Evaluate the Neonatal Screening Program of the Health Secretariat of the State of Santa Catarina for sickle-cell disease, from January 2003 to December 2012, regarding program coverage and disease frequency.
MethodsDescriptive, observational, cross-sectional study with retrospective data collection. The variables analyzed were: number of live births in the State of Santa Catarina; number of screened children; number of children diagnosed with sickle-cell trait and sickle-cell disease; type of sickle-cell disease diagnosed; age at the time of sample collection, ethnicity/skin color, gender, and origin of children with sickle-cell disease. Descriptive measures and frequency tables were used for data analysis.
ResultsDuring the study period, there were 848,833 live births and 730,412 samples were screened by the program, resulting in a coverage of 86.0%. There were 6173 samples positive for sickle-cell trait and 39 for sickle-cell disease. Among children with sickle-cell disease, the median age at the time of sample collection was 6 days. Regarding the ethnicity/skin color, 25 (64.1%) children were white, seven were black, and seven others were not specified. The Midwest and the Highland (Planalto Serrano) of Santa Catarina were the regions with the highest incidence of sickle-cell disease.
ConclusionCoverage by the Neonatal Screening Program of Santa Catarina is good, but did not demonstrate an improvement trend over the years. The frequency of sickle-cell disease is low and lower than in the North, Northeast, and Midwest regions. The median age in days at the time of collection is older than the age recommended by the Ministry of Health.
avaliar o Programa de Triagem Neonatal da Secretaria de Saúde do Estado de Santa Catarina (PTN-SES/SC) para doença falciforme no período de janeiro de 2003 a dezembro de 2012, em relação à sua cobertura e à frequência da doença.
Métodosestudo descritivo, observacional e transversal com coleta retrospectiva dos dados. As variáveis analisadas foram: número de nascidos-vivos no Estado de Santa Catarina; número de crianças triadas; número de crianças diagnosticadas com traço e doença falciforme (DF); tipo de DF diagnosticada; idade da coleta, cor/raça, sexo e procedência das crianças com DF. Foram utilizadas as medidas descritivas e as tabelas de frequência para análise dos dados.
Resultadosno período estudado, houve 848.833 nascidos-vivos e 730.412 amostras triadas pelo programa, gerando cobertura de 86,0%. Das amostras triadas, foram encontradas 6.173 crianças com traço falciforme e 39 com DF. Entre as crianças com DF, a mediana da idade em dias na data da coleta foi de 6. Das 39 crianças doentes, 25 (64,1%) eram da cor/raça branca, 7da negra e 7 de outra cor/raça. As regiões do planalto serrano e do meio-oeste de Santa Catarina foram as regiões com maior incidência de DF.
Conclusõesa cobertura do PTN-SES/SC é boa, contudo não apresentou tendência de melhora ao longo dos anos. A frequência da DF é baixa e menor que nas regiões Norte, Nordeste e Centro-oeste. A mediana da idade em dias no momento da coleta está acima do preconizado pelo Ministério da Saúde.
Sickle-cell disease (SCD) represents a group of autosomal-recessive inherited hematological diseases, which includes several genotypes, with a prevalence of hemoglobin S (HbS).1–3 The presence of this abnormal hemoglobin is responsible for the main clinical manifestations of the disease, which originate from vaso-occlusive phenomena and chronic ischemic disorders.4
The distribution of SCD is a heterogeneous one and is more common among those of African ascendancy. According to 2009 data from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística – IBGE), in Brazil, black and brown individuals represented 6.9% and 44.2% of the population, respectively, reflecting the heterogeneity of SCD in the country.5
In June 2001, through Ministry of Health Decree No. 822, several diseases were added to the list of those screened through the existing National Neonatal Screening Program (NSP) (phenylketonuria and congenital hypothyroidism), to include the detection of SCD and other hemoglobinopathies, as well as cystic fibrosis.6 The diagnosis of SCD is performed by a laboratory through the detection of HbS and its association with quantitative fractions of other hemoglobins.
The NSP, together with multidisciplinary care, has allowed a significantly reduction in morbidity and mortality from the disease, as shown by studies in other countries.6
The aim of this study was to evaluate the NSP of the Health Secretariat of the State of Santa Catarina (Programa de Triagem Neonatal da Secretaria da Saúde do Estado de Santa Catarina [NSP-SES/SC]) for SCD and other hemoglobinopathies in relation to their coverage and the incidence of SCD and sickle-cell trait from January 2003 to December 2012.
