To review the available literature on the association between acute viral respiratory tract infection and the onset of asthma exacerbations, identifying the most prevalent viruses, detection methods, as well as preventive and therapeutic aspects.
SourcesA search was conducted in PubMed, Lilacs, and SciELO databases, between the years 2002 and 2013, using the following descriptors: asthma exacerbation, virus, child, and acute respiratory infection.
Summary of the findingsA total of 42 original articles addressing the identification of respiratory viruses during episodes of asthma exacerbation were selected, mostly cross-sectional studies. There was a wide variation in the methodology of the assessed studies, particularly in relation to the children's age and methods of collection and viral detection. The results indicate that, in up to 92.2% of exacerbations, a viral agent was potentially the main triggering factor, and human rhinovirus was the most frequently identified factor. The pattern of viral circulation may have been responsible for the seasonality of exacerbations. The association between viral infections and allergic inflammation appears to be crucial for the clinical and functional uncontrolled asthma, but few studies have evaluated other triggering factors in association with viral infection.
ConclusionsRespiratory viruses are present in the majority of asthmatic children during episodes of exacerbation. The involved physiopathological mechanisms are yet to be fully established, and the synergism between allergic inflammation and viral infection appears to determine uncontrolled disease. The role of other triggering and protective agents is yet to be clearly determined.
Rever a literatura disponível sobre a relação entre infecção viral aguda do trato respiratório e o desencadeamento de exacerbações da asma, identificando os vírus mais prevalentes, os métodos de detecção, bem como os aspectos preventivos e terapêuticos.
Fonte dos dadosFoi realizada uma busca nas bases de dados PubMed, SciELO e Lilacs utilizando os descritores: asma, exacerbação, vírus, criança e infecção respiratória aguda, entre os anos de 2002 e 2013.
Síntese dos dadosForam selecionados 42 artigos originais que tratavam da identificação de vírus respiratórios durante episódios de exacerbação da asma, em sua maioria estudos transversais. Houve ampla variação na metodologia dos trabalhos avaliados, principalmente em relação à idade das crianças e métodos de coleta e detecção viral. Os resultados apontam que, em até 92,2% das exacerbações, um agente viral foi potencialmente o principal fator desencadeante, sendo o rinovírus humano o mais identificado. O padrão de circulação viral pode ter sido responsável pela sazonalidade das exacerbações. A associação entre infecção viral e inflamação alérgica parece ser determinante para levar ao descontrole clínico-funcional da asma, porém poucos estudos avaliaram outros fatores desencadeantes em associação com a infecção viral.
ConclusõesOs vírus respiratórios estão presentes na maioria das crianças asmáticas durante os episódios de exacerbação. Os mecanismos fisiopatológicos envolvidos ainda não estão totalmente estabelecidos e o sinergismo entre a inflamação alérgica e infecção viral parece determinar o descontrole da doença. O papel dos outros agentes desencadeantes e protetores não estão claramente determinados.
Asthma is a chronic, genetically-determined disease, whose prevalence in the pediatric population ranges between 19.0% and 24.3% among brazilian adolescents and schoolchildren, respectively.1 From the physiopathological viewpoint, it is characterized by chronic inflammation with the involvement of several cell types, associated with airway hyperresponsiveness, with episodes of reversible airflow limitation. It is clinically manifested by recurrent exacerbations, also called “asthma attacks” or, more appropriately, acute asthma, characterized by progressive worsening of dyspnea, coughing, wheezing, chest tightness, or a combination of these.2
The loss of clinical and functional asthma control usually occurs gradually, but it can occur abruptly in a subgroup of patients.2 It is one of the main causes of emergency consultations, having been responsible, in 2007, for 195 deaths in children younger than 19 years in Brazil.3 Public policies have been developed to promote both scientific knowledge about the disease and its management, as well as to organize assistance programs in public health, which include, among others, the dispensing of medications. However, exacerbations continue to represent a significant number in statistics, with great impact on public and private healthcare systems.2
The multifactorial origin of the clinical-functional lack of disease control is well known; since the early 1970s, respiratory viruses have been associated with the triggering of asthma exacerbations in adults and children.3 In the 1990s, the development of more sensitive and specific molecular techniques allowed for the increase in respiratory virus detection and therefore, ways to better explain this association. Studies using reverse transcriptase polymerase chain reaction (RT-PCR) as the detection technique, isolated or combined with traditional methods, observed positivity for respiratory viruses in up to 92.2% of episodes of acute asthma exacerbation in children.4
Considering the possibility of a causal relationship between respiratory virus infection and the triggering of asthma attacks in children, the implications of this association, as well as the possibility of specific prophylaxis and therapy for these agents, special attention to this subject is justified. Therefore, this literature review aimed to analyze articles, published between 2002 and 2013, assessing the association between asthma exacerbation and acute viral airway infection.
MethodsA search was conducted in the PubMed, Lilacs and SciELO databases, using the descriptors: “Asthma Exacerbation”, “Viral Infection”, and “Child”, resulting in a total of 283 references for that period. After selecting the articles published in Portuguese, English, Spanish, or French, 195 articles remained. After reading the titles and abstracts, 42 original articles that assessed respiratory tract viral infection in asthmatic children during exacerbation were selected. Some articles of historical importance or review articles that included the three descriptors were added to generate the bibliography of this review. The list of references was inserted into Endnote X6 (Thompson Corp., CA, USA), a bibliographic citation management software.
