To determine the prevalence of increased serum fibrinogen levels and its association with cardiometabolic risk factors in overweight or obese children and adolescents.
MethodsCross-sectional study with 138 children and adolescents (overweight or obese) followed at a reference outpatient clinic of the public health care network. Fibrinogen concentration was divided into quartiles, and values above or equal to the third quartile were considered high. The association between high fibrinogen values and cardiometabolic risk factors was assessed using Pearson's chi-squared test or Fisher's exact test, as necessary. Logistic regression was used to adjust variables predictive of fibrinogen levels. Analyses were performed using SPSS version 22.0 and SAS software, considering a confidence interval of 95%.
ResultsSerum fibrinogen levels were elevated in 28.3% of individuals, showing association with the presence of high CRP (p=0.003, PR: 2.41, 95% CI: 1.30–4.46) and the presence of four or more risk factors (p=0.042; PR: 1.78, 95% CI: 1.00–3.17). After a logistic regression, only elevated CRP remained associated with altered fibrinogen levels (p=0.024; PR: 1.32; 95% CI: 1.09–5.25).
ConclusionsIncreased fibrinogen was prevalent in the study population and was associated with ultrasensitive C-reactive protein and the presence of four or more cardiovascular risk factors; it should be included in the assessment of individuals at risk.
Verificar a prevalência de níveis séricos elevados de fibrinogênio e sua associação com os fatores de risco cardiometabólicos em crianças e adolescentes com sobrepeso ou obesidade.
MétodosEstudo transversal com 138 crianças e adolescentes (obesos ou com sobrepeso) acompanhados em um ambulatório de referência da rede pública. A concentração do fibrinogênio foi distribuída em quartis, sendo considerada elevada quando os valores eram iguais ou superiores ao terceiro quartil. A associação entre o valor elevado do fibrinogênio com os fatores de risco cardiometabólicos foi verificada através do teste qui-quadrado de Pearson ou teste exato de Fisher, quando necessário. A regressão logística foi utilizada para ajuste das variáveis preditoras do nível do fibrinogênio. As análises foram realizadas no SPSS 22.0 e SAS, considerando-se o intervalo de confiança de 95%.
ResultadosOs níveis séricos de fibrinogênio estiveram elevados em 28.3% dos indivíduos, apresentando associação com a PCR elevada (RP: 2.41; IC 95%: 1.30–4.46, p=0.003) e com a presença de quatro ou mais fatores de risco (RP: 1.78; IC 95%: 1.00–3.17; p=0.042). Após a regressão logística, apenas o PCR elevado continuou associado ao fibrinogênio alterado (RP: 1.32; IC 95% 1.09–5.25; p=0.024).
ConclusõesO aumento do fibrinogênio foi prevalente na população estudada e esteve associado à proteína C reativa ultrassensível e ao número igual ou superior a quatro de fatores de risco cardiovasculares, devendo ser incluído na avaliação de indivíduos sob risco.
The prevalence of excess weight has increased in all age groups in Brazil, similar to what has occurred worldwide.1 Data from the Family Budget Survey2 show that the proportion of obese children has increased four-fold in the last 20 years and three-fold in adolescents in the same period, findings similar to those observed in developed countries.3,4
Obesity represents a subclinical inflammatory condition that results in a considerable number of cardiometabolic risk factors.5 Stoopa et al.,6 when assessing the inflammatory and prothrombotic status of children and adolescents with obesity or normal weight, found elevated fibrinogen levels at an age as young as 6 years in the obese children, regardless of pubertal status.
Inflammation in atherogenesis is caused by the synthesis, secretion, and storage of proinflammatory cytokines by adipocytes, producing a state of low-grade inflammation with vascular and metabolic complications7 that leads to vascular endothelial dysfunction, considered to be the onset of the atherogenic process.8 Coagulation factors, such as fibrinogen, blood flow, and inflammatory factors, have gained importance in establishing the atherosclerotic process and are considered important risk factors for cardiovascular disease.7,9
Fibrinogen, an acute phase protein, is part of the group of inflammatory biomarkers produced by hepatocytes and is considered an important marker to monitor the atherosclerotic inflammatory process evolution,10 as it acts on the genesis of the atherothrombotic process through regulation of cell adhesion and proliferation, vasoconstriction at the site of endothelial injury, stimulation of platelet aggregation, and blood viscosity.7,8
Although the association of obesity with hyperfibrinogenemia has been reported in children, the association of fibrinogen with cardiometabolic risk factors is not yet well-established.11,12 The search for biomarkers for early identification of individuals at higher risk of developing atherosclerosis and the knowledge of its association with other cardiometabolic risk factors is critical for the understanding and development of interventions aimed at reducing morbidity and mortality from cardiovascular disease.
