This study examined the growth status and physical development of Brazilian children with autism spectrum disorders from 4 to 15 years of age. Furthermore, it was examined whether variation in growth patterns and weight status was influenced by the use of psychotropic medications.
MethodsOne-hundred and twenty children aged 3.6–12.1 years at baseline (average=7.2 years, SD=2.3 years) diagnosed with autism spectrum disorders were measured on three repeated occasions across a 4-year period. Stature, body mass, and body mass index were considered. Bayesian multilevel modeling was used to describe the individual growth patterns.
ResultsGrowth in stature was comparable to the age-specific 50th percentile for Centers for Disease Control and Prevention reference data until approximately 8 years, but a substantial decrease in growth rate was observed thereafter, reaching the age-specific 5th percentile at 15 years of age. Both body mass and body mass index values were, on average, higher than both the Brazilian and Centers for Disease Control and Prevention age-specific 95th percentile reference until 8 years, but below the 50th specific-age percentile at the age of 15 years.
ConclusionsBrazilian boys with autism spectrum disorders between 4 and 15 years appear to have impaired growth in stature after 8–9 years of age, likely impacting pubertal growth. A high prevalence of overweight and obesity was observed in early childhood, although a trend of substantial decrease in body mass and body mass index was apparent when children with autism spectrum disorders entered the years of pubertal development.
Este estudo examinou o estado de crescimento e o desenvolvimento físico de crianças brasileiras com transtornos do espectro autista entre 4 e 15 anos de idade. Adicionalmente, examinamos se a variação nos padrões de crescimento e na massa corporal foi influenciada pelo uso de medicamentos psicotrópicos.
Métodos120 crianças com idades entre 3,6 e 12,1 anos no início do estudo (média=7,2 anos, DP=2,3 anos) diagnosticadas com transtornos do espectro autista foram avaliadas em três ocasiões repetidas em um período de 4 anos. Foram considerados estatura, massa corporal e índice de massa corporal. O modelo multinível bayesiano foi utilizado para descrever os padrões de crescimento individual.
ResultadosO crescimento em estatura foi comparável ao percentil 50 específico para a idade para os dados de referência do Centro de Controle e Prevenção de Doenças dos Estados Unidos até cerca de 8 anos. Porém, foi observada uma redução substancial na taxa de crescimento depois dos 8 anos, atingindo o percentil 5 específico para a idade aos 15 anos de idade. Tanto os valores de massa corporal quanto de índice de massa corporal foram, em média, maiores comparativamente ao percentil 95 específico para a idade até aos 8 anos da referência brasileira e do Centro de Controle e Prevenção de Doenças dos Estados Unidos, porém abaixo do percentil 50 específico para a idade aos 15 anos de idade.
ConclusõesOs meninos brasileiros com transtornos do espectro autista entre 4 e 15 anos parecem ter retardo do crescimento na estatura após os 8–9 anos, provavelmente afeta o crescimento púbere. Foi observada uma alta prevalência de sobrepeso e obesidade na primeira infância, apesar de uma tendência de redução substancial na massa corporal e no índice de massa corporal ter sido aparente quando as crianças com transtornos do espectro autista entraram nos anos de desenvolvimento púbere.
Autism spectrum disorder (ASD) is a group of heterogeneous neurodevelopmental conditions, characterized by early-onset impairment in social skills and in social communication, and by restricted and repetitive behaviors.1 The worldwide prevalence of ASD is about 1%; however, population reports have revealed a consistent and significant rise in ASD prevalence. These changes may be explained by advances in diagnostic concepts and criteria and by improved awareness and recognition of ASD.1 In Brazil, the prevalence of ASD in school age children is almost 0.3%.2
Children with ASD likely have an increased risk of obesity and obesity-related metabolic disorders.3 It has been estimated that about 30% of children with ASD were obese, compared to approximately 24% of children without ASD.4 Recent data shows a prevalence of 42.4% for overweight and 21.4% for obesity in children with ASD, compared to 26.1% for overweight and 12.0% for obesity in age-matched children.5
The presence of restrictive and repetitive behaviors in children and adolescents with ASD may impair motor skills development and physical fitness levels, leading to low levels of daily physical activity.6 Consequently, this leads to a high incidence of overweight/obesity and associated complications.4 Other influences on body mass and composition associated with ASD include food selectivity,4 gastrointestinal disturbances,7 sleep problems,8 and psychotropic medication.9 Hence, possible implications for short- and long-term growth of children with ASD should be considered.10
About 30–60% of children with ASD are prescribed with psychotropic medication to treat behavioral abnormalities.4 Antipsychotics use is accompanied by some secondary adverse metabolic effects, such as increased body mass gain and metabolic side effects.11 However, available reports with ASD patients are sparse, based on cross-sectional observations, and mostly inconclusive.