ResultsRespiratory viruses and immune responseThe most frequently identified respiratory viruses in association with asthma exacerbation were human rhinovirus (HRV), respiratory syncytial virus (RSV), human adenovirus (hAdV), influenza (Flu), Parainfluenza (PFlu), human metapneumovirus (hMPV), and human coronavirus (hCoV). Of the listed viruses, most have RNA as the nucleic acid; their biological characteristics and taxonomy5 are described in Table 1.
Biological characteristics and taxonomy of the major respiratory viruses.
| Influenza | Parainfluenza | Syncytial | Adenovirus | Rhinovirus | Coronavirus | Metapneumovirus | Bocavirus | |
|---|---|---|---|---|---|---|---|---|
| Family | Orthomyxoviridae | Paramyxoviridae | Paramyxoviridae | Adenoviridae | Picornaviridae | Coronaviridae | Paramyxoviridae | Parvoviridae |
| Core of nucleic acid | RNA | RNA | RNA | DNA | RNA | RNA | RNA | DNA |
| Symmetry of the capsid | Helical | Helical | Helical | Icosahedral | Icosahedral | Pleomorphic | Pleomorphic | Icosahedral |
| Viral envelope | Enveloped | Enveloped | Enveloped | Naked | Naked | Enveloped | Enveloped | Naked |
| Sensitivity to ether | Sensitive | Sensitive | Sensitive | Resistant | Resistant | Sensitive | Sensitive | Sensitive |
| Type of nucleic acid | Single strand negative | Single negative | Single negative | Double | Single positive | Single positive | Single negative | Single negative |
| Nucleic acid size (kb/kbp) | 10 to 13.6 | 15 | 15 | 40 | 7.2 | 27 to 31.5 | 13 | 5 |
| Particle size (nm) | 80 to120nm | 150 to 300 | 150 to 300 | 70 to 90 | 28 to 30 | 100 to 160 | 150 to 600 | 22 to 28 |
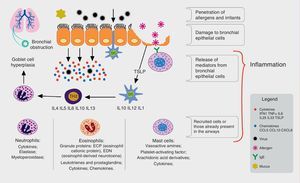
The main transmission methods for these viruses are through contaminated fomites, droplets, aerosols, or direct contamination. By infecting the nasal epithelium cells, these agents trigger an immune response, which involves mainly the dendritic cells and natural killer (NK) cells, inducing the release of a number of pro-inflammatory cytokines and chemokines by the infected epithelial cells, such as interferon (IFN-1) and tumor necrosis factor alpha (TNFα), among others (Fig. 1).
Immune process involved in response to respiratory viruses and their association to allergic inflammation. Respiratory viruses infect bronchial epithelial cells (BECs) through tool-like receptors (TLRs). During replication, they trigger an inflammatory process with induction of cytokine and chemokine production by BECs, among them interferon (IFN-1), tumor necrosis factor alpha (TNF-α), interleukins (IL-33, IL-25), and thymic stromal lymphopoietin (TSLP). The dendritic cells (DCs), components of the innate immunity, are directed to secondary lymphoid organs after capturing viral antigens, where they stimulate the lymphoid cells, the protagonists of the specific immune response. In asthma patients, the production of IFNs is reduced, allowing for greater viral replication and under stimulation of TSLP, there is a deviation from lymphoid profile to helper T lymphocyte 2 (Th2), promoting lower antiviral response and increased allergic inflammation, with bronchial hyperreactivity and increased production of mucus, causing bronchial obstruction and asthma exacerbation.
In asthma patients, the viral infection causes an imbalance in the immune homeostasis of the respiratory system. Several mechanisms related to viral infection and allergic inflammation, as well as their role in triggering acute asthma, have been proposed; among them, the deficient function of the epithelial barrier caused by the virus, which has been implicated as a predisposing factor by some.6 However, both asthma and atopy are associated with epithelial damage, which may contribute to increased susceptibility to infections, including viral diseases and sensitization by aeroallergens.7 Another evaluated factor was mucus production as an airway defense mechanism; in mice studies, it was demonstrated that allergic inflammation and viral infection act synergistically increasing mucus production, which can lead to airway impaction and obstruction in asthma patients.8
Virus-induced alterations in interferon production have also been observed. For instance, in vivo and in vitro studies in epithelial cells from healthy adults and asthma patients infected with HRV demonstrated a decreased production of type I interferon (α and β) in the latter, making them more susceptible to infection associated with viral exacerbation.6,9 Similar results were obtained in studies performed with children, where the production of interferon and Th2 cytokines by bronchial epithelial cells was assessed after HRV-16 infection.
