To compare bacterial growth in pure colostrum versus colostrum with human milk fortifier (HMF) containing iron.
MethodsThe growth of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa in 78 samples of pure colostrum or colostrum with added iron-containing HMF was compared. For qualitative analysis, filter paper discs were immersed in samples from each group and incubated for 48hours with 101 colony forming units (CFUs)/mL of each strain. For quantitative assessment, 1mL of each strain containing 107 CFUs/mL was homogenized with 1mL of either colostrum or colostrum with human milk fortifier, seeded into a Petri dish, and incubated at 37°C. Twenty-four hours later, the number of CFUs was counted.
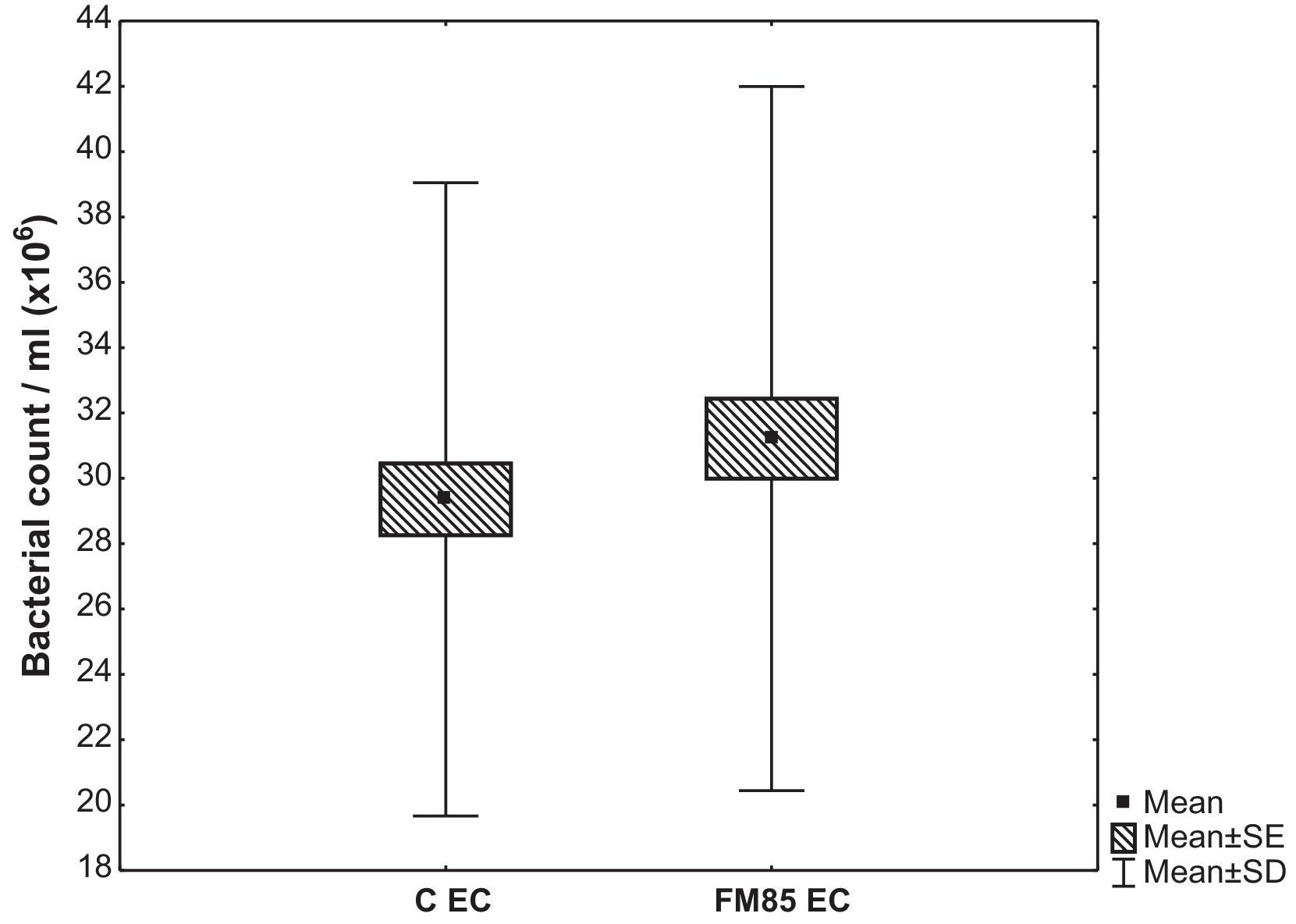
ResultsThe qualitative analysis showed no difference in bacterial growth. In the quantitative evaluation, E. coli growth in the control group was 29.4±9.7×106CFU/mL, while in the HMF group it was 31.2±10.8×106CFU/mL. The difference between the average growth was 1.9±4.9×106CFU/mL (p=0.001). There were no differences in S. aureus and P. aeruginosa growth.
