To longitudinally assess bone mineral content (BMC), bone mineral density (BMD), and whole-body lean mass obtained through bone densitometry by dual-energy X-ray absorptiometry (DXA) in preterm newborns (PTNs) and compare them with full-term newborns (FTNs) from birth to 6 months of corrected postnatal age.
MethodsA total of 28 adequate for gestational age (AGA) newborns were studied: 14 preterm and 14 full-term newborns. DXA was used to determine BMC, BMD, and lean mass in three moments: 40 weeks corrected post-conceptual age, as well as 3 and 6 months of corrected postnatal age. PTNs had gestational age ≤ 32 weeks at birth and were fed their mother's own milk or milk from the human milk bank.
ResultsAll infants had an increase in BMC, BMD, and lean body mass values during the study. PTNs had lower BMC, BMD, and lean mass at 40 weeks of corrected post-conceptual age in relation to FTNs (p<0.001, p<0.001, p=0.047, respectively). However, there was an acceleration in the mineralization process of PTNs, which was sufficient to achieve the normal values of FTNs at 6 months of corrected age.
ConclusionsThis study suggests that bone densitometry by dual-energy X-ray absorptiometry is a good method for the assessment of body composition parameters at baseline, and at the follow-up of these PTNs.
Avaliar longitudinalmente o conteúdo mineral ósseo (CMO), a densidade mineral óssea (DMO) e a massa magra do corpo inteiro obtidos através da densitometria óssea de dupla absorção de Raios-X (DXA) em recém-nascidos pré-termo (RNPT) e comparar com seus pares a termo (RNT) desde o nascimento até 6 meses de idade pós-natal corrigida.
MétodosForam estudados 28 recém-nascidos adequados para a idade gestacional: 14 recém-nascidos pré-termo e 14 recém-nascidos a termo. Utilizando-se a DXA, foram determinados CMO, DMO e massa magra em três momentos: 40 semanas de idade pós-concepcional corrigida, 3 e 6 meses de idade pós-natal corrigida. Os recém-nascidos pré-termo apresentavam ao nascimento uma idade gestacional igual ou inferior a 32 semanas e receberam leite da própria mãe ou leite humano de banco.
ResultadosTodos os recém-nascidos apresentaram um aumento nos valores de CMO, DMO e massa magra durante o estudo. Os recém-nascidos pré-termo apresentaram menor CMO, DMO e massa magra, com 40 semanas de idade pós-concepcional corrigida, em relação aos recém-nascidos a termo (p<0,001, p<0,001, e p=0,047, respectivamente). Entretanto, houve uma aceleração no processo de mineralização nos pré-termos, suficiente para atingirem os valores normais do recém-nascidos a termo aos 6 meses de idade corrigida.
ConclusõesEste estudo sugere que a densitometria óssea de dupla absorção de Raios-X cons-titui um bom método para a avaliação dos parâmetros de composição corporal no início e no seguimento destes recém-nascidos pré-termo.
Metabolic bone disease is characterized by changes in skeletal mineralization due to poor bone mineral content (BMC) accrual. In preterm newborns (PTNs), the BMC is inversely proportional to birth weight and gestational age; decreased BMC is also related to inadequate intake of calcium and phosphorus in extra-uterine life.1–3
Risk factors related to inadequate mineralization are: very-low birth weight, intrauterine growth restriction, prolonged use of parenteral nutrition, use of diuretics and glucocorticoids, bronchopulmonary dysplasia, delay in introduction of food, low mineral supply in the diet, and long immobilization periods.4–6 The use of supplemented human milk is able to provide proper growth and bone mineralization in the short term.7
Metabolic bone disease of PTNs has no characteristic clinical presentation. It can be observed with longitudinal growth arrest, maintenance of head circumference, and even radiological signs similar to rickets, with spontaneous fractures described in 10% of PTNs with very-low birth weight.8
In PTNs with mineral deficiency, some biochemical markers may be altered. Hypophosphaturia and hypercalciuria that precede serum alterations (reduction in calcium and phosphorus, and elevated alkaline phosphatase) and radiological alterations can be observed.5,9 Other more specific serum or urinary markers, such as bone-specific alkaline phosphatase (BAP), deoxypyridinoline (DPD), osteocalcin, C-terminal telopeptide of type I collagen (CTX), and C-type natriuretic peptide (CNP) may be used for growth and bone remodeling assessment.10–12
Additionally, bone densitometry by dual-energy X-ray absorptiometry (DXA) has been considered the gold standard method to assess bone mineralization in newborns, showing high precision and accuracy.4,10,11,13–15
The aim of this study was to evaluate bone mineralization by DXA in the first 6 months of corrected age in PTNs compared with FTNs.