Lower interferon production and higher concentrations of viral RNA have been demonstrated in children with asthma, regardless of their atopic status, and in atopic children without asthma, suggesting that an impaired immune response to viral infection occurs not only in asthma patients, but in children with other disorders associated with Th2 lymphocytes.10 However, other studies failed to demonstrate the same reduction in interferon production; others even found an increase in its production in exacerbated asthma.11,12
The bronchial epithelium produces some cytokines, including interleukin 25 and 33, as well as thymic stromal lymphopoietin, which promotes the differentiation of innate lymphoid cells into Th2. The latter can be induced by viral infection, and its production can be increased by interleukin-4 (IL-4), suggesting that the interaction between viruses and allergic airway inflammation may enhance the inflammatory Th2 response and potentially reduce the antiviral response.11,13
Collection and viral detection methodsViral detection is highly dependent on the quality of the collected sample, on the time of symptom onset to the time of collection (ideally within 72hours), and on transportation and storage of the sample before testing. The analysis for respiratory viruses should be performed in material from the airways. Upper airway secretion is used in most cases, and several methods are employed for this collection, such as nasopharyngeal aspirate (NPA), nasopharyngeal swab (NPS), nasopharyngeal lavage, and the combined nasal-oral swab; the first technique is considered the gold standard.14
Recently, new flocked swabs (Copan, Brescia, Italy) were developed, and presented better performance during data collection. Recent studies using this type of swab presented a sensitivity comparable to that of NPA when the detection is performed by PCR, suggesting that this swab can be used in epidemiological research and surveillance studies, due to its greater technical simplicity.15,16
There are few data to support the use of combined oral-nasal swabs for virus detection, and its sensitivity is lower than that of the nasopharyngeal swab or aspirate, which can be explained by the lower viral load in the oropharynx than in the nasopharynx. The collection can also be performed on material from the lower airways, such as induced sputum and bronchial lavage.17
The methods for detection of respiratory viruses are varied and include rapid tests for antigen detection, culture, direct and indirect immunofluorescence, and nucleic acid amplification reactions, such as RT-PCR, which can detect a single agent (monoplex) or perform multiple detections (multiplex). The sensitivity of the latter is higher, and it was used in most recent studies.18,19 immunofluorescence reactions have lower cost, are faster to perform, and are also able to detect multiple viruses. A panel of seven viruses (IFlu A and B, PFlu 1 to 3, hAdV, and RSV) is generally used. Some viruses, such as HRV and Bocavirus, can only be detected through nucleic acid amplification reactions.17
Virus and exacerbationSeveral authors have performed studies aiming to detect viruses in respiratory secretions of exacerbated asthma patients, showing a prevalence of viral identification that varies with several factors, such as patient age, time of the year, method of sample collection, and method of viral detection. Table 2 presents the original articles that demonstrate results of viral testing in children with exacerbated asthma. Most studies found prevalence rates between 36.0%20 and 92.2%;5 in these cases, the most frequently identified virus was HRV.
Studies that investigated viral infection in exacerbated asthmatic children included in the literature review.
| Author, year, country | n | Age | Control | Collection time | Collection and identification methods | Total detection | More frequently identified virus | Other identified viruses | Observations |
|---|---|---|---|---|---|---|---|---|---|
| Khetsuriani et al., 2007,28USA | 65 cases77 controls | ≥ 2 years | Stable asthma | March/2003 to February/2004 | Nasopharyngeal swabPCR (RT-PCR) for hRV | 37% | hRV (37% of total, with 60% in cases and 18% in controls) | Not researched | Association of asthma exacerbation with hRV infection genogroup C |
| Lopez Perez et al., 2009,22Mexico | 100 cases68 controls | 2 to 17 years | Wheezers | Winter (April to June) | SwabDIF | Identification in 75% of cases and 44% of controls | FLUVA and hRSV in wheezers; hAdV, FLUVB and PF in asthma patients | hRSV, hAdV, FLUV | Co-detection in 17% |
| Olenec et al., 2010,30Korea | 58 cases | 6 to 8 years | No | April and September/2006, 2007 and 2008(501 collected samples) | NPAmultiplex PCR;hRV sequencing | hRV (72 to 99%)hRV C in 16 cases | hRSV A and B, PF 1-4, hAdV, hCoV, enterovirus and hMPV, hBoV. | Virus associated with greater severity and duration; interaction between allergic sensitization and virus | |
| Bizzintino et al., 2011,4Australia | 128 cases | 2 to 16 years | No | April/2003 to February/2010 | NPA or swabDIF and multiplex PCR (18 primers) - nine virusesCharacterization of hRV (types) | 92.2% | hRV (87.5%) | hRSV, hAdV, FLUVA and B, PIV1-4, hMPV, hEV, hCoV and hBoV | hRV C was detected in most children with acute asthma (59.4%) and associated to severity |