ConclusionAddition of iron at this concentration reduces breast milk bacteriostatic action against E. coli.
Comparar o crescimento bacteriano em colostro puro e colostro com aditivo do leite materno contendo ferro.
MétodosForam comparadas 78 amostras de colostro puro ou colostro com adição de aditivo do leite materno contendo ferro para avaliar o crescimento de Escherichia coli, Staphylococcus aureus e Pseudomonas aeruginosa. Para a análise qualitativa, discos de papel-filtro foram imersos em amostras de cada grupo e incubados por 48 horas com 101 Unidades Formadoras de Colônias/mL de cada cepa. Para a avaliação quantitativa, 1mL de cada cepa contendo 107 Unidades Formadoras de Colônias/mL foi homogeneizado com 1mL, tanto de colostro puro quanto de colostro com aditivo do leite materno, espalhado em placa de Petri e incubado a 37°C. O número de Unidades Formadoras de Colônias foi contado 24 horas depois.
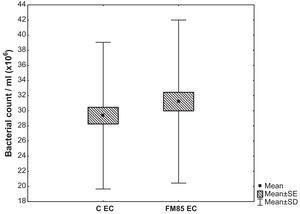
ResultadosA análise qualitativa não mostrou nenhuma diferença no crescimento bacteriano. Na avaliação quantitativa, o crescimento de Escherichia coli (EC) no grupo C foi de 29,4±9,7×106CFU/mL, enquanto no grupo FM85 foi de 31,2±10,8×106CFU/mL. A diferença entre o crescimento médio foi de 1,9±4,9×106CFU/mL (p=0,001). Não houve diferenças no crescimento de Staphylococcus aureus e Pseudomonas aeruginosa.
ConclusãoA adição de ferro a essa concentração reduz a ação bacteriostática do leite materno contra Escherichia coli.
It is well known that breast milk is the optimal food to be offered to the newborn due to its unique growth and immunologic factors.1,2 It has been shown that premature newborns also have better outcomes when fed with breast milk.2–4 However, due to the high-energy needs of premature newborns, breast milk alone is often insufficient to meet their nutritional requirements, especially in premature infants who weigh less than 1,500g.4–6 In such small infants, energy, protein, calcium, phosphorus, iron, and sodium intakes should be increased, and the advantages of breast milk maintained by adding human milk fortifier (HMF) to breast milk.4,6–10 The only HMF available at the moment of this study in Brazil had been modified to contain an increased amount of iron (0.28mg of Fe per 1 gram of product).
Among all immunologic benefits of breast milk, the bacteriostatic capacity of lactoferrin is remarkable.11 Lactoferrin is an iron-binding protein that has been shown to have activity against bacteria, viruses, and fungi;11,12 to stimulate the immune system and the mucosa immune function;11 and to have antioxidant and anti-carcinogenic effects.11–13 Bovine lactoferrin supplementation has been shown to prevent sepsis in very-low-birth-weight neonates, and has been shown to reduce respiratory tract illness and increase hematocrits in healthy bottle-fed infants.14,15 In human breast milk, lactoferrin acts at the newborns’ mucosa and protects them from infection by binding to iron and depriving it from pathologic bacteria that need iron to proliferate.7–11 In order to maintain this bacteriostatic capacity, lactoferrin needs to be in an environment with a low iron concentration. If exogenous iron is added to breast milk, the benefits of lactoferrin might be impaired, which in turn might increase the risk of infection in newborns.7–10
The aim of this study was to compare pathogenic bacterial growth in colostrum versus colostrum supplemented with iron-enriched HMF.