MethodsThis was a longitudinal study involving newborns admitted to the Neonatal Unit of the Hospital Universitário da Universidade de São Paulo (USP), with gestational age ≤ 32 weeks, followed from July of 2006 to September of 2008. As control group, a group of full-term newborns (FTNs) born in the same period were selected.
Newborns with congenital malformations, chromosomal disorders, or genetic disorders were excluded, as well as newborns of diabetic mothers. Newborn small or large for gestational age were also excluded. Informed consent was obtained from all parents of the assessed newborns. The study was approved by the Ethics Committee of Hospital Universitário da USP.
During this period, 42 newborns (17 PTNs and 25 FTNs) were selected. Fourteen newborns (three PTNs and 11 PTNs) were excluded due to: failure to complete follow-up (ten), diagnosis of severe heart disease (one) malnutrition (one), lack of informed consent (one), and inadequate DXA assessment (one). After exclusions, 28 newborns were evaluated: 14 PTNs (nine males and five females); and 14 FTNs (ten males and four females). The PTNs had a mean gestational age of 28.4 to 32.0 weeks (mean 31.1) and FTNs, of 38 to 41.8 weeks (mean 40.1). Birth weight of the PTNs ranged from 1,115g to 2,130g (mean 1,540g), and in FTNs, from 2,900g to 3,700g (mean 3,260g). All had weight between the 10th and 90th percentiles of the reference curve of Alexander et al.16
Sample size calculationAccording to the reference values of BMC for PTNs and FTNs, the estimated variability is approximately 6.5g (SD=6.5g) at 40 weeks of corrected age.2 Assuming a difference in BMC between PTNs and FTNs is found at 6-months of follow-up of at least 7g (with the initial difference being 10g between the two groups), an improvement of at least 30% in PTNs should be expected, with an 80% power and 95% confidence. Based on this calculation, the sample required to perform the study would be 14 patients in each group.
Risk factors for inadequate mineralization (pathologies and medications) found in pre-term infants were sepsis with positive blood cultures, which was observed in 28.5%; necrotizing enterocolitis (Bell's criteria) with clinical therapy, which was seen in 14.3%; and bronchopulmonary dysplasia (requiring oxygen therapy for 28 days or more), observed in 35.7%. Of total PTNs with bronchopulmonary dysplasia, three received hydrochlorothiazide and two, furosemide. Eleven received parenteral nutrition; two for less than one week, and nine for between one week and one month, with 12 days as the mean duration of parenteral nutrition.
Enteral feeding was introduced on the first day of life. During hospitalization in the neonatal unit, all PTNs received human milk, both their own mother's milk and milk from the University Hospital bank. Only four of the seven preterm infants with birth weight < 1,500g received human milk supplemented with an additive (FM85®) in pumped breast milk or pasteurized human milk and administered by orogastric tube or cup. The remaining three PTNs had hypophosphaturia (urinary phosphorus < 1mg kg−1 d−1) and therefore received an additional supply of calcium and phosphorus to achieve a supply of 200mg of calcium/kg and 110mg phosphorus/kg per day.9 All newborns (preterm and full-term) received enteral supplementation of vitamin D at a dose of 400 IU/day, which was maintained during the first two years of age.
