To analyze the profile of blood transfusion reactions in children and to identify the involved factors.
MethodsThis was a cross-sectional study in a tertiary pediatric teaching hospital from the public healthcare system, involving all children admitted from January to July of 2011 (5,437), of which 1,226 received blood transfusions, constituting the sample. A documental study was performed by analyzing files from the hemovigilance service and notification forms of transfusion reactions. The variables investigated were: number and type of blood components transfused, transfusion site, reaction site, age, gender, type of blood components involved, type of incident, and previous history of multiple transfusions. A descriptive and inferential analysis was performed, using statistical tests to establish the association between the variables.
ResultsThere were 57 transfusion incidents involving 47 children and 72 different blood products, thus constituting a prevalence of reactions of 3.8%. At the inferential analysis, the chi-squared test showed that the following variables were significantly associated (p<0.05) with the type of reaction: age range and type of blood component. Similarly, the patient's underlying disease was associated with previous history of transfusion incident and type of blood component.
ConclusionsThe prevalence of transfusion reactions in children is high, and the intervening factors are: type of blood component, age, patient comorbidity, and multiple transfusions; type of blood component and age are also associated with type of reaction.
Analisar o perfil das reações transfusionais em crianças e identificar os fatores intervenientes.
MétodosEstudo transversal realizado em um hospital-escola pediátrico terciário da rede pública, envolvendo todas as crianças internadas de janeiro a julho de 2011 (5437), das quais 1.226 foram hemotransfundidas, constituindo, assim, a amostra. Realizado estudo documental dos relatórios do serviço de hemovigilância e das fichas de notificação de reações transfusionais. As variáveis investigadas foram: número e tipo de hemocomponente transfundido, local de transfusão, local da reação, idade, sexo, tipo de hemocomponente envolvido, tipo de incidente e história prévia de politransfusão. Realizada análise descritiva e inferencial, utilizando-se testes estatísticos para estabelecer associação entre as variáveis.
ResultadosVerificou-se 57 incidentes transfusionais envolvendo 47 crianças distintas e 72 hemocomponentes, constituindo uma prevalência de reações de 3,8%. Na análise inferencial, pelo teste do Qui-quadrado, as seguintes variáveis apresentaram associação estatisticamente significativa (p<0,05) com o tipo de reação: faixa etária e tipo de hemocomponente envolvido. Analogamente, a doença de base do paciente associou-se com a história prévia de incidente transfusional e o tipo de hemocomponente.
ConclusõesA prevalência de reações transfusionais em crianças é elevada, e os fatores intervenientes são o tipo de hemocomponente, a faixa etária, comorbidade do paciente e politransfusão, sendo que os dois primeiros associam-se, também, com tipo de reação ocorrida.
Transfusion reaction is defined as any event that occurs as consequence of blood product transfusion, during or after its administration. These adverse events may vary from mild anaphylaxis to severe hepatitis, sepsis, and death. Therefore, the thorough investigation of cases of transfusion reaction is essential to medical practice.1–3
According to data from the National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA), for every 1,065 transfusions, there is one transfusion reaction notification, of which 85% are mild, 12.7% moderate, and 2.2% severe.4 These figures refer to all Brazilian hospitals that are part of the Hospital Sentinel Network, regardless of the category and age range of patients. A multicenter study involving 35 pediatric teaching hospitals in the United States showed that approximately 0.95% of patients had blood transfusion reactions; children older than 2 years were the most vulnerable to this event, and most reactions were mild.5
Since the pediatric population presents allergic reactions more frequently than adults,5 allergic transfusion reactions may often be underdiagnosed; hence, it is important to analyze them. Similarly, special attention is needed regarding oncologic patients, who undergo many blood transfusions and are therefore more exposed to reactions that may be severe, as many of these patients already have a poor health status. The scarcity of publications available online regarding the pediatric population must be highlighted, after searching the following databases: SciELO, BIREME, PubMed, MEDLINE, Cochrane, and UptoDate. The present study, performed in a teaching hospital, has benefited the training of health professionals, who will be able to work with a greater degree of safety regarding transfusion reactions, with evidence-based practice. Thus, it is expected that the present study will help decision-making, as well as encourage further studies.
Considering the abovementioned facts, this study aimed to analyze the profile of transfusion reactions and to identify associated factors in a tertiary pediatric hospital.