| Miller et al., 2009,29USA | 1,052 cases | ≤ 5 years | No | October/2001 to September/2003 | NPA and swabPCR and sequencing for hRV | hRV (15.9%) | Not studied | hRV type C associated to asthma exacerbation | |
| Chang et al., 2009,32Austrália | 201 cases | 2 to 15 years | No | March/2005 to February/2007 | NPAPCR multiplex | 53.8% | hRV (41%) | hRSV, hAdV, FLUV, PIV, hMPV | Co-detection in ten casesNo difference in evolution for deferment viruses. |
| Ozcan et al., 2011,25Turkey | 104 cases31 controls | 3 to 17 yeras | Stable Asthma patients | 12 months: September/2009 to September/2010 | SwabReal-time PCR | 53.8% | hRV (35.6%) | FLUVA e B, PIV 1-4, hCoV, hRSV, hRV, hMPV and hAdV | |
| Camara et al., 2004,43Brazil | 132 cases65 controls | 0 to 12 years | Non-asthma patients | October/1998 to June/2000 | NPAImmunofluorescence (IIF); culture for hAdV and RT-PCR for hRV and hCoV | Cases (60.8%)Controls (13.3%) | RSV in those younger than 2 yearshRV and hAdV in those older than 2 years | hRSV, FLUV, PIVhAdV, hCoV | There was no association between virus detection and wheezing. Allergic sensitization more associated with exacerbation. |
| Kato et al., 2011,26Japan | 174 cases93 controls | Median4.7 years | StableAsthma patientsNon-asthma patients | November/2003 to October/2003 | NPAKit detection of antigens andRT-PCR | 79% | hRV (33.9%) | hRSV, hAdV, FLUV and PIVhEV | IL1, 5, 6, 10 and ECP were higher in exacerbation than in controls |
| Rawlinson et al., 2003,19Australia | 179 cases79 controls | 1 month to 16 years | Stable Asthma patientsNon-asthma patients with URTI | Winter, spring and summer of 2000 to 2002 | NPADIF PCR for hRV and hMPVCulture for virus | hRV (50% in total: 79% in cases and 17% in controls) | hAdV, FLUVA e B, hRSV, PIV3 | Co-infection was common especially in winter and by hRSV | |
| Fujitsuka et al., 2011,23Japan | 115 | Mean 20.8 months | No | November/2007 to March/2009 | SwabPCR | 86.1% | hRV (31.3%) | hRSV, hAdV, PIV, FLUV, hMPV, hRV, hBoV, hEV | - |
| Dawood et al., 2011,27USA | 701 asthma patients in 2,165 cases of influenza | 2 to 17 years | No | 2003 to 2009 | SwabFLUVA, B, H1N1 | - | Only Influenza was screened | Not screened | Of the children hospitalized with influenza in 2003, 32% and in 2009, 44% were asthmatics. More complications with Influenza A |
| Leung et al., 2010,31China | 209 cases77 controls | 3 to 18 years | StableAsthma patients | 12 monthsJanuary/2007 to February/2008 | NPA or swabmultiplex PCR | 51% | hRV | hRSV, hAdV, PIV, FluV, hMPV, hRV, hBoV, hEV | No agent was correlated with severity |
| Thumerelle et al., 2003,24France | 82 cases27 controls | 2 a 16 years | StableAsthma patients | 9 monthsOctober/1998 to June/1999 | SwabIIF and PCR | Asthma patients (45%) and controls (3.7%) | hRV (12%) | FLUVA and B; PIV1-3; hAdV;RSV;hCoVMycoplasma and chlamydia | Serology 7.4%Atypical 10% |
| Maffey et al., 2010,21Argentina | 209 cases | 3 months to 16 years | No | 12 monthsJanuary/2006 to December/2006 | NPA or swabIIF and PCR | 78% | RSV and hRV | hRSV, hAdV PIV1-3, FLUVA e B)hEV, hMPV, hBoV, mycoplasma and chlamydia | Co-detection in 20%,Seasonal circulation: three peaks: start of October (hRV); winter (hRSV) and spring (hSV, hRV and hMPV) |
| Mandelcwajg et al., 2010,20France | 232 cases107 controls | 1.5 to 15 years | Exacerbated asthma patientsNon-hospitalized | 2005 to 2009November to March | NPADIF and PCR | 47% (hospitalized)31% (non- hospitalized) | hBoV and RSV | FLUVA and B, hAdV, PIV-3, hMPV | hRV not screened |
NPA, nasopharyngeal aspirate; IIF, indirect immunofluorescence; DIF, direct immunofluorescence; PCR, polymerase chain reaction; RT-PCR, real-time polymerase chain reaction; hRV, rhinovirus; hAdV, adenovirus; hRSV, respiratory syncytial virus; FLUV, Influenza; FLUVA, Influenza A; FLUVB, Influenza B; PIV1, Parainfluenza 1; PIV2, Parainfluenza 2; PIV3, Parainfluenza 3; hCoV, coronavirus; hMPV, metapneumovirus; hBoV, Human Bocavirus-infection; hEV, Hepatitis E vírus; ECP, eosinophil cationic protein; URTI, upper respiratory tract infection.
An investigation of respiratory viruses in 209 children aged between three and 16 years hospitalized for asthma exacerbation was performed over a period of 12 months in Buenos Aires, Argentina. Due to the possibility of other diagnoses for wheezing episodes in infants, the definition of asthma was based on the criteria of Castro-Rodriguez for children younger than 3 years, and on the Global Initiative for Asthma (GINA) criteria for those older than 3. Immunofluorescence and PCR were performed in nasopharyngeal secretions of the children and showed a positive rate of 78.0% for viruses in general; hRV and hRSV were the most frequently identified types. There was also 20.0% of dual detection, with the involvement of all analyzed viruses.21
In México, the frequency of the viral positivity at the immunofluorescence was higher in children with asthma (75.0%) than in a control group of wheezing children without asthma (44.0%). hRV was not included in that study, and IFlu, PFlu, and hAdV were the most frequently identified virus in the group of asthma patients.22 In Japan, respiratory viruses were detected by multiplex PCR in 86.1% of 115 children with exacerbated asthma, with a mean age of 20.8 months. The hRSV was related to a single episode of wheezing (p<0.05).23 hRV was more frequently observed in patients with a history of asthma (p<0.05).