Materials and methodsColostrum samples were collected from lactating mothers who delivered at term during the period of 2010 and 2011. 10mL of breast milk was collected from each mother. The inclusion criteria were lactating white healthy mothers who delivered in term. The exclusion criteria were mothers who had cesarean deliveries, receiving antibiotic treatment, on suspicion of infection, or with history of smoking.
The mothers were approached by the researcher after delivery, always accompanied by obstetrics and gynecology resident physicians.
The participants were asked about their pre-pregnancy body weight and age. They were instructed on how to collect the breast milk in an aseptic fashion. Sample collection was performed manually or with a manual suction pump, according to the mother's preference. Mothers who chose to use the manual suction pump received an ethylene oxide-sterilized pump containing a flask, a polypropylene tube, and a latex plunger, and were verbally oriented on how to use the pump (according to manufacturer's instructions).
The samples were collected in sterilized tubes and were closed with sterilized rubber stoppers. Mothers who chose to use a pump collected their samples in a coupled tube, closed with a polypropylene stopper. In the laboratory, each sample was transferred to another sterile tube. Each of these tubes was identified with a label containing the mother's name and the sample number, as well as the day and hour of collection.
The samples were kept in a refrigerator at a temperature of 4°C to 6°C, and analyzed within 72hours. Each sample was divided into two samples of 5mL, one to be analyzed as control (pure human milk), and the other with added HMF. HMF was added immediately before the analyses, in a proportion of 5%, which resulted in 0.25g of fortifier for each 5mL of breast milk (manufacturer's instructions). The fortifier was weighed with an analytical balance.
Proof of sterility was applied to all samples according to the method by Almeida and Novak.16 The samples were seeded in thioglycolate broth and soy tripcasein broth. 0.4mL of breast milk were added to 10mL of each of the broths using an automated, sterilized pipette. Samples were then incubated at 36.4°C for 48hours. The analyses were made after 24 and 48hours.
Qualitative evaluation of bactericidal capacity was evaluated according to the methodology proposed by the American Society for Microbiology17 and by Chan.8Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa were obtained from clinical isolate strains under cultivation 18 to 24hours in brain hearth infusion (BHI) agar.
Each strain of bacteria culture was prepared at 37° C BHI broth in agar plate. For each of the samples, a colony was resuspended in saline and diluted until the desired concentration (101 colony-forming units – CFU/mL) compared to the standard pipe scale of MacFarland. Two Petri dishes containing BHI agar were prepared for each strain of bacteria, and individually seeded with 101 CFU/mL of each strain, identified with the strain and the sample number.
Sterilized discs of filter paper were then immersed in samples of either pure breast milk, denoted as the control (C) group, or breast milk plus HMF, denoted as the HMF group. The discs were placed in the Petri dishes and incubated for 48hours. The inhibition halos were then measured. Four discs were placed in each Petri dish. A standard model, maintaining the same distances between discs, determined the locations of the discs.
For the quantitative assessment of breast milk samples, the samples were evaluated according to the methodology proposed by Hernandez et al.18 The same samples of pure colostrum and fortified colostrum, as well as the same strains of bacteria from the qualitative assessment, were used. However, the concentration of bacteria was now at 107CFU/mL. 1mL of the suspension of bacteria with either 1mL of pure breast milk or 1mL of breast milk plus HMF were homogenized in a magnetic stirrer. 1mL of this mixture was seeded into Petri dishes with BHI agar. The dishes were then incubated at 37°C for 24hours. The number of CFUs was counted after the incubation period.
The sample size was determined by SIGMA, accepting a margin of error of 5% and variation of bacterial growth of 10%. This calculation suggested that a minimum of 40 samples were necessary.
Statistical analysisThe results obtained in the study were expressed as means and standard deviations. To compare the bacterial growth between colostrum and colostrum with HMF, the Student's t-test for paired samples was used. To assess the correlation between the two types of milk, Pearson's correlation coefficient was calculated. This same statistical analysis was used to assess the correlation between the number of bacteria and the mother's age, as well as the pre-pregnancy weight. A p-value <0.05 was considered statistically significant. Data were analyzed with the computer software (StatSoft Inc – USA).
The research protocol was approved by the Research Ethics Committee of the Hospital Angelina Caron and by the Research Ethics Committee of the Hospital das Clínicas – Medical School of the Universidade de São Paulo. All procedures followed the ethical standards of the responsible committee on human experimentation of both institutions. The procedures were also in accordance with the Helsinki Declaration of 1975. All participants received detailed explanation of the protocol and signed an informed consent detailing their participation.
