To verify whether infants with cow's milk protein allergy have inadequate vitamin D levels.
MethodsThis cross-sectional study included 120 children aged 2 years or younger, one group with cow's milk protein allergy and a control group. The children were recruited at the pediatric gastroenterology, allergology, and pediatric outpatient clinics of a university hospital in the Northeast of Brazil. A questionnaire was administered to the caregiver and blood samples were collected for vitamin D quantification. Vitamin D levels <30ng/mL were considered inadequate. Vitamin D level was expressed as mean and standard deviation, and the frequency of the degrees of sufficiency and other variables, as proportions.
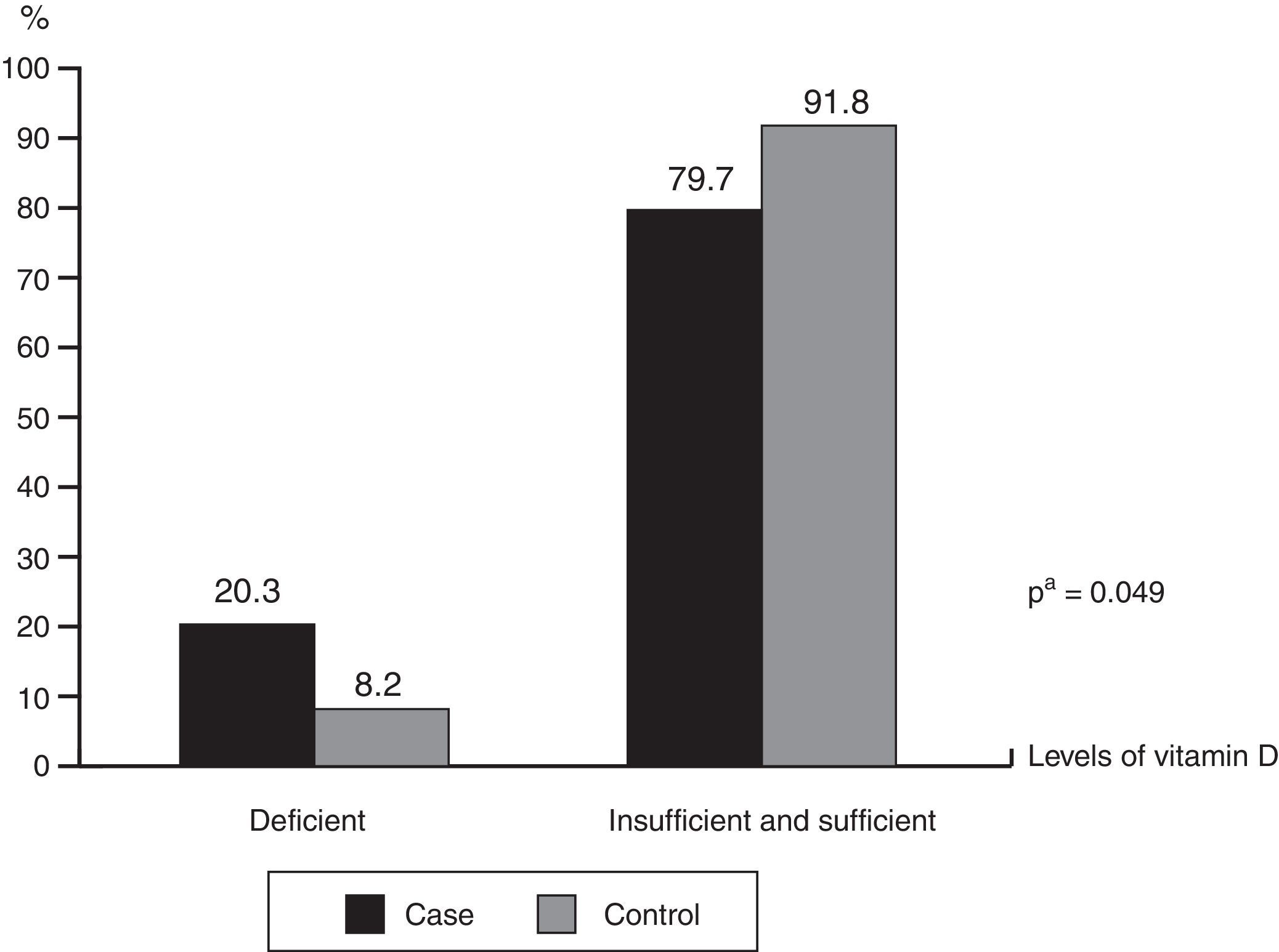
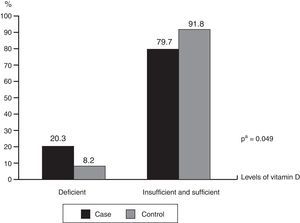
ResultsInfants with cow's milk protein allergy had lower mean vitamin D levels (30.93 vs.35.29ng/mL; p=0.041) and higher deficiency frequency (20.3% vs.8.2; p=0.049) than the healthy controls. Exclusively or predominantly breastfed infants with cow's milk protein allergy had higher frequency of inadequate vitamin D levels (p=0.002). Regardless of sun exposure time, the groups had similar frequencies of inadequate vitamin D levels (p=0.972).