A group of 82 French children with exacerbation treated at home was compared to 27 stable asthmatic children. Immunofluorescence, PCR, and serology for viruses (Mycoplasma pneumoniae and Chlamydophila pneumoniae) detected a pathogen in 45.0% of samples, with significantly higher frequency in cases than in controls (3.7%). Viral detection tests were positive in 38% of cases, and hRV was the most common (12.0%). In 10.0% of cases, the serologic tests were positive for both atypical pathogens.24
Another series of 104 children with exacerbation, compared to 31 stable children, was studied by Turkish authors and showed positivity of 53.8% in the cases and 22.6% in controls, through RT-PCR reaction. hRV was the most commonly found virus in 35.6% of the samples.25 In Japan, 174 children with acute asthma were compared to 79 stable asthmatic children and 14 children without asthma. Using an antigen detection kit and RT-PCR, respiratory viruses were detected in 79.0% of nasal aspirate samples in exacerbated asthmatic children, and hRV was the most common (33.9%). In parallel, the assessment of inflammatory markers showed a significant elevation (p<0.01) of interleukins IL-1, 5, 6, and 10 in serum and in the nasal aspirates of patients in exacerbation, as well as an increase in serum eosinophilic cationic protein (ECP) levels (p<0.01).26
Flu, although less frequently associated with these episodes, appears to be responsible for increased morbidity in patients with an underlying chronic disease, including asthma. Of 2,165 children aged 2 to 17 years admitted with a diagnosis of infections by FLUV-A and B between 2003 and 2009 in the United States, 44.0% were asthma patients, and complications were more significantly associated with FLUV-A (p<0.01). Other viruses were not assessed in that population.27
Another study compared exacerbated children treated in hospitals (n=232) with those treated at home (n=107). Immunofluorescence for Flu, hAdV, hRSV, and PIV was performed, as well as PCR for Bocavirus. A 36.0% rate of viral detection was obtained, but no difference was observed regarding the viral profile between inpatients and outpatients. The most frequently observed viruses were RSV (15.0%) and Bocavirus (12.0%), but hRV was not included in the viral panel of this study.20
A group of 179 Australian children aged up to 16 years had their nasal secretions collected in three periods between 2000 and 2002, and were compared with a control group of non-asthmatic children with upper respiratory tract infection (URTI) in the same period and another group of 28 children with controlled asthma, assessed during routine consultations. hRV and hMPV were screened by RT-PCR and a panel of seven viruses (FLUV- A and B, PIV- 1 to 3, hAdV, and hRSV) was studied by immunofluorescence. hRV infection accounted for 50.0% of the URTI of non-asthmatic children, and co-infection was common, especially with the hRSV, especially in children younger than 2 years.
Children with symptomatic asthma had the highest rates of hRV infection (79.0% vs. 52.0% among all children). Finally, children with controlled asthma had the lowest rates of hRV identification (17.0% vs. 79.0%).19
Studies conducted in 200728 and 200929 aimed to the identification of hRV in exacerbated asthmatic patients through RT-PCR, found an overall frequency of viral identification of 37.0% and 15.9%, respectively. The first study used a group of comparison consisting of stable asthma patients, in which the identification rate was lower (18.0%) than in the case group (60.0%). Both studies found a greater association between exacerbations and the presence of hRV C.