MethodsThis was a cross-sectional, quantitative study with a descriptive and analytical approach, performed in a tertiary pediatric hospital in Fortaleza, state of Ceará, Brazil, in the period between January and July of 2011. The period was chosen for the convenience of the authors.
The hospital is part of the public health care system, specialized in the care of children and adolescents aged 0 to 18 years, providing low, medium, and high complexity treatments. It also has attachment to the hospital, a center specializing in Pediatric Oncology, and all individuals undergoing treatment are therefore considered patients at the institution. It is a national reference in pediatric care and a teaching hospital accredited by the Ministry of Education. It is part of the Brazilian Hospital Sentinel network, which is a strategy for post-marketing surveillance of health products and services, coordinated by the Ministry of Health and ANVISA. Thus, the Hospital Health Risk Department (Gerência de Risco Sanitário Hospitalar – GRSH) is responsible, among other services, for hemovigilance, a system to collect and evaluate information on undesirable or unexpected events after blood product use. This service performs the analysis of records from the Hospital Transfusion Agency and conducts active searches of transfusion reactions in the institution, describing such occurrences in a monthly report filed with the GRSH and sent to ANVISA.
The study variables are defined as follows:
- 1.
Number of transfused blood products: corresponds to the total transfusions performed by the service.
- 2.
Type of transfused blood components: generically classified, similarly to the classification used by ANVISA as concentrated platelets, packed red blood cells, fresh frozen plasma, cryoprecipitate, and whole blood. In order to maintain the standard used by ANVISA, the use of filters or irradiation in blood product classification was not considered, but the proportion of blood products transfused after such treatments is described. According to the service routine, all cancer patients received filtered units, and patients diagnosed with acute myeloid leukemia or medullary aplasia, as well as bone marrow transplant candidates, received filtered and irradiated units of packed red blood cells or concentrated platelets, according to the literature.6 According to the routine of the Blood Center service, premature infants, weighing < 1,200g at birth or up to 28 days after birth also receive irradiated units. Other indications for radiation or filter use are a matter of physician discretion.
- 3.
Place where the transfusion was performed: the institution has chemotherapy beds; four intensive care units (ICUs) - neonatal (NICU), general pediatric, surgical, and oncological; resuscitation, observation, and general wards distributed by specialty (general pediatrics, neurology, genetics, pulmonology, cardiology, gastroenterology, surgery, nephrology and oncology). In order to assess the wards where the number of transfusions was more significant, they were grouped as: oncology (corresponding to all beds of oncological ward, the oncological ICU and the chemotherapy unit), general pediatrics (corresponding to the beds of the respective specialty ward), and others (corresponding to the other beds as, isolated, they had a smaller number of transfusions, as described in the reports produced by the institution's hemovigilance service). Thus, this variable is an indirect way of expressing the comorbidity of the patient.
- 4.
Age: patient age was expressed in years, except for children younger than 1 year, whose ages were expressed in months. Subsequently, patients were classified according to the age group: younger than 1 year, between 1 and2 years, and older than 2 years. The choice of these age groups was based on the literature on blood transfusion from the Brazilian Ministry of Health.
- 5.
Reaction site: corresponds to site where the child had the transfusion reaction, classified similarly to the transfusion site.
- 6.
Gender.
- 7.
Type of blood product involved: corresponds to the type of blood product associated with the transfusion reaction, classified similarly to the type of blood product transfused.
- 8.
Type of incident: corresponds to the type of transfusion reaction that occurred. The classification used followed the Guide for use of Blood Products1. It must be emphasized that, for the investigation of febrile reactions, material from the bag and a patient sample were sent for analysis at the Blood Center of the city, and nonhemolytic reactions were considered when the analysis showed absence of hemolysis.
- 9.
Previous history of blood transfusions: corresponds to patient transfusion history, classified according to the number of transfused blood products before the transfusion incident as no previous transfusion; up to 5; between 5 and 10; between 10 and 20; more than 20; and unknown. This classification follows the same pattern used in the notification form for transfusion reaction used by ANVISA. To allow the inference analysis, data were grouped as patients who underwent any previous transfusion (up to 5; between 5 and 10; between 10 and 20; more than 20) and those who did not undergo transfusion or cases in which this information was not available (no transfusion or unknown information).