For a considerable time, there has been interest in growth and weight status studies in children and adolescents with ASD.12,13 Nonetheless, the body of data on the physical status and potential growth concerns of children with ASD remains limited, contradictory, and inconclusive10 and mostly based on cross-sectional observations. Hence, research is needed to fully assess the physical status and potential growth concerns of children with ASD.10 The present study adopted a mixed-longitudinal approach to examine the growth patterns and weight status of children with ASD from 4 to 15 years of age. Furthermore, it was examined whether variation in growth patterns and weight status was influenced by the use of psychotropic medications.
MethodsStudy design and participantsThis study used a mixed longitudinal design based on three repeated measurements across a 4-year span. The first measurement was in March 2014, the second in March 2015, and the third observation in February 2017. Boys with ASD aged 3.6–12.1 years at baseline (average=7.2 years, standard deviation=2.3 years) volunteered to participate in this study (n=120). The participants were recruited from a specialized pediatric center for populations with ASD located in Maceió, Alagoas (Brazil). All participants and their families received information about the study and signed an informed consent. The study was carried out in accordance with the ethical standards of the Declaration of Helsinki, and approved by the Human Ethics Committee of the Federal University of Alagoas (Protocol No. 1.091.864).
The children and adolescents were heterogenic in terms of etiologic characteristics (e.g., Asperger's syndrome, autism, or developmental disorder without specification) and use of psychotropic medication. In all cases, the diagnosis of an ASD was confirmed by an experienced psychiatrist, based on DSM-IV criteria.14 Information about the psychotropic medication (antiepileptics, antipsychotics, antidepressants, and stimulants) used by the participants was obtained from the participants’ medical records. However, access to children's proportions of the medication doses prescribed was not available.
Thirteen children were classified with Asperger's syndrome, 88 were classified with autism, and 19 were classified with developmental disorder without specification. Regarding medication prescription, 44 children had no prescribed medication during the period of observation; 10 children were medicated with antipsychotics (including risperidone and pericyazine); 49 children were medicated with antipsychotics combined with other stimulant medications (methylphenidate), antidepressants (including nortriptyline and fluoxetine), and/or antiepileptics (including carbamazepine, phenobarbital, and clonazepam); 17 children were medicated with stimulants, antidepressants, and/or antiepileptics, but not with antipsychotics.
ProceduresChronological age was calculated to the nearest 0.1 year as birth date minus testing date. Stature and body mass were measured by a single experienced observer. Stature was measured with a portable stadiometer (Seca model 206, Hanover, MD, USA) to the nearest 0.1cm. Body mass was measured with a calibrated portable scale (Seca model 770, Hanover, MD, USA) to the nearest 0.1kg. Body mass index (BMI) was calculated as body mass (kg) divided by height squared (m2). Reference age-specific data for the US population was used to compare data for stature, body mass, and weight status based on specific BMI cut-off values for reference data,15,16 as well as specific Brazilian BMI cut-off values.17
Statistical analysisBayesian modelingBayesian methods treat parameters as random variables combining both sample data and prior distribution information to estimate posterior information. Multilevel modeling is a flexible and robust framework to naturally deal with unbalanced longitudinal data, as in the present study, overcoming the shortcomings of conventional frequentist methods (ordinary least-squares and maximum likelihood estimations).18
A basic two-level polynomial growth model curve was initially used to separately model stature, body mass, and BMI against chronological age (see the Supplementary Materials for detail about the basic two-level polynomial growth modeling). The model describes each participant's successive measurements over time, defining the individual's change at each measurement point and its variation (level 1), as well as the difference in trajectories between participants and its variation (level 2). To capture growth at infancy and at the onset of puberty for stature, this study considered at least growth coefficients up to the cubic.