Virus and exacerbation severityAnother important issue in the complex association between viruses and asthma is related to the intensity of the association of exacerbations with viral infection. In this sense, several studies4,30–34 presented inconclusive results, although hRV was associated with increased severity or worse response to treatment.30,33,34 The association between viral infection and acute asthma severity was evaluated in 128 children aged 2 to 16 years. A positivity rate of 92.2% for the presence of virus was observed by direct immunofluorescence (DIF) and multiplex PCR; hRV was detected in 87.5% of cases, and type C was observed in half the cases and was associated with greater severity.4
Fifty-eight asthmatic children aged 6 to 8 years were monitored for a period of five weeks between April and September of 2009. They had nasal lavage samples collected weekly for multiplex PCR analysis, in addition to a symptom diary, peak expiratory flow, and notes on rescue medication use. A virus was detected in 36.0% to 50.0% of the specimens; hRV was identified in 72.0% to 99.0% of the positive samples, and was associated with greater symptom severity.30
Nonetheless, viral testing by multiplex PCR for 20 pathogens in 209 children with exacerbated asthma compared with 77 controlled asthma patients, performed in Hong Kong between 2007 and 2008, showed no association between the presence of the virus and exacerbation severity. One virus was identified in 51.0% of cases, and this detection was, in general, more associated with exacerbations (OR 2.77; 95% CI: 1:51 to 5:11; p<0.01). When analyzed individually, no virus was associated with exacerbation, although hRV was the most frequent, being identified in 26.2% of exacerbated and in 13.0% of controlled asthma patients, but with no significant difference (p=0.27).31
Nasopharyngeal aspirate samples of 201 asthmatic children aged between 2and 15 years collected during episodes of exacerbation were referred for viral identification by PCR. The positivity rate was 53.8%;the most frequently observed were hRV (41.0%), followed by hRSV (9.0%). There was no association with exacerbation severity.32
A study compared the response to treatment with bronchodilators between exacerbated children with viral respiratory infection symptoms (n=168) and a group without such symptoms (n=50). The mean age was 6.6 years, and exacerbation severity did not differ between groups. Children with viral symptoms had poor response to bronchodilators, requiring more doses of beta-agonists after 6, 12, and 24hours. The viral screening was conducted in 77.0% of cases; hRV was the most frequently found virus (61.4%).33
In another study, 78 exacerbated children were treated at the hospital and compared to 78 asymptomatic adults. Multiplex PCR reactions for eight respiratory viruses and monoplex PCT for Enterovirus, hRV, and Bocavirus detected the presence of respiratory viruses in 65.4% of cases; hRV was once again the most frequently observed virus (52.6%). Genotyping showed a higher frequency (56.0%) of type C hRV and association with type A showed a worse clinical outcome.34
Seasonality of exacerbationsAsthma exacerbations have seasonal distribution, occurring cyclically in both adults and children, and can be explained by the viral circulation pattern or change in the level of pollutants and aeroallergens. One example is what occurs in temperate countries, where a higher rate of occurrence is more likely to be observed in the fall and spring among schoolchildren.35 A combination of factors may explain this phenomenon, such as increased circulation of hRV in late summer and early autumn, increased circulation of pollutants and aeroallergens, and the return to school after the summer vacations. The influence of the return to school activities may be explained by lower adherence to maintenance treatment during the vacation period. The circulation of other viruses has been reported in other countries in the northern hemisphere, especially hRSV during autumn-winter, Flu in winter, PIV- 1 and 2 in the fall, and PIV- 3 in the spring.36,37
In Brazil, data on viral circulation were collected from the Brazilian system of epidemiological surveillance on Flu viruses and their counterparts in the period between 2000 and 2010. Samples obtained from nasopharyngeal swabs of patients in different sentinel units distributed throughout the country were analyzed by immunofluorescence. They showed a predominance of FLUV and hRSV, with circulation throughout the year, with peaks for the latter between March and June, and between May and August for FLUV.38 No data were located concerning the movement of hRV in Brazil.
Few published data regarding the seasonality of exacerbations were found. To make a parallel to virus circulation and the occurrence of exacerbations, the authors analyzed data obtained in some studies, such as the study conducted in the Federal District, which observed a higher frequency in the month of March.39 Still in the Midwest region, in the state of Goiás, an increased frequency of respiratory symptoms, not specified as asthma, was observed in winter.40 An observation regarding the distribution of the occurrence of asthma in the state of Minas Gerais also showed higher concentrations in fall-winter, between May and July,41 indicating a predominance of respiratory and/or asthma symptoms in the Brazilian fall-winter seasons.
Viral infection and allergic sensitizationIn addition to the seasonal variation of the virus, other factors involved in the genesis of asthma exacerbation may explain this variation, such as aeroallergens and pollutants, which also vary throughout the different seasons. It is likely that the combination of these and other factors result in the observed seasonal peaks in exacerbations. In the month of April of the years 2006 and 2008, a study was conducted in Korea aiming to monitor viral infection and to identify sensitization to aeroallergens in 58 children with acute asthma or diagnosis of a cold, whose mean age was 6.5 years. Children with allergic sensitization presented the same number of viral infections, but with more symptoms than those non-sensitized.30
In another study, conducted in Manchester, England, 84 children hospitalized for exacerbation were compared to children with stable asthma and children hospitalized for non-respiratory disease. The authors concluded that the association between viral infection and allergen exposure increased the risk of hospital admission by 19.4-fold.42
In Brazil, Camara et al.43 investigated the role of viral infections, sensitization, and exposure to aeroallergens as risk factors for wheezing in children aged up to 12 years. In those younger than 2 years, the frequency of viral positivity was significantly higher in cases (60.8%) than in controls (13.3%). In older children, there was no significant difference: 69.7% of cases and 43.4% of the positive controls. They concluded that in children younger than 2 years, the risk factors associated with wheezing were viral infection and a family history of atopy; among older children, sensitization to inhalant allergens was the most important event for the onset of crises.