Thus, this study aimed to determine the prevalence of elevated serum levels of fibrinogen and its association with cardiometabolic risk factors in overweight or obese children and adolescents.
MethodsCross-sectional study, conducted between June of 2011 and April of 2012, at the Centro de Obesidade Infantil (Childhood Obesity Center – COI), located at Instituto de Saúde Elpídeo de Almeida (ISEA), Campina Grande-PB. COI is a referral service for childhood obesity in the city and consists of researchers and a multidisciplinary team that includes doctors (pediatrician and endocrinologist), pharmacists, nutritionists, psychologists, nurses, social workers, and physical education professionals.
When the research began, there were 450 obese or overweight children and adolescents enrolled at COI. The study included those who attended COI throughout the study period, comprising a convenience sample of 138 children and adolescents. Those with diseases receiving medication that interfered with glucose or lipid metabolism; those with a diagnosis of genetic syndrome; and those who achieved a nutritional status of normal weight were excluded. For study purposes, the calculation of the sample statistical power was performed retrospectively in order to allow estimation of altered fibrinogen prevalence of 28.3%, to ensure that the sample size allowed attaining the proposed objectives. For measures of association, the sample showed a statistical power of 85% (β=15%) for the confidence level of 95%.
The anthropometric variables (weight, height, and waist circumference) were verified in duplicate, considering the mean value of two measurements, according to the recommendations of the World Health Organization (WHO).13
Nutritional status was classified according to body mass index (BMI), as recommended by the Centers for Disease Control and Prevention (CDC) as overweight (BMI ≥85th percentile and <95th percentile), obesity (BMI ≥95th percentile and <97th percentile), and severe obesity (BMI ≥97th percentile).14 Waist circumference (WC) was considered increased when ≥the 90th percentile, according to the International Diabetes Federation (IDF).15 with a maximum limit of 88cm for girls and 102cm for boys, according to the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII).16
Blood pressure was measured by the oscillometric method in a Tycos® (Welch Allyn Inc., NY, USA) device, according to the guidelines established in the VI Brazilian Guidelines on Arterial Hypertension.17
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and glycemia were evaluated by the enzymatic colorimetric method in automated equipment (BioSystems, Model 310, Applied Biosystems, CA, USA); fibrinogen was measured by the coagulometric method; high-sensitivity C-reactive protein (hs-CRP) and insulin by chemiluminescence in IMMULITE 1000 automated equipment (Siemens®, Siemens Healthcare, Erlangen, Germany). Low-density lipoprotein (LDL-c) was calculated using the Friedewald formula.18 Blood collection was performed after a 12-h fasting period.
The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was used as the criterion for diagnosis of insulin resistance (IR), classified as such when values ≥2.5 were found.19
The following were considered cardiometabolic risk factors: BMI ≥97th percentile, WC ≥90th percentile for gender, age, and ethnicity, with a maximum limit of 88cm for girls and 102cm for boys16; systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) ≥90th percentile for gender, height, and age17; TG ≥130mg/dL, TC ≥170mg/dL; HDL-c<45mg/dL; LDL-c ≥130mg/dL; fasting glucose ≥100mg/dL; insulin ≥15μIU/mL; HOMA-IR ≥2.5; and CRP>3mg/L.20,21 Individuals with CRP ≥10mg/L were excluded from the analysis of this variable, as in these cases, it is recommended to rule out the presence of an acute inflammatory process.21
Fibrinogen levels were divided into quartiles; values ≥the 75th percentile of the sample, which corresponds to 3.4g/L, were considered high, as there is no established cutoff for the pediatric population.22
The project was approved by the Research Ethics Committee of Universidade Estadual da Paraíba (UEPB) (CAEE – 0256.0.133.000-1) and was performed after parents or guardians signed an informed consent, authorizing the participation of their children in the study.