MethodsThis was a descriptive, observational, cross-sectional study approved by the Ethics Committee of Hospital Infantil Joana de Gusmão under opinion No. 029/2013.
The assessed variables were obtained retrospectively from a database at the Central Laboratory (Laboratório Central [LACEN]) of the Health Secretariat of the State of Santa Catarina (Secretaria da Saúde do Estado de Santa Catarina [SES/SC]) and the Live Birth Information System (Sistema de Informações sobre Nascidos Vivos [SINASC]). The collected data comprise the period from January 2003 to December 2012.
The variables included: number of live births in the State of Santa Catarina; number of children screened by the NSP-SES/SC for SCD and other hemoglobinopathies; number of children diagnosed with SCD and sickle-cell trait by the NSP-SES/SC; type of SCD diagnosed by the aforementioned program; final diagnosis of children whose first samples were inconclusive; age at collection, ethnicity/skin color, gender, and origin of children with SCD according to the macro-regions of Santa Catarina.7
Regarding the variable ethnicity/skin color, it used the criterion established by the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística [IBGE]).5 The information on this variable was obtained from the file completed by the nursing staff at the time of blood sample collection.
Blood sample collection was carried out by the nursing staff in hospitals, maternity hospitals, or basic health units in the municipalities of Santa Catarina. The sample, taken from the child's heel and deposited on filter paper discs, was later sent to LACEN for analysis.
The method used to test blood samples was high-performance liquid chromatography associated with the cation-exchange chromatography, using the Variant II device (Bio-Rad®, CA, USA).
Samples with results different from the Hb FA pattern were submitted to another distinct chromatography test. The isoelectric focusing test was usually used for this assessment. When the results of both tests were distinct, constituting an inconclusive result, the child was called for a new sample collection.
Children whose samples had an indeterminate result, i.e., a hemoglobin type different from the hemoglobin that can be identified by standard screening tests, were referred for diagnostic clarification at Hospital Infantil Joana de Gusmão (HIJG), a referral center in the State of Santa Catarina for neonatal screening tests.
Descriptive measures and frequency tables were used for analysis of the results.
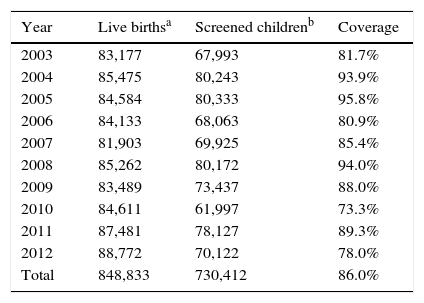
ResultsFrom January 2003 to December 2012, 730,412 samples were submitted to screening test of the NSP-SES/SC. During this period, 848,833 children were born in the state, according to SINASC, which indicates a program coverage of 86.0% (Table 1).
Number of live births, screened children, and percentage of coverage of the Neonatal Screening Program of the Health Secretariat of the State of Santa Catarina, from January 2003 to December 2012.
| Year | Live birthsa | Screened childrenb | Coverage |
|---|---|---|---|
| 2003 | 83,177 | 67,993 | 81.7% |
| 2004 | 85,475 | 80,243 | 93.9% |
| 2005 | 84,584 | 80,333 | 95.8% |
| 2006 | 84,133 | 68,063 | 80.9% |
| 2007 | 81,903 | 69,925 | 85.4% |
| 2008 | 85,262 | 80,172 | 94.0% |
| 2009 | 83,489 | 73,437 | 88.0% |
| 2010 | 84,611 | 61,997 | 73.3% |
| 2011 | 87,481 | 78,127 | 89.3% |
| 2012 | 88,772 | 70,122 | 78.0% |
| Total | 848,833 | 730,412 | 86.0% |
No justifications were found for the low coverage in 2010.