Pollutants and aeroallergensThe effect of air pollutants is usually disregarded in the presence of viruses or allergens. However, there is evidence that acute exposure to specific pollutants may contribute to the symptoms and severity of exacerbations. For instance, cigarette smoke induces a model of non-eosinophilic inflammation with relative resistance to corticosteroids.44 Passive smoking is quite common in homes of asthmatic children, causing a negative impact on disease control. In Scotland, the 2006 legislation that banned smoking in public places reduced hospitalizations for asthma by 18.2%.45
Other pollutants appear to contribute to asthma exacerbations, such as those resulting from the combustion of natural gas and engine oil, such as nitrogen dioxide (NO2). Children spend most of their time outside and breathe in a greater amount of pollutants per kilogram of weight when compared to adults, and the increased levels of NO2 are associated with the severity of virus-induced exacerbations. This emphasizes a potential synergism between these two inflammatory stimuli.44
Moreover, controlled exposure in asthma patients demonstrated that NO2 increases the response to inhaled allergens. A cohort of 114 asthmatic children aged between 8 and 11 years were monitored for symptoms, measurement of peak expiratory flow, measurement of exposure to NO2, and presence of virus in nasal secretion during a period of 13 months. One or more viruses were detected in 78% of the reported episodes of respiratory symptoms; it was demonstrated that exposure to high concentrations of NO2 in the week before the onset of a viral respiratory infection was related to the exacerbation severity.46
A longitudinal study conducted in the United States measured exposure to cigarette smoke in 1,444 children with asthma and NO2 in a subset of 663 of them, over a period of nine months. They demonstrated increased symptoms in those exposed to NO2, but only among non-atopic children, with a relative risk of 1.8 (95% CI: 1.1 to 2.8). There was no association between symptoms and increased cigarette smoke exposure.47 Two cross-sectional studies compared children exposed to different levels of cigarette smoke and showed that those exposed to high levels had higher symptom scores (p<0.01), nocturnal symptoms (OR 3.4; 95% CI: 1.3 to 8.8), and need for relief (p=0.03) and control (p=0.02) medications.48
A study in which 937 children aged between 5 and 11 years were randomized to intervention with environmental education guidelines aimed at reducing exposure, showed a reduction in exposure in the group that received instructions for a period of 14 months. The intervention group had fewer days with symptoms (p<0.01) after one year of follow-up, in addition to a decrease in the levels of dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae) and cockroach antigens in the home environment.49
Fungal sensitization is prevalent in children with asthma, although few studies have addressed this issue, compared to studies related to dust mites. One study demonstrated that children with a positive skin test for fungi had more days of symptoms when compared to those with negative tests (6.3 vs. 5.7 for two weeks, p=0.04). During the study period, fungi were grown from the intra- and extra-domestic environment; increased exposure to fungi was associated with increased days of symptoms and unscheduled physician visits for asthma.44
Preventive and therapeutic aspectsThe preponderance of virus participation among the infectious agents in exacerbations makes the indiscriminate prescription of antibiotics in this situation pointless in most cases. Previous studies suggest that chest radiography is improperly and unnecessarily used in children and adults with acute asthma treated in emergency rooms.50 Due to the alterations that are usually found in patients during asthmatic crises, such as hyperinsufflation, fluid extravasation, and atelectasis associated with hypoxemia,51 the misinterpretation of these findings as a sign of pneumonia is common and, consequently, unnecessary prescription of antibiotics.
A multicenter study of 734 asthmatic patients treated in emergency rooms evaluated the request for additional tests, in this case, chest radiography and blood tests. Severely ill patients, those under 1 year, and those with a comorbidity were excluded. A total of 302 (41.0%) children underwent additional tests, such as chest radiography (27.0%) and blood tests (14.0%). After excluding febrile or hypoxic patients, 32.0% were still subjected to at least one of the exams.50 Despite the lack of Brazilian data, the routine of pediatric emergency care services in the country appears to adhere to this rule.
In order to prevent the dissemination of viral agents, due to the high capacity of viral spread through droplets and fomites, hand washing, and the use of respiratory masks are simple strategies that have been proven to be effective.52 Staying away from situations that favor clusters of people during periods of increased viral circulation has been recommended, although there are no studies that proved the effectiveness of this strategy.52,53 The use of substances such as herbal preparations including Echinacea and vitamin C has been evaluated, but double-blind, placebo-controlled studies failed to demonstrate their benefit.54
The prevention of viral infections through vaccines has been the most effective way to control diseases caused by viruses. In the case of respiratory viruses, the only vaccine available is for Flu, although there are ongoing studies for the development of vaccines for other respiratory viruses, especially HRV. However, their great antigenic diversity hinders research success; recent studies have tried to establish a more adequate antigenic target in the viral structure.54,55 Specific RSV immunoglobulin has been successfully used in reducing hospitalizations for viral bronchiolitis, and new perspectives for the treatment of exacerbations triggered by viral infections have emerged from studies directed to synthetic agonists of TLR3 receptor, IFN-β agonist, and IL33- and IL25-antagonists, among others.56,57
There is no specific treatment for most respiratory viruses. Some antivirals have been successfully used, as in the case of Flu infection, such as amantadine, rimantadine, oseltamivir phosphate, and zanamivir; the latter is not indicated for patients with asthma. Ribavirin is indicated for the treatment of severe infections caused by RSV. Other antiviral agents are being studied and have not yet been approved for clinical use, such as pleconaril, vapendavir, pirodavir, and rupintrivir.54
Glucocorticoids have potent anti-inflammatory effects and have been successfully used in maintenance treatment in patients with persistent asthma, controlling inflammation and preventing exacerbations. Some studies have assessed its effect on virus-induced asthma. The suppression of the release of pro-inflammatory mediators induced by HRV infection in vitro in bronchial epithelial cells, such as CCL5, CCL10, CXCL8, and IL6, as well as the reduction of factors associated with remodeling, was achieved after the use of budesonide.58 Other in vitro studies documented the action of other corticosteroids alone or in combination with bronchodilators or leukotriene antagonists in reducing the release of several inflammatory molecules, with potential modulation of the deleterious effects of viruses on the asthmatic population.51,59 Despite the proven benefits of inhaled corticosteroids in the control of asthma triggered by multiple factors, their action on virus-induced exacerbations is unclear. The use of low-to-moderate doses of inhaled corticosteroids as maintenance therapy cannot prevent intermittent viral-induced wheezing.60,61
However, better results have been obtained with the intermittent use of inhaled corticosteroids at high doses.62
DiscussionThe use of viral detection techniques with high sensitivity and specificity has increased the identification of some respiratory viruses in children with asthma exacerbation. The direct or indirect immunofluorescence reactions still have great practical importance, as they can detect a panel of seven viruses (FLUV- A and B, PIV- 1-3, hAdV and hRSV), being an affordable and fast method, with good sensitivity, especially in children.18 It is currently used by the Brazilian Ministry of Health for the screening of respiratory viruses in the diagnosis of severe acute flu-like illness and Flu-like illness in sentinel units.