After discharge, the children were assessed monthly. During the study period, all infants were fed exclusively human milk. Children with indication for additive use received a combined solution of calcium gluconate and dibasic calcium phosphate between breast-feedings, which was maintained for the first six months of age (age corrected for the PTNs and chronological age for the FTNs), and complementary feeding was not introduced.
All newborns were weighed on an electronic scale (Baby Model; Filizola - São Paulo, Brazil) and height was obtained using an anthropometric ruler graduated in centimeters.
In the group of PTNs, serum calcium, phosphorus, and alkaline phosphatase measurements were performed at the ages of 40 post-conceptual weeks and 6 months of corrected postnatal age. Furthermore, the concentration of calcium and phosphorus was determined in 6-hour urine samples between the third and fourth weeks of life (uncorrected age).
In the group of FTNs, measurements of serum calcium, phosphorus, and alkaline phosphatase were performed only at 40 weeks post-conceptual age, as blood collection at 6 months was not approved by the Ethics Committee.
Bone densitometry was performed at the Laboratory for Bone Metabolism of Rheumatology, Faculdade de Medicina da USP. The following parameters were evaluated: BMC, bone mineral density (BMD), and lean mass in three periods: 40 weeks of corrected post-conceptual age, as well as 3 and 6 months of corrected postnatal age. BMC reflects the total amount of material (mineral bone) measured by absorptiometry, in grams; BMD is defined as bone mineral content divided by bone area in grams per square centimeter, and lean body mass is fat-free mass.
A dual X-ray absorptiometry (DXA) apparatus was used (DXA: Discovery A; Hologic Inc. - Bedford, MA, USA) with the Infant Whole-Body scanning mode (software version 12.3.3; Hologic Inc.).
The software used is considered superior to pediatric software for the analysis of bone mineral, accurately validated for both PTNs and FTNs.17 In addition, the fan-beam technique, used in the study, makes the Discovery A scanner more accurate when compared to the prior pencil-beam technique.18 The study by Blake et al. demonstrated that the Discovery A scanner has additional advantages, as it requires a lower radiation dose when compared to the previously used Discovery W (Hologic Inc. - Bedford, MA, USA) and QDR 4500 Hologic Inc. - Bedford, MA, USA) models. For a newborn, the effective radiation dose is 8.9 mSv for the whole body, and it is 7.5 mSv for a child aged 1 year.19
Examinations were performed without sedation after breastfeeding. The coefficient of variation for whole body BMD was 0.004g/cm2 (0.4%), and the minimum significant difference for newborns evaluated in the study was 1.2% (95% confidence interval). These values are appropriate, as the literature describes coefficient values ranging from 0.8 to 2.2%.20
Statistical analysisStatistical analysis was performed using the Statistical Analysis System, release 9.1.3 (SAS Institute, Cary, NC, USA), and analysis of variance (ANOVA) with repeated measurements was performed in the PROC MIXED module of SAS (SAS Institute, Cary, NC, USA). Tukey multiple comparisons were performed after the ANOVA, which allowed for detection of the differences. To assess the association of variables with the group, Fisher's exact test was used. A significance level of 5% was adopted in all analyses.
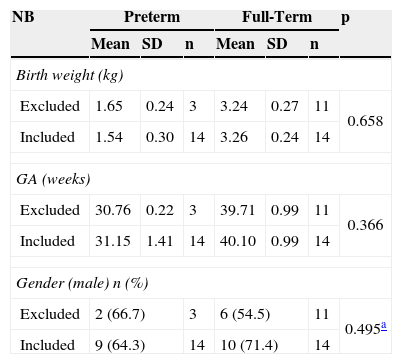
ResultsA total of 28 newborns were assessed: 14 PTNs and 14 FTNs. The gestational age of the PTNs ranged from 28.4 to 32.0 weeks (mean 31.1 weeks). Birth weight was 1,115-2,130g (mean 1,540g) and 2,900-3,700g (mean 3,260g) for PTNs and FTNs, respectively, and all of were adequate for gestational age. Fourteen infants (11 FTNs and three PTNs) were excluded, as they did not complete the assessment, and they showed similar weight, gestational age, and gender to the infants included in the study (p>0.05) (Table 1).