The data are shown as percentage and median, with the respective interquartile range (IQ). The association between high fibrinogen values (values ≥the 75th percentile) with gender, age, nutritional status, WC, SBP, DBP, TG, HDL-c, LDL-c, TC, blood glucose, insulin, HOMA-IR, and hs-CRP was evaluated by Pearson's chi-squared test or Fisher's exact test, as necessary, with prevalence ratios (PR) and their respective confidence intervals.
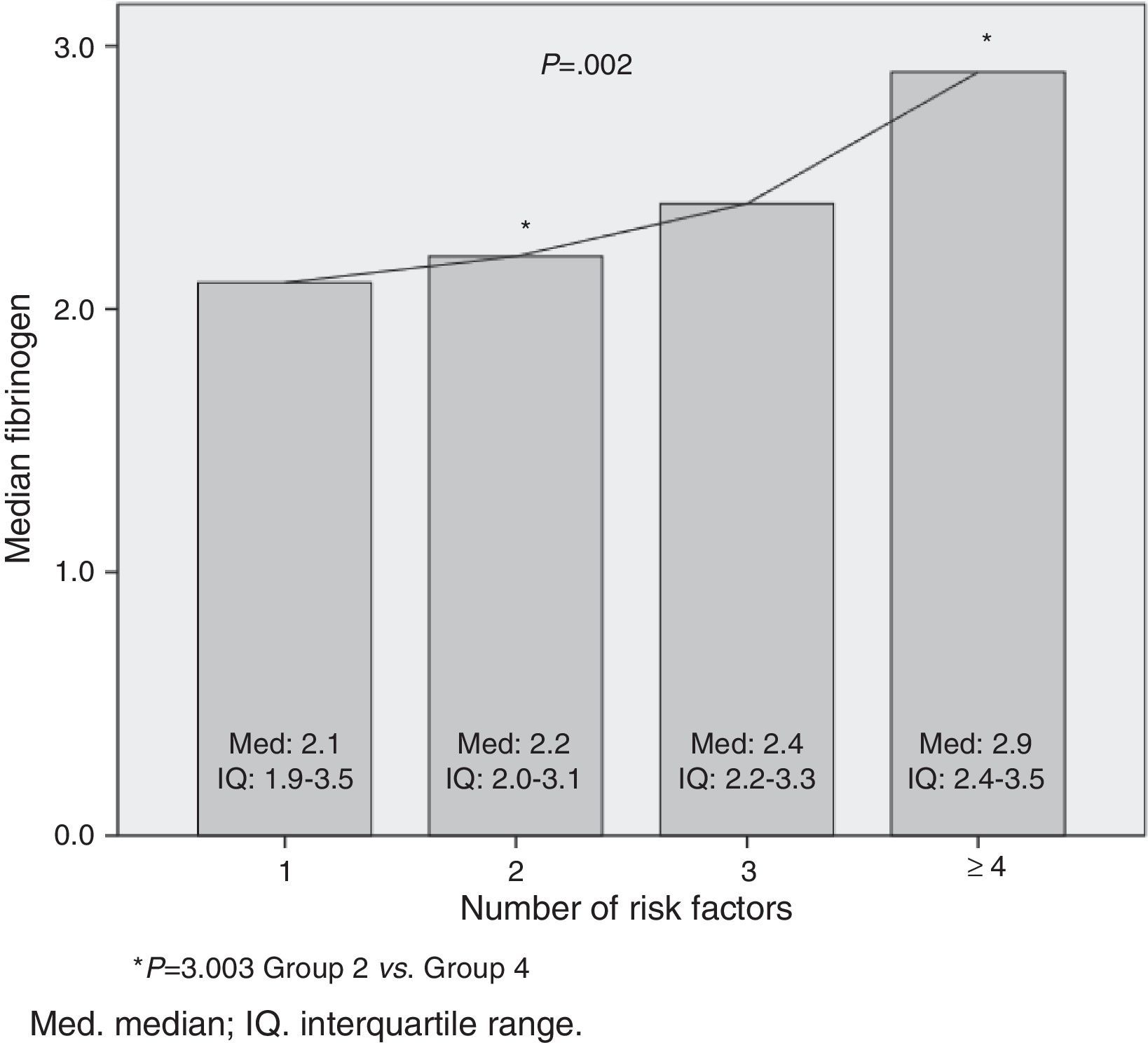
After performing the Kolmogorov–Smirnov test, which verified a non-parametric distribution of the “fibrinogen” variable, the Kruskal–Wallis test was applied to test the association of the fibrinogen median with nutritional status (overweight, obesity, and severe obesity) and with the number of cardiometabolic risk factors (1=one risk factor; 2=two risk factors; 3=three risk factors, 4=four or more risk factors). Subsequently, the Mann–Whitney test was used to evaluate the difference of these measures between each group, adjusted to ensure that the α error did not exceed the value of 0.05.