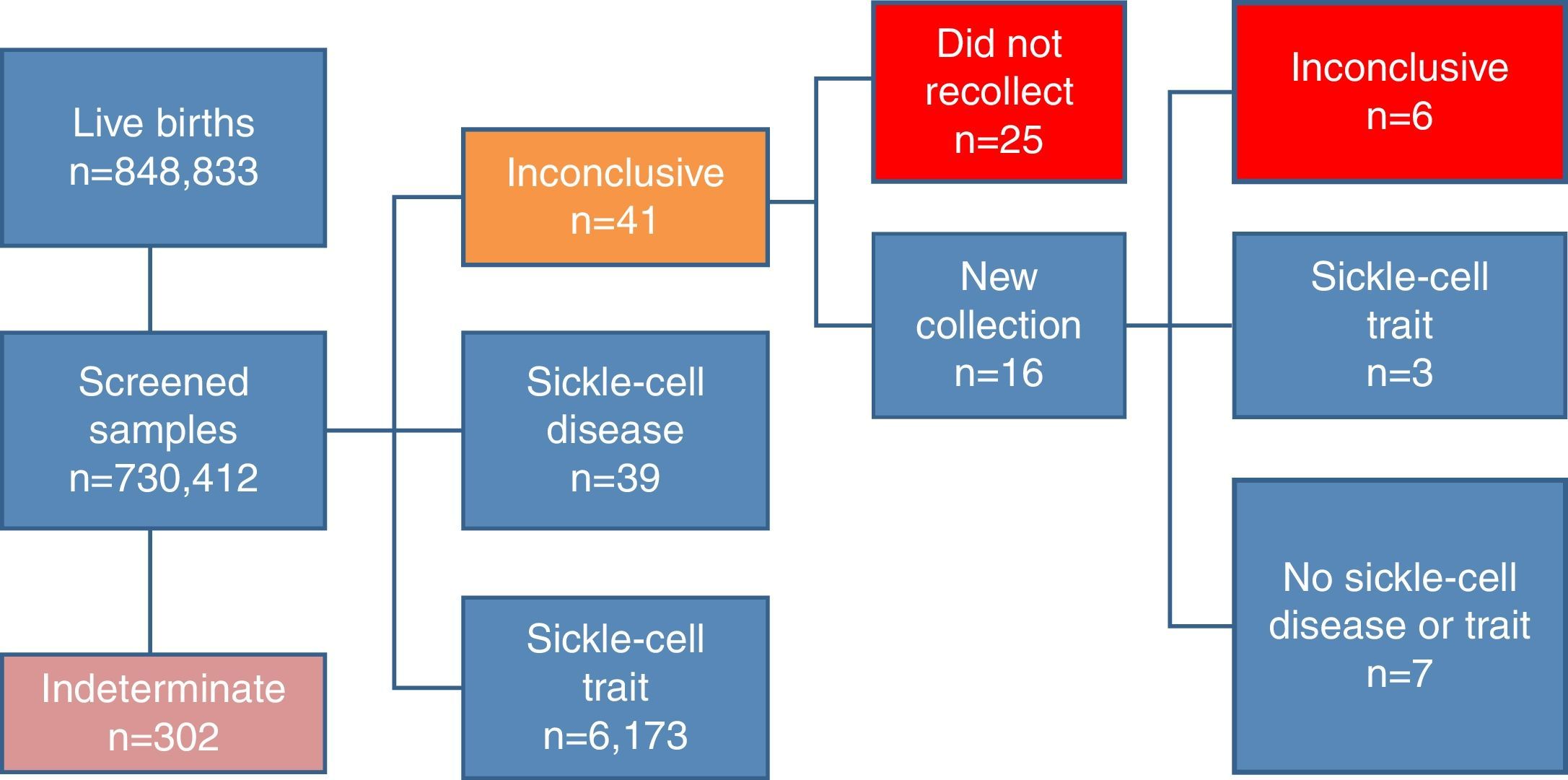
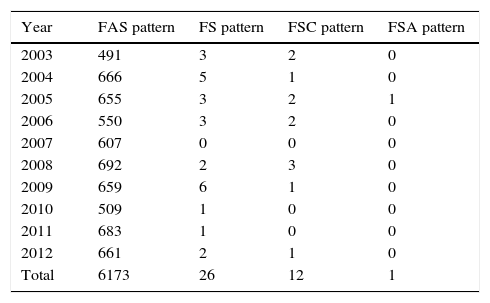
SCD was identified in 39 children of the screened samples. Of these, 26 cases showed the FS pattern (in order of decreasing quantity, presence of hemoglobins F and S, in the absence of hemoglobin A); 12 FSC (presence of hemoglobins F, S and C, in the absence of hemoglobin A); and one FSA (presence of hemoglobins F, S and A; Table 2). Each of these samples was submitted to two tests – high-performance liquid chromatography and isoelectric focusing – with equivalent results. Whether there was further confirmation of the results with other diagnostic tests was not assessed.