Comparison of birth weight, gestational age, and gender of children excluded from and included in the study.
| NB | Preterm | Full-Term | p | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | ||
| Birth weight (kg) | |||||||
| Excluded | 1.65 | 0.24 | 3 | 3.24 | 0.27 | 11 | 0.658 |
| Included | 1.54 | 0.30 | 14 | 3.26 | 0.24 | 14 | |
| GA (weeks) | |||||||
| Excluded | 30.76 | 0.22 | 3 | 39.71 | 0.99 | 11 | 0.366 |
| Included | 31.15 | 1.41 | 14 | 40.10 | 0.99 | 14 | |
| Gender (male) n (%) | |||||||
| Excluded | 2 (66.7) | 3 | 6 (54.5) | 11 | 0.495a | ||
| Included | 9 (64.3) | 14 | 10 (71.4) | 14 | |||
ANOVA result.
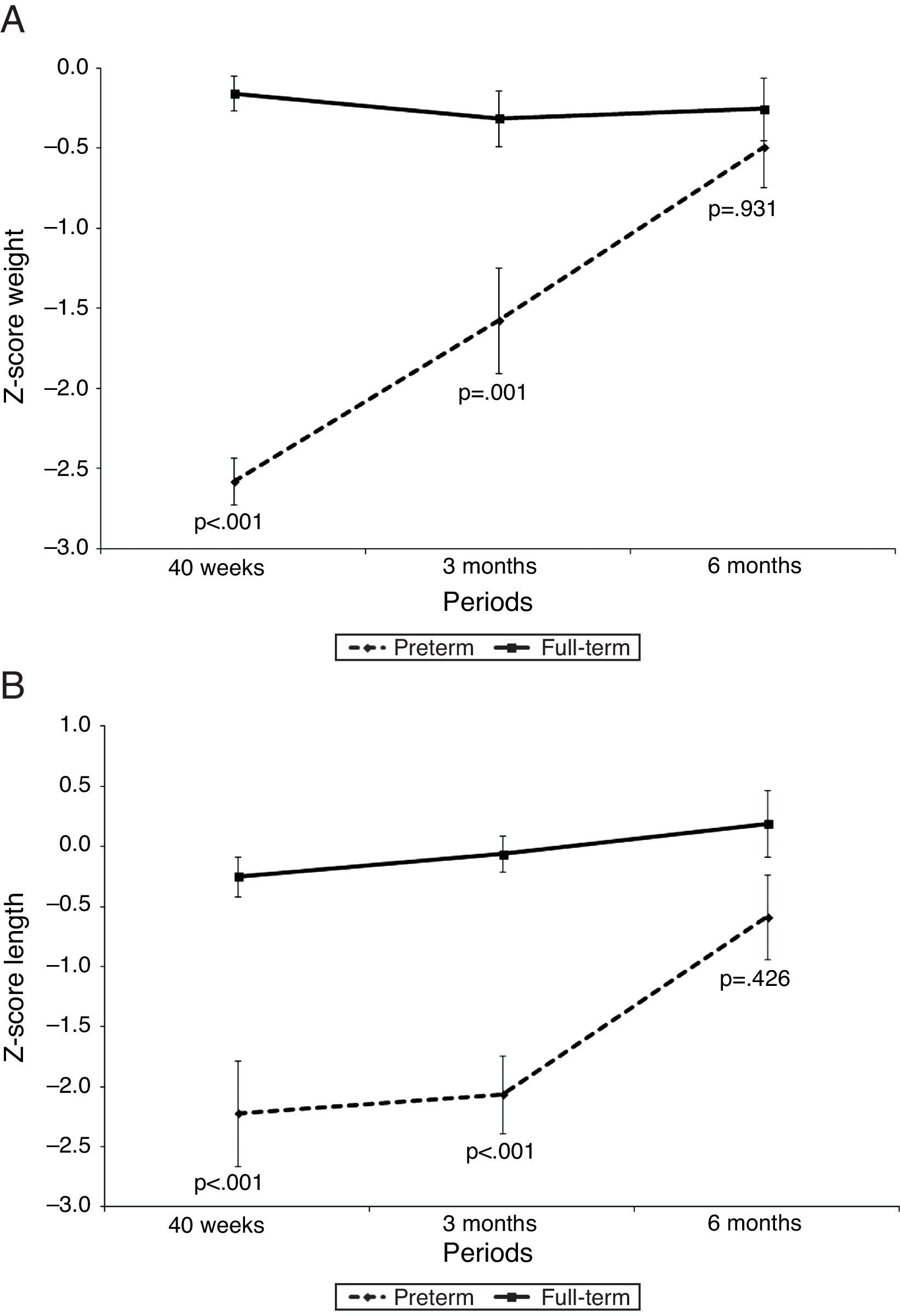
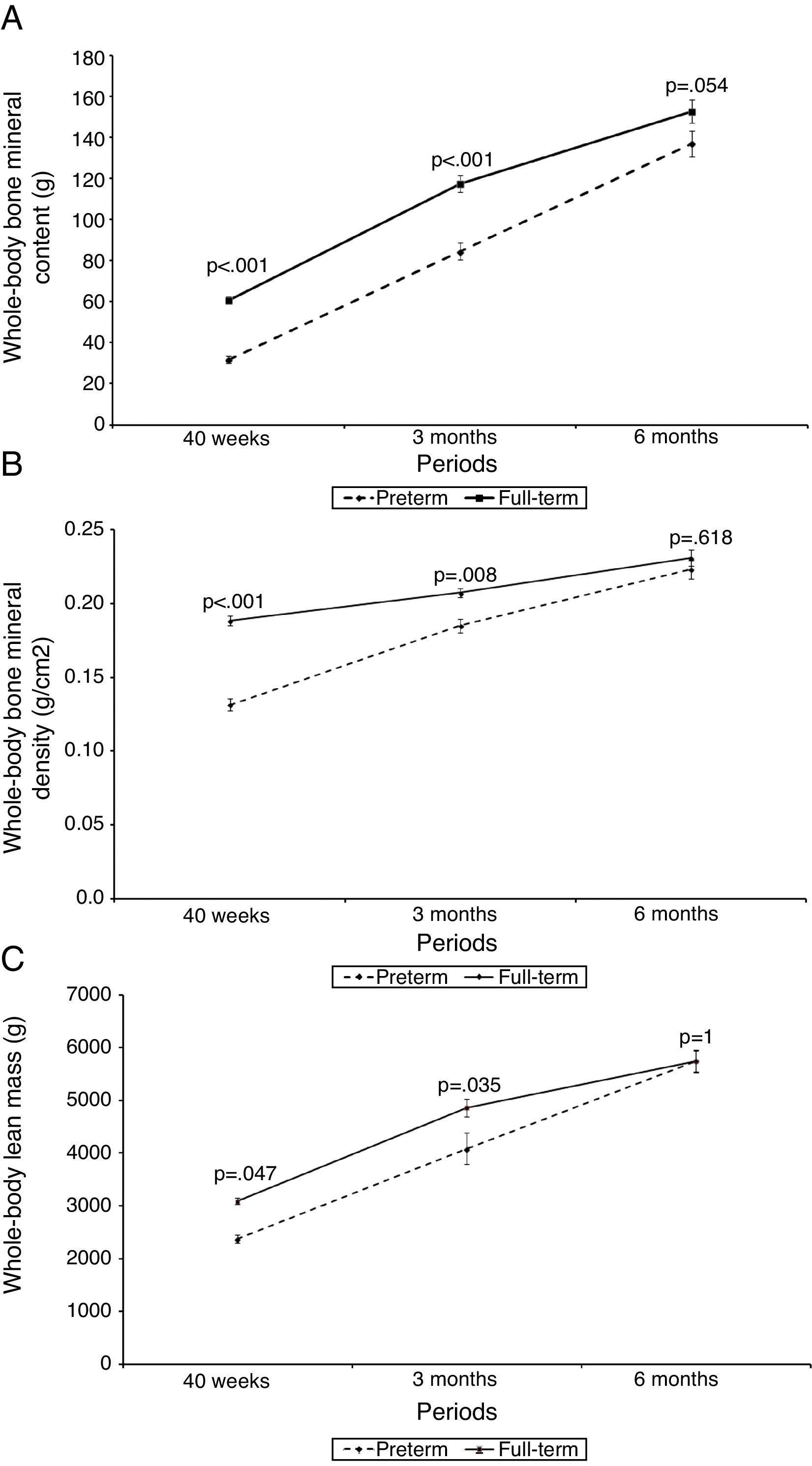
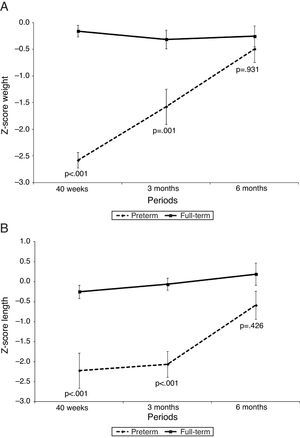
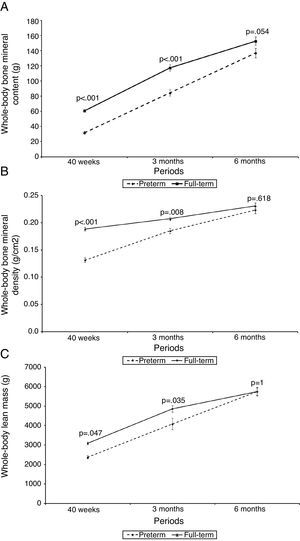
Fig. 1 shows the z-score for weight (kg) and height (cm), and Fig. 2 shows BMC (g), BMD (g/cm2), and lean mass (g) of PTNs and FTNs throughout the follow-up period, at 40 weeks of post-conceptual age, and at 3 and 6 months of corrected gestational age. At all times, differences were observed between the PTNs and FTNs, in both Z-score measures of weight and height, as well as BMC, BMD, and lean mass. The comparison between the PTNs and FTNs showed that at the initial assessment (40 weeks of post-conceptual age), PTNs had lower values when compared to FTNs regarding weight, Z-scores for weight and height, BMC, BMD, and lean mass (p<0.05) (Table 2). However, these differences disappeared at the 6-month evaluation of postnatal age, when all parameters showed similar means between the PTNs and FTNs (p>0.05) (Table 2).
Evolution of growth with Z-scores for weight and length of preterm infants in relation to their full-term peers at 40 weeks, 3 months, and 6 months of corrected age.
No. of sample: 40 weeks (preterm and full-term)=14; 3 months (preterm and full-term)=12; 6 months (preterm and full-term)=13.