Chronological age was centered at the beginning of the average of the observations (4.1 years). This allows for the model to provide predicted values with meaningful information within the range of observations, in particular the intercept term. To examine the best fit to describe the growth trends, competing polynomial models were compared, and group-level effects were assessed by the widely applicable information criterion (WAIC).18 After establishing the polynomial trends of change in body dimensions, it was explored variation in the trajectories between participants exposed to psychotropic medication use (level 3; binary variable—no medication or other non-psychotropic medication coded 0, psychotropic medication use coded 1). It was determined whether there was variation between participants at the group level (levels 2 and 3) across the intervals of observations.
Prior distributions of the parametersFor each of the dependent variables, i.e., stature, body mass, and BMI, informative priors were considered for the intercept term, based on the reference estimates for stature, body mass, and BMI for the US population.15 It was considered the 97th percentile estimate for both body mass and BMI, as a prevalence of obesity, to be expected in children with ASD.3–5,10 For the polynomial growth estimates at the population level, the estimates were regularized using weakly informative prior distributions, normal priors (0, 10). For group-level effects, weakly informative prior distributions were used, half-Cauchy priors (0, 2). For the correlations of group-level effects, a non-informative LKJ prior was used (1), allowing for all correlations matrices to be equally likely a priori.18,19
Bayesian estimationFour chains with 10000 iterations with a warm-up length of 5000 iterations were used to ensure convergence of the Markov chain. The convergence of the Markov chain was assessed by a visual inspection of the trace plots. Visual inspection of standardized residuals against fitted values, observed values, and the independent variable in the models plots and observed against fitted values plots were explored to determine the models’ validity to describe the data. Posterior predictive checks were used to confirm that relevant interactions were not omitted.20 WAIC was used to compare models and to ensure that the data was not overfit.18,20
All models were estimated by using Bayesian methods implemented via Markov Chain Monte Carlo (MCMC) simulation and by using Hamiltonian Monte Carlo and its extension, the No-U-Turn Sampler using Stan,21 obtained using brms package19 available as a package in the R statistical language.
ResultsDescriptive estimates at each observation across the 35-month period in the Brazilian children and adolescents with ASDs are summarized in Table 1. Also, the distribution of etiologic characteristics (Asperger's syndrome, autism, or developmental disorder without specification) of the present sample at the baseline of observations is presented in Table 2. Exploratory analysis of growth trends showed that stature and BMI were best described by a cubic model, whereas body mass was best described by a quadratic model.
Sample characteristics at each observation across the 35-month period in children and adolescents with ASDs (children were grouped at baseline by yearly age group, e.g., 4 years from 3.5 to 4.4 years).