Multiple logistic regression was used to adjust the variables, whose criteria for inclusion was the association with the dependent variable in the bivariate analysis with a p-value<0.20. The variables were included in the regression analysis using the “Enter” method, according to the decreasing value of the PR. The Hosmer and Lemeshow test was used as a measure of goodness-of-fit of the logistic regression models, in which a p≥0.05 indicates that the model is adjusted.
The analyses were performed using the SPSS program, version 22.0 (SPSS Inc, Chicago, USA) and SAS University Edition (SAS Institute Inc., Cary, NC, USA), considering a confidence interval of 95% (95% CI).
ResultsBiological, clinical, and laboratory characteristics of the 138 assessed subjects are described in Tables 1 and 2. The observed percentage for cardiometabolic risk factors was 5.8% for only one factor, 13.0% for two, 28.3% for three, and 52.9% for four or more factors.
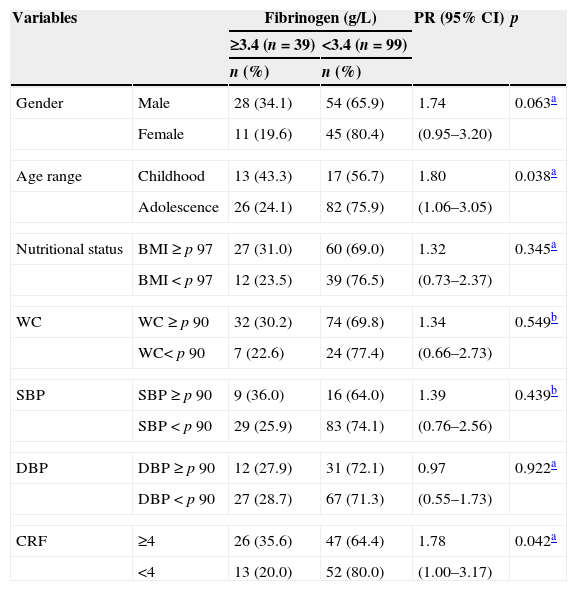
Frequency distribution of the biological and clinical variables according to fibrinogen levels in children and adolescents with overweight or obesity.
| Variables | Fibrinogen (g/L) | PR (95% CI) | p | ||
|---|---|---|---|---|---|
| ≥3.4 (n=39) | <3.4 (n=99) | ||||
| n (%) | n (%) | ||||
| Gender | Male | 28 (34.1) | 54 (65.9) | 1.74 | 0.063a |
| Female | 11 (19.6) | 45 (80.4) | (0.95–3.20) | ||
| Age range | Childhood | 13 (43.3) | 17 (56.7) | 1.80 | 0.038a |
| Adolescence | 26 (24.1) | 82 (75.9) | (1.06–3.05) | ||
| Nutritional status | BMI≥p 97 | 27 (31.0) | 60 (69.0) | 1.32 | 0.345a |
| BMI<p 97 | 12 (23.5) | 39 (76.5) | (0.73–2.37) | ||
| WC | WC≥p 90 | 32 (30.2) | 74 (69.8) | 1.34 | 0.549b |
| WC< p 90 | 7 (22.6) | 24 (77.4) | (0.66–2.73) | ||
| SBP | SBP≥p 90 | 9 (36.0) | 16 (64.0) | 1.39 | 0.439b |
| SBP<p 90 | 29 (25.9) | 83 (74.1) | (0.76–2.56) | ||
| DBP | DBP≥p 90 | 12 (27.9) | 31 (72.1) | 0.97 | 0.922a |
| DBP<p 90 | 27 (28.7) | 67 (71.3) | (0.55–1.73) | ||
| CRF | ≥4 | 26 (35.6) | 47 (64.4) | 1.78 | 0.042a |
| <4 | 13 (20.0) | 52 (80.0) | (1.00–3.17) | ||
WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRF, cardiometabolic risk factors.