ResultsSeventy-eight samples of breast milk were collected. Only three mothers refused to donate milk sample, two because of pain and one for personal reasons. The average age of the participants was 25.2±6.6 years and the mean pre-pregnancy body weight was 60.6±10.1kg.
The proof of sterility confirmed that all samples were sterile. There was no bacterial growth inhibition in any sample by the qualitative analysis, as there was no evidence of inhibition halos around the discs of the C group or the HMF group.
The quantitative evaluation showed that the mean E. coli growth in the C group was 29.4±9.7×106CFU/mL, while in the HMF it was 31.2±10.8×106CFU/mL. The difference between the average growth in C and HMF group was 1.9±4.9×106CFU/mL (p=0.001) (Fig. 1).
The mean growth of S. aureus in the C group was 43±11.6×106CFU/mL, and in HMF group it was 43.2±12.6×106CFU/mL. The mean difference between the two groups was 0.3±4.5×106CFU/mL (p=0.614). The mean growth of P. aeruginosa in the C group was 51.1±12.0×106CFU/mL, and in the HMF group it was 51.5±12.0×106CFU/mL. The mean difference between the two groups was 0.4±3.0×106CFU/mL (p=0.285).
The variables pre-pregnancy body weight and age were not correlated with bacterial growth (the correlation coefficient for all strains was p>0.05).
For each bacterial strain, the correlation coefficient between the number of bacteria in both the C group and the HMF group was estimated. The correlation coefficient between C group's E. coli and HMF group's E. coli was 0.89 (p<0.001); between C group's S. aureus and HMF group's S. aureus was 0.94 (p<0.001), and between C group's P. aeruginosa and HMF group's P. aeruginosa was 0.97 (p<0.001).
DiscussionAll samples were collected within two days of delivery. There were no bacterial or fungal growth in the samples as determined by the proof of sterility, with no contamination in both collection and storage, which validates the applied methodology.
The variables age and pre-pregnancy body weight showed no correlation with bacterial growth. Prepregnancy weight was the only nutritional parameter evaluated in this study. Collado et al.19 have associated the immunomodulatory function of human milk with overweight and excessive weight gain during pregnancy. In their study, overweight mothers presented lower levels of TGF- β2 and CD14 when compared with normal-weight mothers. Maternal weight and weight gain during pregnancy were also shown to affect infants’ fecal microbial composition, with higher levels of Staphyloccocus sp. and lower levels of Bifidobacterium sp. in infants of normal-weight mothers with normal weight gain during pregnancy.19,20 Previous studies have found no relationship between maternal hemoglobin and iron levels and iron and lactoferrin content in their breast milk.21,22 Conversely, other studies have shown that maternal nutritional status affects human milk composition.23,24
The variable age also did not show correlation with bacterial growth, and this was probably because an adult population (25.2±6.6 years) was studied. It is known that human milk from teenager mothers has nutritional deficiencies due to their fast growth during this period.25,26
It was decided to collect colostrum, similarly to other studies, because of its higher concentration of lactoferrin,22 and also because it would be possible to standardize lactoferrin concentration in samples. The collection was also easier because samples were collected immediately after birth, while mothers were still at the hospital.
The qualitative analysis used in this study did not allow for the confirmation of a bacteriostatic effect of milk; since there was no formation of a bacterial growth inhibition zone, it was not possible to measure the halos, in contrast to the results found by Chan et al.7,8 They found inhibition of bacterial growth by pure breast milk and by breast milk with HMF without the addition of iron (0.2mg/100mL of breast milk), but found no inhibition in bacterial growth when the breast milk was supplemented with HMF that contained additional iron (1.5mg of iron in 100mL of breast milk).
Santiago et al.10 compared the growth of Gram-positive and Gram-negative bacteria in breast milk samples supplemented with the same HMF used by Chan et al.7,8 The authors performed total counts of bacteria in frozen samples at time 0, 24hours, and 72hours with and without the addition of HMF. They found no difference in bacterial growth reduction in both groups, and also concluded that the antibacterial properties of human milk, with or without HMF, can last for 72hours. These results also validate the applied methodology of refrigeration. Similarly, Yuen et al.27 analyzed 25 colostrum and 11 mature samples of milk after being stored at 4°C and - 20°C, and concluded that all nutritional and immunological factors were adequately preserved after three days of refrigeration.