| Age group at baseline | Age (years) | Stature (cm) | Body mass (kg) | BMI (kg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months after | 35 months after | Baseline | 12 months after | 35 months after | Baseline | 12 months after | 35 months after | Baseline | 12 months after | 35 months after | |
| 4 years | 4.1 (0.2) | 5.2 (0.2) | 7.1 (0.2) | 101.0 (6.5) | 111.3 (5.1) | 118.9 (5.3) | 26.3 (15.7) | 27.8 (16.2) | 28.7 (1.4) | 25.8 (14.8) | 22.2 (11.8) | 20.0 (10.8) |
| 5 years | 5.1 (0.2) | 6.2 (0.2) | 8.0 (0.2) | 107.2 (9.2) | 114.7 (10.0) | 121.1 (5.5) | 22.8 (5.6) | 23.9 (5.3) | 24.6 (5.1) | 19.7 (2.8) | 18.0 (2.8) | 16.6 (2.6) |
| 6 years | 6.0 (0.2) | 7.1 (0.2) | 9.0 (0.2) | 112.3 (8.6) | 121.5 (7.6) | 127.0 (7.9) | 30.2 (10.7) | 32.2 (11.5) | 33.6 (11.7) | 24.1 (9.1) | 21.7 (7.3) | 20.8 (6.8) |
| 7 years | 6.9 (0.2) | 8.0 (0.2) | 9.9 (0.2) | 125.4 (14.3) | 130.8 (14.3) | 135.4 (14.0) | 34.0 (15.2) | 34.9 (14.5) | 35.9 (13.6) | 20.8 (6.0) | 19.7 (5.0) | 19.0 (4.2) |
| 8 years | 8.0 (0.2) | 9.1 (0.2) | 11.0 (0.2) | 125.1 (9.5) | 133.9 (8.1) | 138.0 (8.0) | 29.9 (5.7) | 33.7 (9.7) | 35.2 (9.8) | 19.0 (2.8) | 18.7 (4.5) | 18.4 (4.4) |
| 9 years | 9.0 (0.1) | 10.1 (0.1) | 12.0 (0.1) | 131.3 (11.7) | 136.6 (11.3) | 140.9 (12.0) | 40.3 (10.5) | 42.1 (10.4) | 44.2 (10.3) | 23.9 (8.5) | 23.0 (7.5) | 22.9 (7.6) |
| 10 years | 10.1 (0.2) | 11.2 (0.2) | 13.1 (0.2) | 149.9 (27.5) | 154.5 (25.9) | 158.6 (23.9) | 57.4 (25.2) | 59.9 (24.9) | 59.2 (24.7) | 26.3 (13.8) | 25.5 (11.8) | 24.3 (10.5) |
| 11 years | 11.1 (0.2) | 12.2 (0.2) | 14.1 (0.2) | 147.7 (7.6) | 163.4 (8.1) | 166.0 (7.6) | 52.2 (11.0) | 51.5 (8.0) | 54.2 (6.9) | 24.1 (6.0) | 19.6 (3.3) | 19.8 (3.1) |
| 12 years | 11.8 (0.2) | 12.9 (0.2) | 14.8 (0.2) | 146.0 (27.1) | 151.2 (25.3) | 154.3 (26.0) | 61.5 (31.9) | 62.3 (32.1) | 64.0 (28.5) | 26.6 (7.1) | 25.2 (7.5) | 25.4 (5.1) |
Distribution of etiologic characteristics (Asperger's syndrome, autism, or developmental disorder without specification) of children and adolescents with ASDs at the baseline of observations.
| Age group at baseline | Asperger's syndrome | Autism | Developmental disorder without specification | Total sample |
|---|---|---|---|---|
| 4 years | – | 15 | 2 | 17 |
| 5 years | 3 | 14 | 2 | 19 |
| 6 years | – | 9 | 3 | 12 |
| 7 years | 3 | 15 | 2 | 20 |
| 8 years | 2 | 9 | 3 | 14 |
| 9 years | 3 | 11 | 2 | 16 |
| 10 years | 1 | 7 | 2 | 10 |
| 11 years | – | 5 | 1 | 6 |
| 12 years | 1 | 3 | 2 | 6 |
The results from the Bayesian multilevel modeling growth for stature, body mass, and BMI for children and adolescents between 4 and 15 years of age with ASD are summarized in Table 3. The results showed that changes in body mass and BMI did vary between children grouped by medication use (Table 3). These results imply that medication had an influence on body mass and BMI, which was independent of chronological age across the range of observations.