ConclusionsLower vitamin D levels were found in infants with CMPA, especially those who were exclusively or predominantly breastfed, making these infants a possible risk group for vitamin D deficiency.
Verificar se lactentes com alergia à proteína do leite de vaca (APLV) apresentam níveis inadequados de vitamina D.
MétodosEstudo transversal, envolvendo 120 crianças de até 2 anos de idade, sendo um grupo com APLV e outro de comparação, captadas dos ambulatórios de Gastroenterologia Pediátrica, Alergologia Pediátrica e Puericultura de um hospital universitário, no Nordeste brasileiro. Foi aplicado um formulário e coletadas amostras sanguíneas para a análise da vitamina D, sendo considerados inadequados os níveis<30ng/mL. Níveis de vitamina D foram expressos em média e desvio padrão, e a frequência dos graus de suficiência e demais variáveis, em proporções.
ResultadosLactentes com APLV quando comparados aos saudáveis, apresentaram uma menor média do nível da vitamina D (30,93 vs 35,29ng/mL) (p=0,041) e maior frequência de deficiência (20,3% vs 8,2) (p=0,049). Maior frequência de níveis inadequados de vitamina D foi observada nas crianças com APLV que estavam em aleitamento materno exclusivo/predominante (p=0,002). Independentemente do período de exposição solar, a frequência de um status inadequado de vitamina D foi semelhante entre os grupos (p=0,972).
ConclusõesMenores níveis de vitamina D foram observados em lactentes com APLV, especialmente naquelas em aleitamento materno exclusivo/predominante, configurando este como um possível grupo de risco para essa deficiência.
Currently, vitamin D deficiency has been recognized as a frequent problem, which is observed in adults, children, and sometimes even in the neonatal period.1 Classically, vitamin D deficiency is associated with bone diseases, but in recent years it has been associated with several extraosseous outcomes, including immune diseases.2 It appears to occur more frequently in certain groups of individuals; therefore, the Endocrinology Society recommends screening for vitamin D deficiency only in the group of individuals considered to be at risk. This risk group would comprise individuals of black ethnicity, as well as those who are obese, pregnant and lactating women, and individuals with certain diseases, such as endocrine and renal diseases, among others. Individuals with cow's milk protein allergy (CMPA) are not included in the risk group.3
In turn, some international studies, mainly carried out in children with the immediate forms of CMPA, mediated by IgE, suggested a higher frequency of vitamin D deficiency in this group.4–7 Low levels of vitamin D in children are associated with factors such as poor maternal–fetal transfer due to maternal hypovitaminosis, inadequate sun exposure, inappropriate use of vitamin D supplements, and low dietary intake.1,8,9
Studies on vitamin D levels in children with CMPA are important to assess whether they are a risk group for vitamin D deficiency. Since most studies that assessed vitamin D levels in children with CMPA were carried out in children without gastrointestinal manifestations,4–7 there are gaps on the knowledge of this problem. Additionally, few studies have been performed in children with CMPA living in sunny regions, where adequate exposure to ultraviolet rays is assumed. The present study aimed to verify whether infants with CMPA present inadequate levels of vitamin D.
MethodsThis was an observational, cross-sectional study comparing vitamin D levels in a group of infants with clinical diagnosis of CMPA and a group of healthy infants (control group). The study was carried out from March 2013 to April 2015 in the pediatric gastroenterology, pediatric allergology, and pediatric outpatient clinics of Hospital das Clínicas at Universidade Federal de Pernambuco (HC/UFPE).
The group of children with CMPA consisted of 59 infants of both genders who had clinical manifestations related to CMPA. The diagnosis was attained considering the clinical history, physical examination, and previous history, and confirmed by the open oral challenge test, as indicated by the main current guidelines.10,11 The challenge test was contraindicated by the investigators in the following situations: report of immediate manifestations associated with a positive IgE result; late manifestations compatible with CMPA, associated with malnutrition. The comparison group consisted of 61 healthy children. Children with clinical suspicion of other pathologies or factors that could influence vitamin D levels, such as acute or chronic infectious diseases, other inflammatory bowel diseases, and renal or gastrointestinal diseases were excluded.