- 10.
Previous history of reactions to blood transfusion: corresponds to the occurrence of reactions before the studied event.
The population consisted of all patients admitted at ward beds, chemotherapy unit, ICUs, resuscitation, or observation units of the institution, totaling 5,437 children, according to data from the Service of Medical Records and Statistics (Serviço de Arquivo Médico e Estatístico – SAME) of the hospital. The sample consisted of all patients, including neonates, who received transfusion of one or more blood products, from January to June of 2011, totaling 1,226 children, according to data from the hospital transfusion agency. Separate analysis of neonates (approximately 10% of the sample) was not performed in order to follow the standard procedures used by the Brazilian Ministry of Health in publications on hemovigilance.1
The total sample of 1,226 patients was used, considering that the expected prevalence of transfusion reactions in the pediatric population, of approximately 1%,5 would result in an insufficient number of reactions to be studied if a smaller sample was used.7,8 Since the reactions are the focus of the study and considering time availability, the authors chose to work with all transfused patients in order to increase research safety.
The technique used for data collection was the documental analysis of hemovigilance service reports and notification forms of transfusion reactions. A semistructured form developed by the authors was used as tool, based on transfusion incident report forms used by the institution.
The data were stored in an EXCEL spreadsheet and processed using the Predictive Analytics Software for Windows (PASW) software, release 17.0. For the descriptive analysis, data were interpreted by frequencies (absolute and percentage), and the inferential analysis involved the association between the outcome (transfusion) and explanatory (demographic data) variables. Transfusions were represented by the following variables: blood products, history of previous transfusions, and previous transfusion incidents. Sociodemographic data considered in the analysis were: age, gender, and place of transfusion. The chi-squared test was used in the bivariate analysis or the maximum likelihood test, when the first was not adequate to measure the association between variables, with a significance level of 5%. Additionally, an association was observed between the variable type of reaction and the following explanatory variables: age, type of blood product transfused, and previous history of transfusions.9,10
The study was approved by the Research Ethics Committee of the Hospital Infantil Albert Sabin (CAAE: 1707.0.000.035-11/Protocol No. 076/2011).
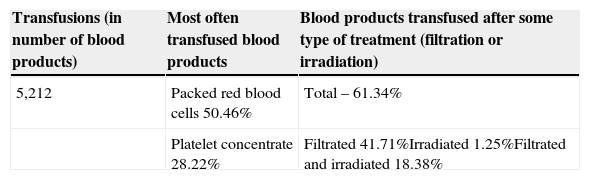
ResultsThe general characteristics of transfusions and patients who had reactions during the study period are shown in Table 1. A proportion of transfusions per patient of approximately 4.25 (5,212/1,226) was observed. It was also observed that transfusions occurred mainly in patients admitted to oncology beds, i.e. patients with neoplasias (46.5% of transfusions).
General characteristics of blood transfusions and patients with transfusion reaction reported in Hospital Infantil Albert Sabin, 2011.
| Transfusions (in number of blood products) | Most often transfused blood products | Blood products transfused after some type of treatment (filtration or irradiation) |
|---|---|---|
| 5,212 | Packed red blood cells 50.46% | Total – 61.34% |
| Platelet concentrate 28.22% | Filtrated 41.71%Irradiated 1.25%Filtrated and irradiated 18.38% |
| Age range | Gender | Place of reaction (inpatient patient unit) |
|---|---|---|
| < 1 year – 15.8% | Male – 52.6% | Oncology – 64.9% |
| Between 1 and 2 years – 33.3% | Female – 47.4% | General pediatrics– 15.8% |
| >2 years – 50.9% | Othersa – 19.3% |
A total of 57 reactions were reported, involving 47 patients among the 1,226 studied individuals and 72 blood products. According to the Dirichlet's principle, better known as the pigeonhole principle, at least one of these patients had recurrence of the studied event. It was observed that approximately 12.3% of the reactions involved more than one blood product, and approximately 10.6% of patients had more than one transfusion reaction. The prevalence of transfusion reactions was 3.8% of patients and involved 1.3% of total blood products transfused in the hospital. It is noteworthy that none of the children who had a reaction were neonates.