Bayesian multilevel modeling growth for stature, body mass, and BMI between 4 and 15 years (chronological age centered at 4.1 years) of children and adolescents with ASDs (number of participants=120, number of observations=360).
| Stature (cm) | Body mass (kg) | BMI (kg/m2) | |
|---|---|---|---|
| Population-level effects (95% credible interval) | |||
| Intercept | 106.91 (103.77 to 110.14) | 31.82 (26.41 to 36.64) | 26.94 (23.35 to 30.05) |
| Age | 7.83 (6.45 to 9.13) | 0.81 (0.26 to 1.34) | −3.10 (−3.83 to −2.40) |
| Age2 | −0.65 (−0.94 to −0.34) | 0.03 (−0.03 to 0.08) | 0.43 (0.28 to 0.59) |
| Age3 | 0.03 (0.01 to 0.05) | – | −0.02 (-0.03 to −0.01) |
| Group-level effects (95% credible interval) | |||
| Level 1 standard deviation (within children) | |||
| Within-individuals | 3.88 (3.54 to 4.29) | 2.18 (1.94 to 2.47) | 1.81 (1.61 to 2.02) |
| Level 2 standard deviation (between children) | |||
| Intercept | 13.00 (10.33 to 15.80) | 16.28 (13.58 to 19.18) | 10.05 (8.51 to 11.84) |
| Age | 0.29 (0.01 to 0.70) | 0.51 (0.03 to 1.10) | 0.65 (0.43 to 0.89) |
| Age2 | – | 0.07 (0.02 to 0.12) | – |
| Level 3 standard deviation (between children grouped by psychotropic medication use) | |||
| Intercept | – | 3.02 (0.13 to 9.42) | 1.97 (0.15 to 5.59) |
Although both growth in stature and BMI were described by cubic models, substantial age-matched variation between children with ASD (group-level effects at intercept) and substantial variation between children for the linear growth coefficient was observed. Notably, for body mass, the quadratic pattern for population effects was adopted given the substantial variation at the group level in the quadratic term (between individual's trajectory variation).
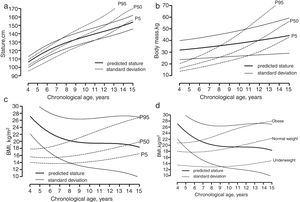
Brazilian boys with ASD had similar statures and growth around the age-specific 50th percentile for reference North American data until about 8 years. Thereafter, the growth in stature tended to approach the age-specific 10th percentile until 15 years (Fig. 1A). The average body mass values and growth rate were higher than the 95th specific-age percentile for reference North American data until about 8 years, with the rate of growth in mass decreasing thereafter, approaching the 5th specific-age percentile at the age of 15 years (Fig. 1B). As for BMI, average values were higher than the 95th specific-age percentile for reference North American data until 9 years, with the rate of growth in BMI approaching the age-specific 50th percentile at the age of 15 years (Fig. 1C). Hence, at least until 9 years of age, the children with ASD were obese, and a substantial part remained overweight at the age of 15 years. Considering Brazilian BMI references, the observed trend for obesity among infants with ASD was confirmed. However, at 9 years of age, the children in the present sample had normal weight considering the Brazilian specific-age references, and a trend of more pronounced decreasing BMI was noted entering the pubertal years, approaching the age-specific 5th percentile at the age of 15 years.
Average growth in (a) stature, (b) body mass, and (c) body mass index of children and adolescents with ASDs plotted on reference centiles for American boys (CDC data available online from: http://www.cdc.gov/growthcharts/percentile_data_files.htm), and (d) body mass index of children and adolescents with ASDs plotted on reference centiles for Brazilian boys.
The main findings of these longitudinal observations were that the body mass and weight status of children with ASD was influenced by the use of psychotropic medication alone, or combined with stimulants and/or antiepileptics, independent of the age. This study also showed that children with ASD appear to have statures and growth rates similar to reference populations15 until about 8–9 years of age. After this age, there was a decrease in stature and growth rate for the children with ASD compared with reference growth charts. To the best of our knowledge, this is the first study presenting longitudinal data to describe physical growth and weight status of children with ASD while accounting for variation in the psychotropic medication used.