For the anthropometric evaluation, the infant's weight was obtained and the weight/age (W/A) indicator proposed by the World Health Organization (WHO) was used. For the evaluation of W/A, the following cutoffs were used: <−2 z-score for low, ≥−2 and <+2 z-score for age-appropriate, and ≥+2 z-score for elevated.12
Regarding vitamin D, levels and degrees of sufficiency were analyzed, categorized as insufficient/deficient and sufficient. Vitamin D levels were considered deficient when 25(OH)D was ≤20ng/mL; insufficient, when between 21 and 29ng/mL; and sufficient, when ≥30ng/mL.3,13 For this analysis, a blood sample (4mL) was collected by a trained professional, stored in a tube with separator gel, and then taken to the laboratory connected with the research. 25(OH)D was measured using the chemiluminescence technique using the LIAISON® commercial kit. Parents of children who had inadequate levels of vitamin D were warned of the need for supplementation.
The WHO categorization was used to classify the current practice of breastfeeding (BF); children were considered as in exclusive breastfeeding (EBF) when they received only breast milk, without other liquids or solids; in predominant BF, when they received, in addition to breast milk, water or water-based preparations; in supplemented BF, when they received, in addition to breast milk, any solid or semi-solid food for the purpose of complementing it, not replacing it; and in mixed or partial BF, when they received breast milk and other types of milk.14
When evaluating sun exposure, the guidelines of the Brazilian Society of Pediatrics (SBP) were used, which recommends that the child should receive direct exposure of the skin to sunlight after the second week of life; a weekly quota of 30min of sun exposure with the child wearing only diapers is considered sufficient.15
The construction of the database was performed using the Epi-Info software, version 6.04d (Epi-info 6.04, WHO/CDC, Atlanta, USA). Statistical analyses were performed using the SPSS software, version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were tested for normality of distribution using the Kolmogorov–Smirnov test, being expressed as mean and standard deviation when showing a symmetric distribution, and as median and quartiles when the distribution was asymmetric. Differences in frequency and association between categorical variables were assessed by Pearson's chi-squared or Fisher's exact tests, when indicated. For all tests, the significance level was set at 5%.
The children's parents or tutors were verbally informed about the study aims and signed the free and informed consent form. The study project was registered at the National Committee of Research Ethics (CONEP/Plataforma Brasil) and submitted to the approval of the Ethics Committee on Research in Human Beings of the Centro de Ciências da Saúde at Universidade Federal de Pernambuco, under CAAE No. 12878313.4.0000.5208/2013.
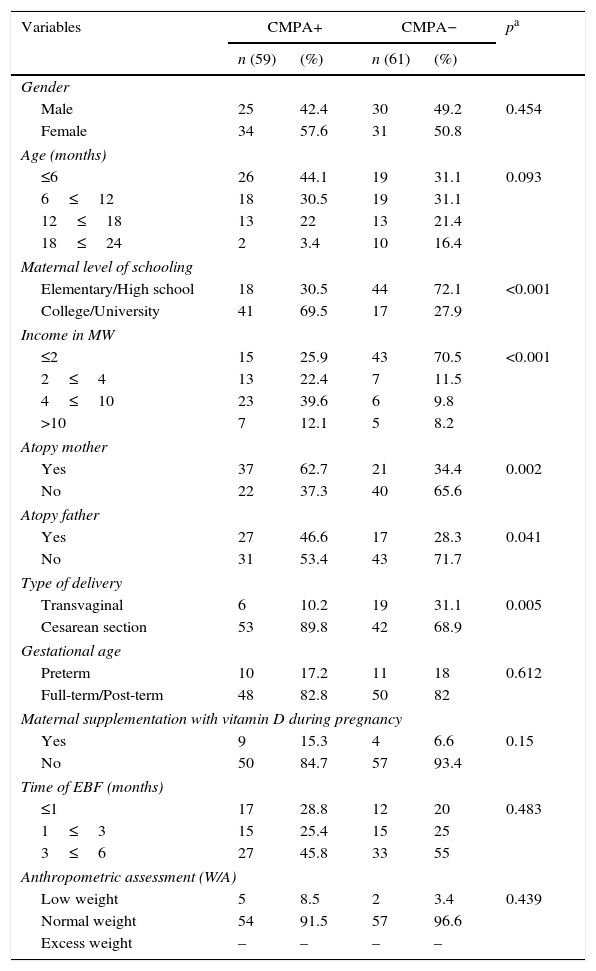
ResultsThe median age of the total sample was 8.5 months (P25: 6.0, P75: 14.0), whereas in children with CMPA the median was 7.0 months (P25: 4, P75: 13), and in the control group, it was 9.0 months (P25: 6.0, P75: 16.0). The socioeconomic and biological characteristics of the sample are shown in Table 1, and among them, it was observed that the mothers of children with CMPA had a better level of schooling than those of the children in the comparative group (69.5% vs. 27.9, college/university level of schooling), as well as higher family income (51.7% vs.18%, >four Brazilian minimum wages). A predominance of cesarean delivery (89.8%) was observed in the group of children with CMPA. There was also a higher frequency of atopy among mothers (62.7% vs. 34.4%) and fathers (46.6% vs. 28.3%) of children with CMPA. When the nutritional status was evaluated, a higher percentage of normal weight was observed in the sample, with similar frequencies in both groups (91.5% in the CMPA group and 96.6% in the control group).