Regarding the blood products involved in the reactions, platelet concentrate was the one most frequently associated, corresponding to 50.9% of reactions, followed by packed red blood cells (33.3%), and fresh frozen plasma (5.3%). Regarding the total number of transfusions, adverse events occurred involving 1.1% of packed red blood cells, 2.4% of platelet concentrate and 0.8% of plasma. Additionally, 79.17% of these blood products were filtered and/or irradiated (65.28% filtered, 1.39% irradiated, 12.5% filtered and irradiated).
Most reactions (56.1%) occurred in patients who had not had previous transfusion reactions, and 14% of cases lacked detailed information on the existence of reactions before the studied event. It was observed that 64.9% of these incidents involved multitransfused patients (24.6% had received up to 5 units; 17.5%, between 5 and 10; 15.8%, from 10 to 20; and 7%, more than 20), whereas 21.1% had never been transfused; in 14% of cases no information was available on multiple transfusions.
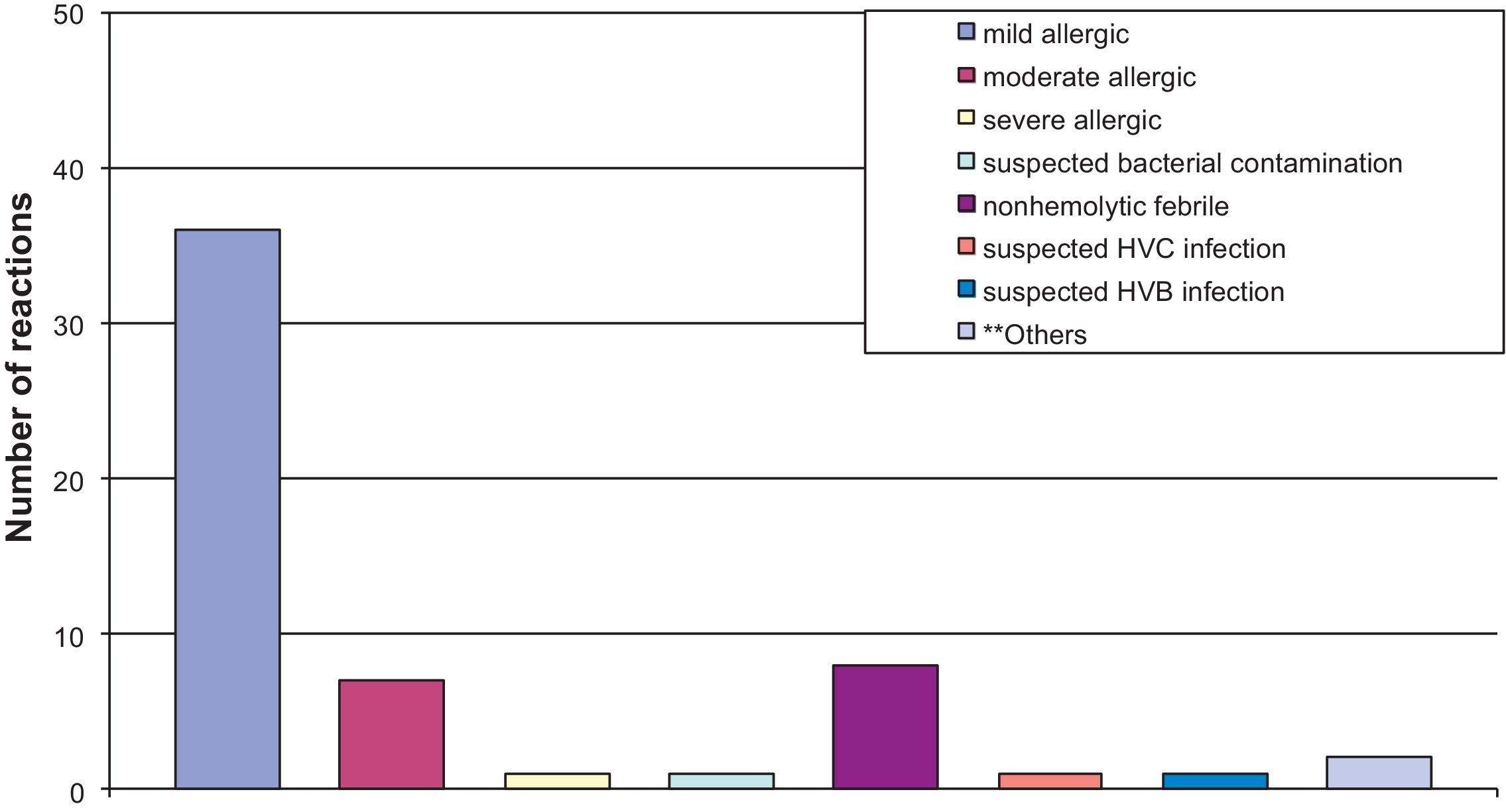
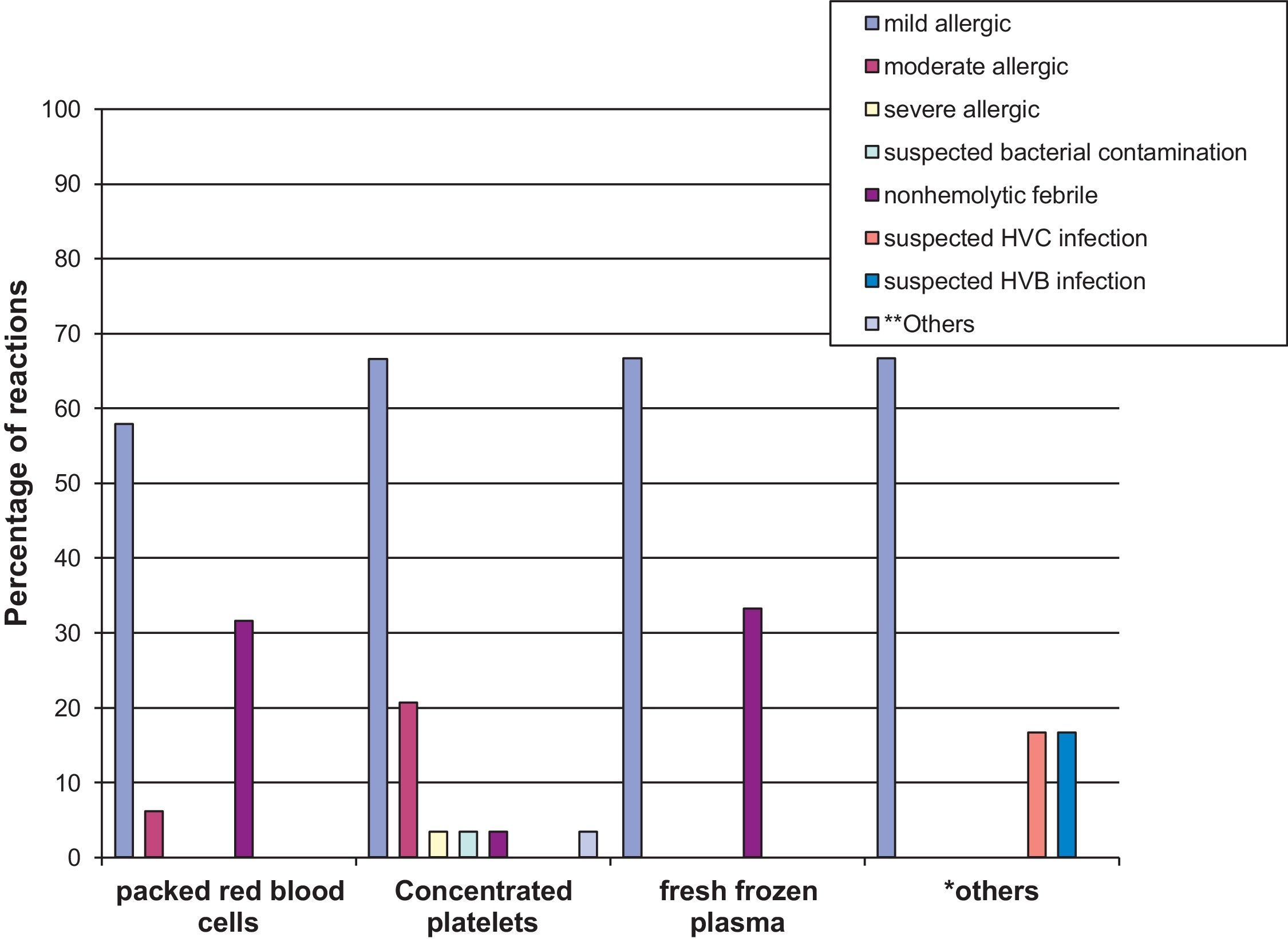
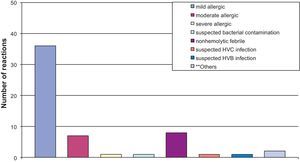
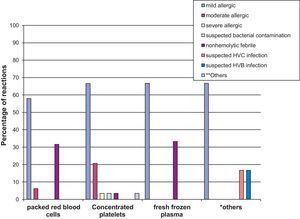
Regarding the type of transfusion reaction, 77.2% were allergic reactions (mild, moderate, and severe) and 14% were nonhemolytic febrile reactions, as shown in Fig. 1. The associations between type of reaction and blood products involved are shown in Fig. 2, emphasizing that most allergic reactions (mild, moderate, or severe) involved platelet concentrate (68.4%).