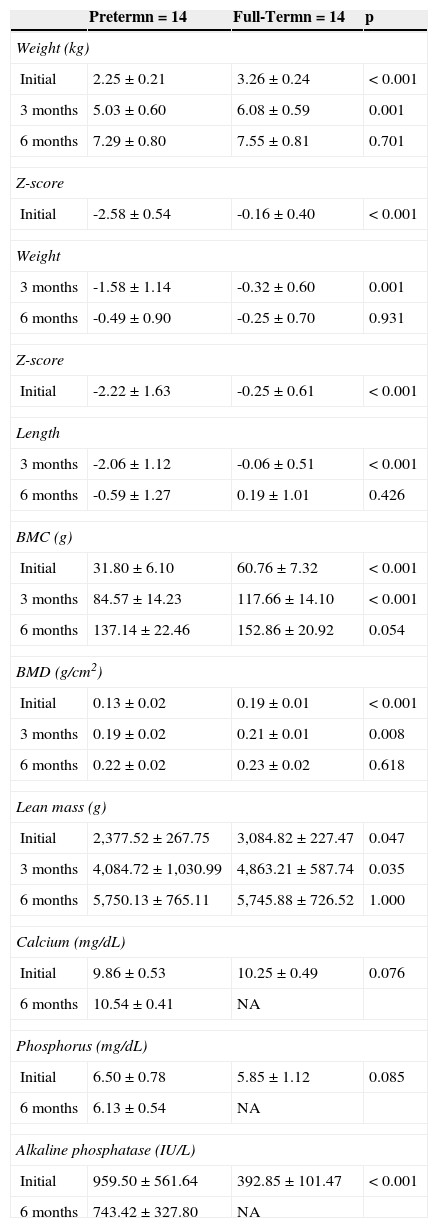
Measurements of weight, z-score for weight and length, bone mineral content (BMC), bone mineral density (BMD), lean mass, calcium, phosphorus, and alkaline phosphatase in preterm newborns and full-term newborns at 40 weeks corrected post-conceptual age, as well as 3 and 6 months of corrected postnatal age.
| Pretermn=14 | Full-Termn=14 | p | |
|---|---|---|---|
| Weight (kg) | |||
| Initial | 2.25±0.21 | 3.26±0.24 | < 0.001 |
| 3 months | 5.03±0.60 | 6.08±0.59 | 0.001 |
| 6 months | 7.29±0.80 | 7.55±0.81 | 0.701 |
| Z-score | |||
| Initial | -2.58±0.54 | -0.16±0.40 | < 0.001 |
| Weight | |||
| 3 months | -1.58±1.14 | -0.32±0.60 | 0.001 |
| 6 months | -0.49±0.90 | -0.25±0.70 | 0.931 |
| Z-score | |||
| Initial | -2.22±1.63 | -0.25±0.61 | < 0.001 |
| Length | |||
| 3 months | -2.06±1.12 | -0.06±0.51 | < 0.001 |
| 6 months | -0.59±1.27 | 0.19±1.01 | 0.426 |
| BMC (g) | |||
| Initial | 31.80±6.10 | 60.76±7.32 | < 0.001 |
| 3 months | 84.57±14.23 | 117.66±14.10 | < 0.001 |
| 6 months | 137.14±22.46 | 152.86±20.92 | 0.054 |
| BMD (g/cm2) | |||
| Initial | 0.13±0.02 | 0.19±0.01 | < 0.001 |
| 3 months | 0.19±0.02 | 0.21±0.01 | 0.008 |
| 6 months | 0.22±0.02 | 0.23±0.02 | 0.618 |
| Lean mass (g) | |||
| Initial | 2,377.52±267.75 | 3,084.82±227.47 | 0.047 |
| 3 months | 4,084.72±1,030.99 | 4,863.21±587.74 | 0.035 |
| 6 months | 5,750.13±765.11 | 5,745.88±726.52 | 1.000 |
| Calcium (mg/dL) | |||
| Initial | 9.86±0.53 | 10.25±0.49 | 0.076 |
| 6 months | 10.54±0.41 | NA | |
| Phosphorus (mg/dL) | |||
| Initial | 6.50±0.78 | 5.85±1.12 | 0.085 |
| 6 months | 6.13±0.54 | NA | |
| Alkaline phosphatase (IU/L) | |||
| Initial | 959.50±561.64 | 392.85±101.47 | < 0.001 |
| 6 months | 743.42±327.80 | NA | |
Data shown as mean ± SD.
IU/L, international units/liter; Initial, 40 weeks of corrected gestational age; NA, not assessed.