Socioeconomic and biological characteristics of infants with CMPA and of the control group followed at the outpatient clinic of a university hospital, Recife-PE, Brazil (2013–2015).
| Variables | CMPA+ | CMPA− | pa | ||
|---|---|---|---|---|---|
| n (59) | (%) | n (61) | (%) | ||
| Gender | |||||
| Male | 25 | 42.4 | 30 | 49.2 | 0.454 |
| Female | 34 | 57.6 | 31 | 50.8 | |
| Age (months) | |||||
| ≤6 | 26 | 44.1 | 19 | 31.1 | 0.093 |
| 6≤12 | 18 | 30.5 | 19 | 31.1 | |
| 12≤18 | 13 | 22 | 13 | 21.4 | |
| 18≤24 | 2 | 3.4 | 10 | 16.4 | |
| Maternal level of schooling | |||||
| Elementary/High school | 18 | 30.5 | 44 | 72.1 | <0.001 |
| College/University | 41 | 69.5 | 17 | 27.9 | |
| Income in MW | |||||
| ≤2 | 15 | 25.9 | 43 | 70.5 | <0.001 |
| 2≤4 | 13 | 22.4 | 7 | 11.5 | |
| 4≤10 | 23 | 39.6 | 6 | 9.8 | |
| >10 | 7 | 12.1 | 5 | 8.2 | |
| Atopy mother | |||||
| Yes | 37 | 62.7 | 21 | 34.4 | 0.002 |
| No | 22 | 37.3 | 40 | 65.6 | |
| Atopy father | |||||
| Yes | 27 | 46.6 | 17 | 28.3 | 0.041 |
| No | 31 | 53.4 | 43 | 71.7 | |
| Type of delivery | |||||
| Transvaginal | 6 | 10.2 | 19 | 31.1 | 0.005 |
| Cesarean section | 53 | 89.8 | 42 | 68.9 | |
| Gestational age | |||||
| Preterm | 10 | 17.2 | 11 | 18 | 0.612 |
| Full-term/Post-term | 48 | 82.8 | 50 | 82 | |
| Maternal supplementation with vitamin D during pregnancy | |||||
| Yes | 9 | 15.3 | 4 | 6.6 | 0.15 |
| No | 50 | 84.7 | 57 | 93.4 | |
| Time of EBF (months) | |||||
| ≤1 | 17 | 28.8 | 12 | 20 | 0.483 |
| 1≤3 | 15 | 25.4 | 15 | 25 | |
| 3≤6 | 27 | 45.8 | 33 | 55 | |
| Anthropometric assessment (W/A) | |||||
| Low weight | 5 | 8.5 | 2 | 3.4 | 0.439 |
| Normal weight | 54 | 91.5 | 57 | 96.6 | |
| Excess weight | – | – | – | – | |
CMPA, cow's milk protein allergy; MW, Brazilian minimum wages; EBF, exclusive breastfeeding; W, weight; A, age.
In children with a diagnosis of CMPA, the most frequent clinical manifestations were of the immediate type (59.3%): angioedema, urticaria and skin hyperemia, vomiting, diarrhea, cough, and wheezing. The late manifestations were: regurgitation and vomiting compatible with gastroesophageal reflux disease (GERD), diarrhea, diarrhea with blood (compatible with colitis), proctitis, low weight gain or weight loss, excessive crying and abdominal distension, bowel disorders, and atopic eczema. Among the immediate manifestations, the most prominent were urticaria in 28.8%, angioedema in 15.3%, and cough in 15.3%. In the group with late manifestations, 40.7% had vomiting and diarrhea, 35.6% had signs of colitis, and 22% had symptoms compatible with GERD.