It was also observed that most reactions that occurred in oncology and general pediatrics involved platelet concentrate (73.0% and 88.9%, respectively). In the other wards, packed red blood cells were responsible for most reactions (54.5%).
When associating type of transfusion incident with age, it was found that 77.2% of the reactions in all age groups were of the allergic type, distributed as follows: 100% in children younger than 1 year, 68.4% in children aged 1 to 2 years, and 75.9% in those older than 2 years. Nonhemolytic febrile reactions accounted for 10.5% of the total, all observed in children between 1 and 2 years of age.
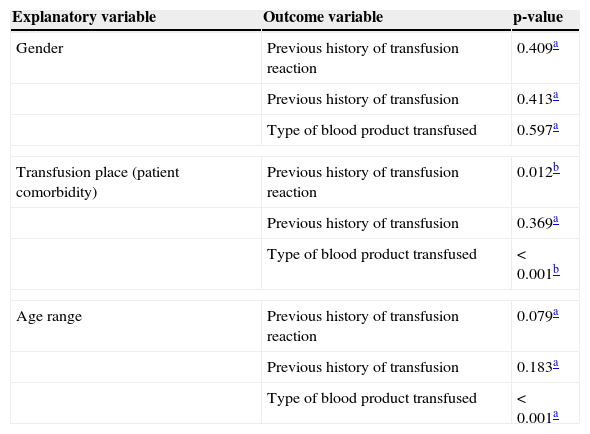
The results shown in Table 2 were obtained when associating the outcome and explanatory variables, with a level of significance of 5%. Similarly, statistical associations were performed between type of reaction and the following variables: age, type of blood product, and previous history of transfusion. The maximum likelihood test showed a statistically significant association between age and type of reaction (p-value = 0.025). Similarly, it was found that reaction type and previous history of transfusions were not statistically associated (p-value=0.391); however, there was an association between the type of transfusion reaction and type of blood products transfused (p-value=0.024). Thus, this study demonstrated that the place of admission is an indirect way of determining patient comorbidity. Therefore it cannot be stated that the sample is multitransfused because it consists mainly of oncology patients, as according to this study, type of comorbidity was not significantly associated with the previous history of transfusions (Table 2).
Association between outcome and explanatory variables, with significance level of 5%.
| Explanatory variable | Outcome variable | p-value |
|---|---|---|
| Gender | Previous history of transfusion reaction | 0.409a |
| Previous history of transfusion | 0.413a | |
| Type of blood product transfused | 0.597a | |
| Transfusion place (patient comorbidity) | Previous history of transfusion reaction | 0.012b |
| Previous history of transfusion | 0.369a | |
| Type of blood product transfused | < 0.001b | |
| Age range | Previous history of transfusion reaction | 0.079a |
| Previous history of transfusion | 0.183a | |
| Type of blood product transfused | < 0.001a | |
According to a multicenter study performed in pediatric teaching hospitals in the United States, approximately 0.95% of patients had a blood transfusion reaction.5 In the present study, a prevalence of 3.8% was observed. However, the profile of these patients regarding comorbidities and multiple transfusions is different from that of the study in the United States. The latter had a larger sample (51,720), who presented a lower rate of multitransfusion and were more heterogeneous regarding comorbidities, as well as proportionally fewer oncology patients involved in the study. Therefore, there may be a bias when trying to compare the two studies. Regarding the use of filters or irradiation of transfused units, both studies used at least one of these treatments in most transfusions. Thus, the higher prevalence is justified by the profile of the studied population, but it is necessary to perform a cross-sectional study with a larger sample size to increase the reliability of this information.