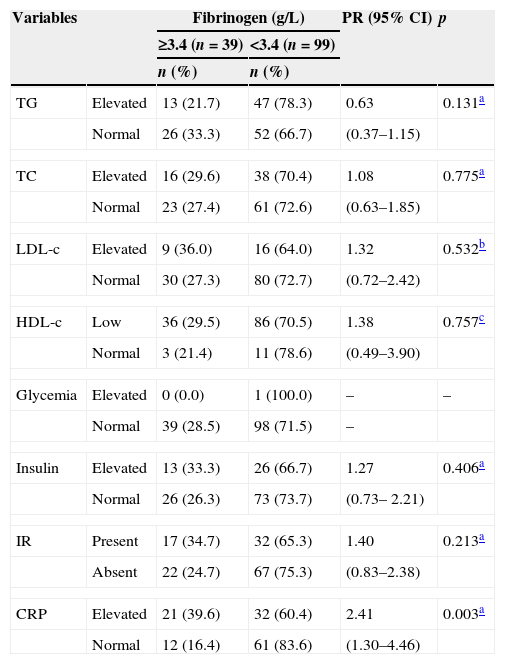
Frequency distribution of laboratory variables according to fibrinogen groups in children and adolescents with overweight or obesity.
| Variables | Fibrinogen (g/L) | PR (95% CI) | p | ||
|---|---|---|---|---|---|
| ≥3.4 (n=39) | <3.4 (n=99) | ||||
| n (%) | n (%) | ||||
| TG | Elevated | 13 (21.7) | 47 (78.3) | 0.63 | 0.131a |
| Normal | 26 (33.3) | 52 (66.7) | (0.37–1.15) | ||
| TC | Elevated | 16 (29.6) | 38 (70.4) | 1.08 | 0.775a |
| Normal | 23 (27.4) | 61 (72.6) | (0.63–1.85) | ||
| LDL-c | Elevated | 9 (36.0) | 16 (64.0) | 1.32 | 0.532b |
| Normal | 30 (27.3) | 80 (72.7) | (0.72–2.42) | ||
| HDL-c | Low | 36 (29.5) | 86 (70.5) | 1.38 | 0.757c |
| Normal | 3 (21.4) | 11 (78.6) | (0.49–3.90) | ||
| Glycemia | Elevated | 0 (0.0) | 1 (100.0) | – | – |
| Normal | 39 (28.5) | 98 (71.5) | – | ||
| Insulin | Elevated | 13 (33.3) | 26 (66.7) | 1.27 | 0.406a |
| Normal | 26 (26.3) | 73 (73.7) | (0.73– 2.21) | ||
| IR | Present | 17 (34.7) | 32 (65.3) | 1.40 | 0.213a |
| Absent | 22 (24.7) | 67 (75.3) | (0.83–2.38) | ||
| CRP | Elevated | 21 (39.6) | 32 (60.4) | 2.41 | 0.003a |
| Normal | 12 (16.4) | 61 (83.6) | (1.30–4.46) | ||
TG, triglycerides; TC, total cholesterol; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; IR, insulin-resistance; CRP, C-reactive protein.
Elevated fibrinogen levels were present in 28.3% of the participants, and were more common in children than in adolescents (p=0.038; PR: 1.80; 95% CI: 1.06–3.05); those with four or more cardiometabolic risk factors (p=0.042; PR: 1.78; 95% CI: 1.00–3.17), and in those with high CRP levels (p=0.003; PR: 2.41; 95% CI: 1.30–4.46). The latter was present in 52.9% of the participants.