Ovali et al.9 compared the bacteriostatic capacity of pure breast milk, breast milk with HMF without addition of iron, and breast milk with ferrous sulfate (0.38mg in 30mL of breast milk), using the same methodology proposed by Chan, and with the same bacterial strains used in the present study. In contrast to the results of the present study, the authors concluded that the addition of ferrous sulfate at 0.38mg in 30mL of breast milk inhibited the bacteriostatic capacity against all strains.
All abovementioned studies analyzed milk samples of mothers who delivered prematurely, while the present study included mothers who delivered in term, since the number of premature births in this hospital is very small. It is important to note that this might be a reason for the differing results.
Previous reports on lactoferrin levels in preterm milk have shown either significantly higher values than term milk28 or no difference between them.29 Ronayne de Ferrer et al.30 found no difference between preterm and term lactoferrin values, in spite of the trend observed in colostrum samples, where term milk tended to show higher levels than preterm milk. The same study,30 similarly to others,22 showed that lactoferrin concentration values decrease, mainly in term milk. It is also important to observe that studies have demonstrated that lactoferrin levels vary in different populations.31
In the present study, term colostrum was used instead of preterm colostrum, and this might be a limitation, although lactoferrin levels between preterm and term colostrum appear not to be clearly defined. When the present results are extrapolated to clinical practice, it is important to highlight that, although HMF is currently used for preterm infants, in Brazil a large number of preterm infants receive HMF added to human milk from the Human Milk Bank, and in that case they usually receive term milk.
None of the previous studies evaluated bacterial growth with the addition of the HMF used in this study, which is the only fortifier available in Brazil (0.28mg of iron in 1g of fortifier). It is also important to consider that in the studies by Chan et al., different bacteria strains were analyzed: E. coli, Staphylococcus, Enterobacter sakazakii, and group B Streptococcus. In the present study, E. coli growth was higher in the samples with HMF, similarly to the studies by Chan et al.7,8
The bacterial strains selected for this study are the most frequent reported by the Commission of Hospital Infection Control of the hospital where the study was performed.
The correlation coefficients between C group samples and HMF group samples were significant for all strains of bacteria, which demonstrates that the bacterial growth was similar in all samples when the two groups were compared, indicating that bacterial growth followed the same pattern in both groups, regardless of the addition of HMF.
The mechanism through which lactoferrin inhibits bacterial growth remains unclear. The bacteriostatic capacity of lactoferrin is often attributed to its ability to chelate iron, restricting this essential nutrient for pathogenic bacteria proliferation. However, it is important to highlight that studies have also shown that the bactericidal activity is not dependent on the degree of iron saturation of lactoferrin. Since lactoferrin has been shown to affect the immune system and the mucosa immune function, the addition of iron may affect these actions. However, since the present study was in vitro, it was not possible to analyze those functions.7,11–13,32,33
Care should be taken when extrapolating the present results to clinical practice. In vitro, it appears that the HMF currently available in Brazil may increase the risk for E. coli proliferation. However, in vivo studies are needed to test the clinical significance of these results.
Contradictory results found in similar studies are probably due to different milk samples, which may vary depending on ethnic characteristics, infant age at the delivery time, milk maturity, time of sample collection, added iron amount, and strain of bacteria analyzed. Additional studies are needed to identify the degree to which iron can interfere with the bacteriostatic capacity for each bacterial strain, since the bacteriostatic function of breast milk appears to depend upon all these factors.
ConclusionThis study demonstrated that iron supplementation with 0.28mg of iron per 1g of HMF reduced the bacteriostatic action of breast milk against E. coli in vitro; however, this effect was not observed for P. aeruginosa and S. aureus strains. Further in vivo studies are needed to determine how to extrapolate these data to clinical practice.
Conflicts of interestThe authors declare no conflicts of interest.
The authors want to acknowledge all mothers who participated in this research that generously donated their breast milk, and Frederick Schwenk, MD, for his critical reading of the manuscript.
Please cite this article as: Campos LF, Repka JCD, Falcao MC. Effects of human milk fortifier with iron on the bacteriostatic properties of breast milk. J Pediatr (Rio J). 2013;89:394–9.