn regarding a causal association between BO and lower quality of life scores. Another limitation is that the assessed sample was chosen by convenience. Considering PIBO as a rare and chronic disease, the results appear to meet the hypothesis of the present study. These data allow a first diagnosis of HRQoL in patients with PIBO. Differences in quality of life between individuals with PIBO and their healthy peers could be attributed to the disease impairment, since the other characteristics of the control group were paired.
Finally, due to the similarities between PIBO and severe asthma regarding symptoms and functional and imaging tests, the possibility that some patients may have both diseases cannot be completely discarded, despite fact that the chronic respiratory clinical picture was observed after the acute episode of PIBO.
The authors conclude that patients with PIBO have lower HRQoL scores than healthy individuals in the total score and in the health and school domains. The present study reinforces the importance of the inclusion of a more subjective and multidimensional tool in the assessment of patients with BO.
FundingCAPES and CNPQ provided grants to the postgraduate students.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Sarria EE, Mundstock E, Machado DG, Mocelin HT, Fischer GB, Furlan SP, et al. Health-related quality of life in patients with bronchiolitis obliterans. J Pediatr (Rio J). 2018;94:374–9.
Study carried out at Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Universidade Federal do Rio Grande do Sul (UFRGS), Centro Universitário Ritter dos Reis (UniRitter), Porto Alegre; and Universidade de Santa Cruz do Sul (UNISC), Santa Cruz do Sul, RS, Brazil.