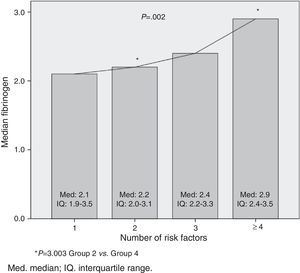
There was no statistically significant difference in relation to fibrinogen median with nutritional status categories: overweight=3.0 (IQ: 2.3–3.6); obesity=2.5 (IQ: 2.1–3.2); severe obesity=2.6 (IQ: 2.2–3.5); p=0.284. In the relation to the cardiometabolic risk, it was observed that the fibrinogen median increased as the number of risk factors increased (p=0.002). This difference occurred in the groups with two and four or more risk factors (p=0.003; Fig. 1).
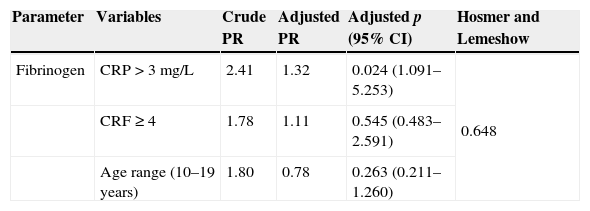
At the multiple logistic regression, CRP level>3mg/L was independently associated with high fibrinogen levels (≥the 75th percentile), with a risk of 1.32 (p=0.024; 95% CI: 1.09–5.25). The final model, which included as explanatory variables CRP levels >3mg/L, the number of risk factors ≥4, and the age group (10–18 years) showed a good fit, assessed by the Hosmer and Lemeshow test (0.648; Table 3).
Values of crude and adjusted prevalence ratio (95% CI) of fibrinogen alterations in children and adolescents with overweight or obesity.
| Parameter | Variables | Crude PR | Adjusted PR | Adjusted p (95% CI) | Hosmer and Lemeshow |
|---|---|---|---|---|---|
| Fibrinogen | CRP>3mg/L | 2.41 | 1.32 | 0.024 (1.091–5.253) | 0.648 |
| CRF≥4 | 1.78 | 1.11 | 0.545 (0.483–2.591) | ||
| Age range (10–19 years) | 1.80 | 0.78 | 0.263 (0.211–1.260) |
CRP, C-reactive protein; CRF, cardiometabolic risk factors.
In the present study, most assessed individuals were obese and had four or more cardiometabolic risk factors. It was also observed that high level of fibrinogen, one of the prothrombotic state evaluation factors, was present in more than one quarter of the sample. The prothrombotic state is considered as the imbalance between procoagulant and pro-fibrinolytic factors, characterized, mainly from laboratory analysis, by increase in fibrinogen levels, by plasminogen activation inhibitor-1 (PAI-1), and by activation of the coagulation pathways, as well as associated with endothelial dysfunction.23
In obese individuals, the increase in fibrinogen levels and other coagulation factors, such as PAI-1 and von Willebrand factor (vWF-Ag), has been associated with an increase in cardiovascular events.24 Some studies8,22 have found significantly higher fibrinogen levels in overweight children, when compared with levels found in children with normal weight.
The high levels of fibrinogen in obese children may be explained by the increase in its synthesis. Although there is no report of its production by adipose tissue, the release of adipocytokines into the portal circulation would influence the production of fibrinogen and other coagulation factors in the liver.7
A study carried out with 313 obese children and adolescents detected a lower prevalence of increase in fibrinogen levels (10.7%)24 when compared to that in the present study (28.3%). This can be explained because the abovementioned study used a cutoff (4.0g/L to 4.5g/L) to consider fibrinogen levels as elevated, which was higher than that used in the present study (3.4g/L). Such comparisons between studies are difficult, as there is no consensus for the normal value of fibrinogen levels in childhood.25 Additionally, the behavior of cardiovascular risk factors may vary according to ethnicity.24
Fibrinogen levels found in current studies are of concern, especially because they are elevated both in prepubertal and pubertal obese children.6 Although the process of fatty streak and atheromatous plaque formation have been identified pathologically, mainly in adolescents, the significant increase in markers such as fibrinogen and PAI-1 in obese children suggests the increase in cardiovascular risk in this age group, which may cause the formation of atherosclerotic plaques before puberty onset in young obese individuals.26
Regarding the assessed cardiometabolic risk factors, all children and adolescents had at least one risk factor. The median of fibrinogen serum levels was associated with an increased number of risk factors. This can be explained by the potentiation of cardiometabolic risk due to the aggregation of several biomarkers that are unfavorable for cardiovascular health.22 This finding reinforces the importance of carrying out an early intervention in overweight or obese children and adolescents, especially in the presence of four or more risk factors, as the normalization of fibrinogen levels has been reported after weight loss, changes in lifestyle, and practice of physical exercise.5
Studies have also shown the association of some cardiometabolic risk factors with elevated fibrinogen levels from childhood, such as BMI,6,24 HOMA-IR,6,25 fasting insulin,6,24,25 glycemia,26 triglycerides, total cholesterol, LDL-c, and low HDL-c.24
In this study, fibrinogen was associated only with hs-CRP and the presence of four or more cardiometabolic risk factors. This may be due to the characteristics of the sample, which includes only children and adolescents with overweight and obesity, conditions associated with a proinflammatory state, unlike other abovementioned studies.6,24,25 After the logistic regression was performed, the value of hs-CRP remained a predictor of high fibrinogen levels and was associated with a 1.3-fold higher chance of developing this condition.