The results of the present study showed that children with similar ASD statures and growth rates compared to reference populations15 until about 8–9 years of age. Thereafter, a marked decrease in stature and growth rate was observed for the children with ASD entering pubertal ages compared with reference growth charts. These results are consistent with observations showing a tendency for physical growth delays in children with ASD.12 The decreases in growth rate in stature are around the onset of puberty, impacting pubertal growth. This possible deviant changes pubertal growth in stature may reflect the long-term effects of compromised dietary intake associated with typical behaviors of children with ASD.10,22 There a growing body of cross-sectional evidence suggesting that children with ASD may be at elevated risk for unhealthy weight.3 In the present study, the values of body mass and BMI, particularly between 4 and 8 years, were high compared to the age-specific reference of normal children,15,16 and comparable with specific Brazilian BMI cut-off values for obesity.17 Generally, the prevalence of overweight and obesity in children with ASD can be explained by lower levels of physical activity, delayed levels of psychomotor skills, and poor levels of associated physical fitness.9 However, after about 8 years, there was a marked decrease trend of growth in body mass and BMI, approaching the age-specific 5th percentile at the age of 15 years, i.e., the specific Brazilian BMI cut-off values for underweight.17
Despite an initial trend of obesity in early infancy, children and adolescents with ASD may have physical growth delays. As noted above, it may reflect the long-term effects of compromised dietary intake associated with the typical behaviors of children with ASD. Also, it has been reported hormonal dysregulation in children with ASD compared to age-matched children, particularly elevated levels of leptin, total testosterone, and free testosterone, and lowers level of growth hormone, des-acyl ghrelin, and acyl ghrelin.23 This suggests that potential dysregulation of hormonal mechanisms can be one of the factors responsible for obesity and physical growth delays. Unfortunately, the available data is scarce and based on cross-sectional observations, hence the present results add to the need for further longitudinal endocrine investigation among children and adolescents with ASD.
However, children with ASD are often prescribed psychotropic medications to treat behavioral abnormalities.3,4 Antipsychotics use is accompanied by some secondary adverse metabolic effects, such as increased weight gain, metabolic side effects, and the onset of type-2 diabetes mellitus.24,25 Longitudinal observations have showed substantial weight gain in psychotic patients treated with risperidone, an antipsychotic drug.26 Children treated with risperidone or quetiapine were observed to be at a higher risk to develop obesity, elevated waist circumference, and dyslipidemia during 12 months of treatment.27 These observations were confirmed by an increase in metabolic markers, including fasting glucose, fasting insulin, HOMA-IR, triglycerides, ratio of triglycerides to HDL cholesterol, and ratio of total. Altogether, the previous observations emphasize the importance of regular monitoring for early identification and treatment of metabolic side effects and also the development of other treatments (such as exercise) for ASD symptoms.28 The present results showed a substantial influence of the use of psychotropic medication alone, or combined with stimulants, antidepressants, and/or antiepileptics, on body mass and weight status in Brazilian children with ASD. It was also observed that this influence was independent of the age within the range observed.
There are limitations in the present study analysis worth noting. The analysis considered only boys with ASD, as the available sample of girls was very limited for modeling. The lower number of girls with ASD in this sample is consistent with the higher prevalence of ASD in boys generally reported.29 References from the United States were used to compare the data. These references may be insufficiently sensitive to describe Brazilian children and adolescents’ normal growth, in particular considering ethnic and socioeconomic variability, and its possible implications on the growth status of children and adolescents. Hence, caution in the interpretation and generalization of the present results is warranted. However, to the best of our knowledge, no consistent Brazilian growth references are available. Unfortunately, the World Health Organization growth standards, which include a subsample from the South Region of Brazil, do not provide body mass references for the age range of the present study.30
In summary, the present longitudinal observations in Brazilian boys with ASD between 4 and 15 years showed a pattern of normal growth in stature until the age close to the onset of puberty, followed by a marked decrease in the growth rate during adolescent years within the range of observation. Furthermore, there was a trend of high prevalence of overweight and obesity among Brazilian boys with ASD, although a trend of decrease in BMI was apparent when children entered the years of pubertal development. There was an apparent influence of psychotropic medications use on the weight status of children with ASD. Future longitudinal designs should also consider the interactions between levels of physical activity, food selectivity behaviors, and dietary intake with somatic growth.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Toscano CV, Ferreira JP, Gaspar JM, Carvalho HM. Growth and weight status of Brazilian children with autism spectrum disorders: A mixed longitudinal study. J Pediatr (Rio J). 2019;95:705–12.