Number of children with sickle-cell trait and disease diagnosed by the Neonatal Screening Program of the Health Secretariat of the State of Santa Catarina, from January 2003 to December 2012.
| Year | FAS pattern | FS pattern | FSC pattern | FSA pattern |
|---|---|---|---|---|
| 2003 | 491 | 3 | 2 | 0 |
| 2004 | 666 | 5 | 1 | 0 |
| 2005 | 655 | 3 | 2 | 1 |
| 2006 | 550 | 3 | 2 | 0 |
| 2007 | 607 | 0 | 0 | 0 |
| 2008 | 692 | 2 | 3 | 0 |
| 2009 | 659 | 6 | 1 | 0 |
| 2010 | 509 | 1 | 0 | 0 |
| 2011 | 683 | 1 | 0 | 0 |
| 2012 | 661 | 2 | 1 | 0 |
| Total | 6173 | 26 | 12 | 1 |
Source: Central Laboratory (Laboratório Central [LACEN]) of the Health Secretariat of the State of Santa Catarina.
The result was indeterminate in 302 samples, i.e., the hemoglobin was different from hemoglobins that can be diagnosed by neonatal screening methods. These children were referred to the HIJG for diagnostic clarification.
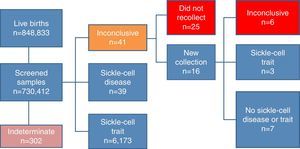
Of the samples analyzed by the NSP-SES/SC, 41 showed inconclusive results and 25 children with these results did not undergo a second test, despite the program's recommendation. Of the remaining 16 children that had a new sample collected, six once again showed an inconclusive pattern, seven showed a normal pattern, and three had sickle-cell trait pattern. Thus, 31 children with an inconclusive pattern did not have a definite diagnosis (Fig. 1).
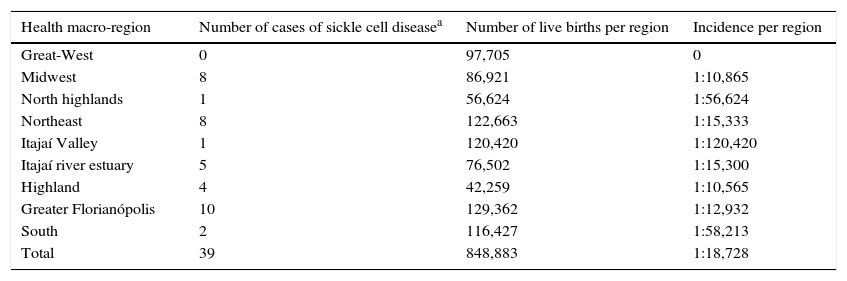
Regarding the origin, the Midwest and Highland macro-regions had the highest percentages of SCD cases (Table 3).
Distribution of sickle-cell disease per health macro-region of the State of Santa Catarina, from January 2003 to December 2012.
| Health macro-region | Number of cases of sickle cell diseasea | Number of live births per region | Incidence per region |
|---|---|---|---|
| Great-West | 0 | 97,705 | 0 |
| Midwest | 8 | 86,921 | 1:10,865 |
| North highlands | 1 | 56,624 | 1:56,624 |
| Northeast | 8 | 122,663 | 1:15,333 |
| Itajaí Valley | 1 | 120,420 | 1:120,420 |
| Itajaí river estuary | 5 | 76,502 | 1:15,300 |
| Highland | 4 | 42,259 | 1:10,565 |
| Greater Florianópolis | 10 | 129,362 | 1:12,932 |
| South | 2 | 116,427 | 1:58,213 |
| Total | 39 | 848,883 | 1:18,728 |
Source: Live Birth Information System (Sistema de Informações sobre Nascidos Vivos [SINASC])7 and Central Laboratory (Laboratório Central [LACEN]) of the Health Secretariat of the State of Santa Catarina.