Serum biochemical parameters did not differ between PTNs and FTNs, except alkaline phosphatase, which was statistically higher in PTNs in relation to the FTNs (Table 2). Only two patients, both preterm, had alkaline phosphatase levels>1,200 IU/L, which is considered suggestive of metabolic bone disease.
Among the PTNs, three (21.4%) had results of urine tests suggestive of phosphorus deficiency syndrome (urinary calcium>4mg/kg per day, and urinary phosphorus < 1mg/kg per day). For this reason, the use of the oral solution of calcium and phosphorus was indicated, which was maintained until the corrected age of 6 months.
In addition to this solution with calcium and phosphorus oral supplementation, human milk additive was used in four PTNs, totaling 50% of preterm infants requiring human milk supplementation.
DiscussionIn Brazil, this was the first study conducted on the evolution of body composition of PTNs and FTNs fed human milk, assessed by DXA after discharge.
Bone mineralization of PTNs is frequently addressed in the literature, and DXA has become the method of choice for assessing body composition of newborns.21–24
The present study demonstrated, through evaluation by DXA, that PTNs reach BMC and BMD similar to those of FTNs after 6 months of corrected age.
The study sample consisted of 14 PTNs, of whom 50% had very-low birth weight. Nutritional support was required for proper growth of these infants weighing less than 1,500g, with a high supply of calcium, phosphorus, and protein, as these infants show an accelerated bone-remodeling rate. At birth, these PTNs had lower BMC and BMD in relation to FTNs, which persisted until 6 months of corrected age. This observation is in agreement with the literature, where there are reports of PTNs who, although receiving human milk and supplementation, did not significantly improve bone mineralization until they reached full term.4
This study demonstrated, through analysis by DXA, that the process of bone mineralization showed a significant acceleration in PTNs, but was still far from that observed in FTNs up to 6 months of corrected age, suggesting that mineral supplementation should be carried out for a prolonged period in very-low birth weight newborns.
In PTNs, calcium and phosphorus urinary concentrations depend on a complex interaction between ingestion, absorption, and renal function in these infants. Some authors recommend a urinalysis of these ions as a method to determine the need for supplementation, aiming to improve BMC and reduce the incidence of metabolic bone disease. However, these analyses do not appear to substitute the direct measurement of BMC and BMD.9,25 In fact, the present study demonstrated that the BMC and BMD were significantly lower in PTNs when compared to FTNs, even in infants with normal urinary and serum measurements.
Among serum markers of metabolic bone disease, the most widely used is alkaline phosphatase. However, the cutoff value for osteopenia definition varies widely in the literature, between 300 and 1,200 IU/L. In this sense, Figueras-Aloy et al. evaluated alkaline phosphatase and BMD in 336 PTNs and considered metabolic bone disease when both variables were altered (alkaline phosphatase>500 IU/L and BMD<0.068g/cm2) at hospital discharge.26
Although metabolic bone disease of prematurity is a self-limiting process, the rapid recovery of BMC (catch up) has many advantages: better growth in height and head circumference, prevention of fractures, and reduction of osteopenia in adulthood.27
Lean mass also normalized in PTNs at 6 months of corrected postnatal age, a finding similar to that reported by Cooke et al., albeit in children assessed at 12 months of corrected age.28 These authors found that lean mass was lower in PTNs when corrected for age. However, when corrected for weight, PTNs had lean mass values similar to the reference values for the FTNs.
In conclusion, the present study showed that PTNs recover BMD, BMC, and lean body mass at 6 months of corrected age, and suggests that bone densitometry is a good method for the assessment of these parameters at the initial assessment and especially at follow-up of these infants.
FundingConselho Nacional de Ciência e Tecnologia (CNPQ), Process No. 305691/2006-6.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Quintal VS, Diniz EM, Caparbo VF, Pereira RM. Bone densitometry by dual-energy X-ray absorptiometry (DXA) in preterm newborns compared with full-term peers in the first six months of life. J Pediatr (Rio J). 2014;90:556–62.