Most adverse events occurred in children older than two years, as expected by the literature review.5 However, according to the inferential analysis, it was not possible to associate the children's age to event recurrence, as the age group showed no statistically significant association with a previous history of transfusion reaction. In fact, most children who had a transfusion reaction had no prior incident. This fact may be related to the intervention of the Transfusion Committee of the institution, which promotes preventive measures against the occurrence of these reactions, especially in patients who have had a prior incident.
Transfusion reactions consist mostly of the allergic type, which are generally mild. Furthermore, children aged 1 to 2 years are more susceptible to febrile nonhemolytic reaction, while those older than 2 years are more prone to allergic reactions. It is also possible to associate the allergic event to platelet concentrate transfusion, suggesting that this component may increase children's susceptibility to these events. This fact, however, has not been analyzed in the reviewed literature,5,11,12 whose authors only emphasized the higher prevalence of allergic reactions in relation to others in children, without suggesting explanations. Therefore, the importance of further studies, classifying the type of reaction that occurred, is highlighted. The higher number of allergic reactions is justified by the increased susceptibility of children to such events.11,13
Recurrence of reactions was shown to be associated with child comorbidity. Therefore, in the studied population, oncology patients had a higher susceptibility to transfusion incident recurrence than others. This fact is not mentioned in the reviewed literature, and it is important for the preventive care of these patients once a reaction occurs, as they may have a new event in future transfusions.14
According to the literature,15–17 the most often transfused blood products are packed red blood cells and platelets, respectively, but the one most frequently involved in transfusion reactions is platelet concentrate (50.9%). More reactions are expected when packed red blood cells are transfused, when compared to platelets;5 this may be due to the different populations in which these products are used.
It was verified that age and comorbidity were associated with the transfused blood product in children who had reactions. Thus, children older than 2 years were more susceptible to reactions when they received platelet concentrate, as well as those aged 1 to 2 years who received packed red blood cells. Moreover, children with cancer had more reactions when transfused with platelet concentrates, while in those affected with general pediatric disorders, such incidents were associated with packed red blood cells. This explains why in the study of Slonin et al.5 packed red blood cells showed a higher incidence of reaction occurrence when compared to platelets, since their population consisted mainly of children with general pediatric disorders.
Children older than 2 years who had transfusion reactions were more likely to have received multiple transfusions, and were more prone to have received platelet concentrate transfusion. However, the present study demonstrated that oncology patients were also more likely to receive platelet concentrates. Thus, there may be a bias, as this same population consists of many patients with cancer, making it difficult to distinguish which variable (neoplasia or age) has a greater influence. Moreover, it is clear that allergic reactions occurred more often in children receiving platelet concentrate and in those older than 2 years.
ConclusionsIn this population, the profile of transfusion reactions consisted mainly of allergic events involving multitransfused patients, with low recurrence, and most often related to platelet concentrate. Children older than 2 years were most frequently affected by these incidents. Moreover, it is clear that several factors are directly related to transfusion incidents: age, type of blood products, patient comorbidity, and multiple transfusions. Of these, the type of blood product and age also are associated with the reaction type. These data should be taken as a warning for interventions involving these variables, as a prophylactic measure and to improve the prognosis of these patients.
The present study could not define which was the most important variable regarding the incidence of transfusion reactions, considering that the study population was exposed to all of them.
It is noteworthy that there are few studies involving the pediatric population, especially in Brazil, making it difficult to compare data. Furthermore, the sample available for the analyzed period was small compared to the number of all transfused children, partly limiting the external validity of the study.
Case-control studies are suggested, evaluating each variable separately in the same population, for further comparison between the significance of the variables, as well as a cohort study to assess their individual susceptibility to the occurrence of new reactions. However, that does not appear possible to achieve under conventional conditions, as it would undermine study effectiveness.
Therefore, a new study is proposed, with a similar population and larger sample, analyzed in view of the literature and clinical experience of qualified professionals, in order to consolidate the observed findings.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Pedrosa AK, Pinto FJ, Lins LD, Deus GM. Blood transfusion reactions in children: associated factors. J Pediatr (Rio J). 2013;89:400–6.