Regarding the skin color/ethnicity of children with SCD (patterns FS, FSC, and FSA), 25 (64.1%) were white; seven were black, and seven were of ethnicity/skin color not specified in the sample collection form. As for gender, 24 were males and 15 were females. The mean age on the sample collection day was 9.08 days and the median was 6 days.
DiscussionThe main result of this study was to confirm the frequency of SCD (1:18,728) and sickle-cell trait (1:118) in the State of Santa Catarina, as well as the coverage of the neonatal screening program in the state (86.0%).
The incidence of SCD was low, possibly due to the small proportion of African descendants in the State of Santa Catarina. Blacks, according to IBGE, accounted for only 2.2%, and mixed-race, 11.7% of the state population in the year 2009.5 The highest SCD incidence occurred in the Midwest and Highland macro-regions of Santa Catarina.
In Brazil, in 2003, the incidence of SCD was 1:650 in Bahia, the state with the highest proportion of blacks in the population,8 while in Rio de Janeiro it was 1:1288 in 2007.9 However, a study carried in 2004 in Rio Grande do Sul showed an incidence of SCD of 1:39,100,10 and another performed in Paraná between 2002 and 2004 showed an incidence of 1:20,320.11
The observed coverage may not reflect the actual screening coverage of all live births. Even though all the municipalities in the state have joined this program, some children may have been screened in the private health care network, which does not generate record for the NSP-SES/SC and reflects a failure of the state's monitoring program.
In studies conducted in Brazil, it was observed that the program coverage in the Federal District was 83.4% in 200612; in Bahia, 88.9% between the years 2007 and 200913; and in Mato Grosso do Sul, 91.8% between 2006 and 2010.14 The goal of 100% coverage of the neonatal screening programs is still a challenge, even in more developed countries.9 Canada and Belgium, in 2006, achieved coverage of 76%15 and 87%,16 respectively.
Among the children with SCD in the period analyzed in this study, the mean age on sample collection day was 8.58 days and the median was 6 days, showing the need to improve these variables, as the Ministry of Health recommends that the collection be performed between 3 and 5 days of life. Although the test can be performed later, it is of utmost importance to collect the sample within the ideal period for some of the screened diseases, in order to provide early diagnosis and treatment, as well as to benefit from the prevention of sequelae.
A higher number of white patients was observed (64.1%) among the 39 SCD cases. The higher incidence of the disease in whites can be explained by the higher number of these individuals among the State of Santa Catarina's population, which, according to 2009 IBGE data, accounted for 85.7% of the total population,5 in addition to the white ethnicity being misattributed to the mixed-race population. The data limitation regarding skin color/ethnicity originates from the fact that this information is filled out by the person who completed the blood sample form and not based on a detailed family background questionnaire, considering the interpretative disagreement between skin color and ethnicity of a particular individual. It is essential, however, to be aware that none of these limitations affect the major aspects assessed in this study.
Regarding children with inconclusive and indeterminate results, it is important to create mechanisms to intensify the search for these individuals, aiming to expand the program's scope and to identify and provide early treatment to any SCD carriers – which in this study was of a low frequency, considering the number of screened samples.
One limitation of this study is due to lack of patient monitoring after diagnosis through the neonatal screening, in order to assess patient adherence to the interdisciplinary outpatient follow-up and health measures proposed by the program. Recently, a study performed in Minas Gerais found that neonatal screening in that state, even if carried out in a comprehensive and effective manner, was not enough to reduce SCD mortality, which was 7.4% in the first 14 years, with slightly more than half of the deaths occurring before 2 years of age.17
The drastic reduction in mortality related to SCD in the early years of life, from 26% to 1–2%, occurred due to comprehensive care provided to this population at pediatric referral centers. Therefore, early diagnosis and treatment of SCD helps to increase survival and significantly improves the quality of life of these individuals.6,18
Thus, it can be concluded that the NSP has good coverage in Santa Catarina and that the incidence of SCD in the state is quite low. Nevertheless, it is essential to assess patients’ adherence to health measures recommended by the NSP, as without this follow-up, the diagnosis alone becomes pointless. With the information provided by this study, the authors intend to seek the affected children and assess adherence to outpatient medical follow-up, as well as the health support offered to patients, in order to actually evaluate the program's efficacy.
Conflicts of interestThe authors declare no conflicts of interest.