The techniques for nucleic acid amplification (RT-PCR) are more expensive, but more sensitive; thus, they are used in research and by the Brazilian Ministry of Health for the identification and genotyping of Flu.38 Furthermore, it allows for the identification of some viruses with significant clinical and epidemiological importance, such as hRV and Bocavirus, not identified by immunofluorescence.17,54
As for the method used to obtain the sample, it is worth mentioning the controversial issue of nasopharyngeal swab in viral research. Although its use has been consolidated for bacterial infections (S. pneumoniae and S. aureus), its role in viral infections still deserves some consideration. The authors agree that, from the practical point of view, it is more feasible, eliminating the use of suction systems, probes, and more specialized training, when compared to aspirate or lavage samples. However, only those with more advanced technology (flocked swab), which provides best capture and release of cells and, therefore, of the virus, are equivalent to the aspirate in terms of sample quality. Nevertheless, this swab is not routinely used in services and researches in Brazil.38
Regarding the association between viral infection and asthma exacerbation, a wide variation was observed concerning the methods of studies that assessed viral infection in exacerbated asthmatic children in the studies included in this review. For instance, sample size varied from 58 to 1,052 cases and the age ranged from 0 to 17 years. This finding is important, given the difficulty in defining asthma in children younger than 3 years, which should be considered in case selection.2,21 Moreover, it is known that there is a considerable difference between the age groups and the most prevalent viruses, such as the hRSV.60 Regarding the methods of respiratory secretion collection in the included studies, there was no uniform means of collection; the aspirate was used in 43.8% of studies, the swab in 31.6%, both in 25%, and the flocked swab was not used. There was a wide variation regarding the detection methods and in relation to some outcomes.
In addition to the differences in sample collections, all these studies were cross-sectional, which does not allow for the establishment of a cause-effect association between viral infection and the onset of exacerbation, but suggest such an association. In relation to other factors known to be associated with uncontrolled asthma, such as allergens and irritants, most studies did not include these variables in the evaluation. When the inflammatory process typical of asthma is associated with a viral respiratory infection, there is a tendency to greater severity and duration, as well as a poorer response to conventional treatment of the acute episode.32,33 The involved mechanisms still need to be fully elucidated, evidencing the synergistic effect between viral infection and allergic airway inflammation in the pathogenesis of exacerbations.30,43
Another pertinent issue is the role of inhaled corticosteroids in attenuating the inflammation triggered by the virus, also seldom mentioned in these studies. Its action in the control and reduction of morbidity associated with asthma is well established,2 but it is still a controversial subject regarding the prevention of viral-induced wheezing. Its effectiveness in the inflammatory process triggered by a virus has been demonstrated in in vitro studies,51,58,59 but studies evaluating its clinical benefit have yet to reach conclusive results.61,62
Regardless of the direction of virus-allergen interaction, the present findings strongly suggest that an adequate strategy to prevent virus-induced exacerbations should focus on two courses, namely the improvement of antiviral response and the reduction of allergic sensitization or inflammation. The latter can be achieved with appropriate treatment of the asthma patient at risk with medications that reduce airway inflammation. Conversely, the preventive measures for viral infection acquisition and its timely diagnosis allow for a proper management of exacerbations, and reduction of the number of hospitalizations and unnecessary additional tests, especially in children who are febrile at the time of assessment.
The association between viral infection and asthma in childhood still has several points that need clarification, especially the actual role of viruses in triggering exacerbations and that of inhaled corticosteroids in its attenuation.
FundingFundação de Amparo à Pesquisa do Estado de Goiás (N° 20120267001128).
Conflicts of interestThe authors declare no conflicts of interest.
To the Laboratory of Virology of the Instituto de Patologia Tropical e Saúde Pública of the Universidade Federal de Goiás.
Please cite this article as: Costa LD, Costa PS, Camargos PA. Exacerbation of asthma and airway infection: is the virus the villain? J Pediatr (Rio J). 2014;90:542–55.
Study conducted at the Department of Pediatrics, Universidade Federal de Goiás (UFG), Goiânia, GO, Brazil.