In relation to the lipid profile, although no association was found with fibrinogen, the highest prevalence of total cholesterol and LDL-C was observed among those who had high fibrinogen levels. The association between vascular alterations and lipoproteins, especially those of low-density, has been explained as originating from the damage induced by oxygen-free radicals, which promote the destruction of endothelial cells by oxidizing these lipoproteins, triggering a cascade of alterations including hypercoagulability and decreased fibrinolysis, signaled by increased fibrinogen production.24
This promotes atherosclerosis through several mechanisms: by binding to endothelial cell receptors (Intercellular Adhesion Molecule 1-ICAM1); triggering the release of vasoactive mediators, smooth-muscle cell proliferation, and monocyte chemotaxis induction; and by playing a role in foam cell formation and facilitating the transfer of cholesterol from platelets to macrophages and monocytes.6
It is important to note that fibrinogen is an acute-phase inflammation protein with procoagulant activity and, together with hs-CRP, has been used to predict cardiovascular disease in different groups. Both seem to be more reliable markers than interleukin-6 (IL-6), which has a short half-life.27 It should also be noted that inflammation plays a key role in the onset and promotion of atherosclerosis and can lead to acute coronary syndrome (ACS), by inducing plaque instability.23
This study has some limitations, such as the lack of a cutoff in literature for normal fibrinogen levels in children and adolescents, making it difficult to compare studies; the scope of age range, as there are peculiar characteristics in the different groups, such as pubertal stage, which interferes with the metabolism and fat deposition, although this fact has not been observed in relation to fibrinogen; the isolated use of fibrinogen as a marker for the assessment of the prothrombotic state, although some researchers consider it a more reliable marker of cardiovascular disease than IL-627; and the use of a convenience sample, which may not reflect the characteristics of the overall population.
It is worth mentioning that these limitations do not diminish the importance of this study, as it innovates by assessing the fibrinogen behavior in relation to cardiometabolic risk factors in Brazilian children and adolescents with excess weight. That is due to the fact that studies published in the literature on this topic have been conducted in other countries and, as mentioned previously, this ratio can vary with ethnicity.28
Therefore, the findings reinforce the concept that the fibrinogen and hsCRP are potential biomarkers that can be used in obese children, even in those without associated complications, for the screening of children and adolescents at risk of developing cardiovascular disease. It is also noteworthy that longitudinal studies are needed to elucidate the role of these biomarkers in the genesis of atherosclerotic disease in childhood.
FundingResearch funded by the Programa de Incentivo à Pós-Graduação e Pesquisa (PROPESQ) Proclamation 01/2008 – Pró-Reitoria de Pós-Graduação, Universidade Estadual da Paraíba (PRPGP/UEPB), concession term No. 98/2008.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Azevedo WF, Cantalice AS, Gonzaga NC, Simões MO, Guimarães AL, de Carvalho DF, et al. Fibrinogen: cardiometabolic risk marker in obese or overweight children and adolescents. J Pediatr (Rio J). 2015;91:464–70.
Study conducted at Núcleo de Estudos em Pesquisas Epidemiológicas (NEPE), Centro de Obesidade Infantil (COI), Universidade Estadual da Paraíba (UEPB), João Pessoa, PB, Brazil.