The mean levels of vitamin D among children with CMPA were 30.93±12.33ng/mL and 35.29±10.74ng/mL in the control group (p=0.041). Fig. 1 shows the frequencies of the different levels of vitamin D sufficiency in both groups, indicating that among the total number of children, there was a higher frequency of deficiency levels in those with CMPA (20.3% vs. 8.2%; p=0.049). It is noteworthy that none of the children were receiving vitamin D supplementation at the time of the interview and only nine of the mothers of children with CMPA reported receiving vitamin D supplementation during the gestational period.
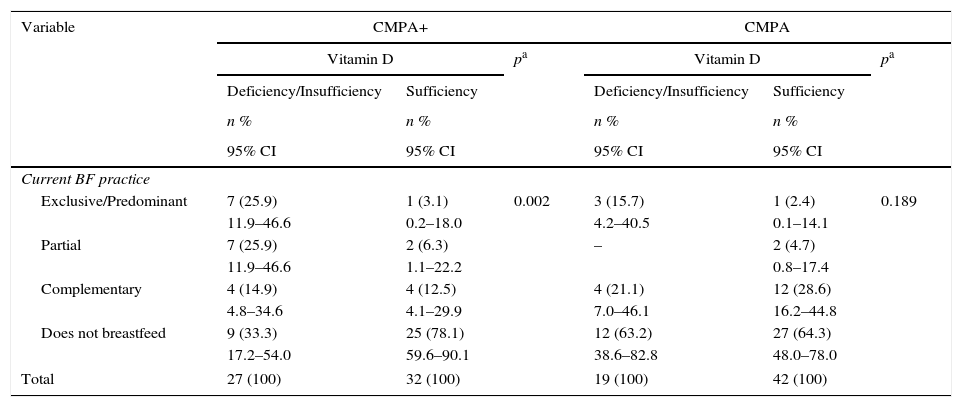
Table 2 indicates that among the children with CMPA, those who received exclusive/predominant BF at the time of the interview had a higher frequency of vitamin D deficiency; 25.9% of children were deficient and only 3.1% showed sufficient levels. This finding was also observed in those receiving partial BF (25.9% vs. 6.3%, p=0.002). Most children with CMPA who were not breastfed (i.e., who were fed infant formulas) showed sufficiency levels (78.1%). In the comparison group, there was no association between different dietary practices and vitamin D sufficiency levels (p=0.189).
Vitamin D status of infants with CMPA and of the control group followed at an outpatient clinic of a university hospital, in relation to the feeding practice, Recife, PE, Brazil (2013–2015).
| Variable | CMPA+ | CMPA | ||||
|---|---|---|---|---|---|---|
| Vitamin D | pa | Vitamin D | pa | |||
| Deficiency/Insufficiency | Sufficiency | Deficiency/Insufficiency | Sufficiency | |||
| n % | n % | n % | n % | |||
| 95% CI | 95% CI | 95% CI | 95% CI | |||
| Current BF practice | ||||||
| Exclusive/Predominant | 7 (25.9) | 1 (3.1) | 0.002 | 3 (15.7) | 1 (2.4) | 0.189 |
| 11.9–46.6 | 0.2–18.0 | 4.2–40.5 | 0.1–14.1 | |||
| Partial | 7 (25.9) | 2 (6.3) | – | 2 (4.7) | ||
| 11.9–46.6 | 1.1–22.2 | 0.8–17.4 | ||||
| Complementary | 4 (14.9) | 4 (12.5) | 4 (21.1) | 12 (28.6) | ||
| 4.8–34.6 | 4.1–29.9 | 7.0–46.1 | 16.2–44.8 | |||
| Does not breastfeed | 9 (33.3) | 25 (78.1) | 12 (63.2) | 27 (64.3) | ||
| 17.2–54.0 | 59.6–90.1 | 38.6–82.8 | 48.0–78.0 | |||
| Total | 27 (100) | 32 (100) | 19 (100) | 42 (100) | ||
CMPA, cow's milk protein allergy; BF, breastfeeding.
In the group of allergic children, the frequency of sun exposure did not interfere with the level of vitamin D sufficiency; the results were similar in those with deficiency/insufficiency and in those with sufficient degrees (among those with deficient/sufficient degrees, 14 were adequately exposed to sunlight, whereas 12 were not; among those with sufficient degrees, 17 were adequately exposed and 13 were not, p=0.972).
DiscussionIn this study, carried out among children who mostly had normal weight and were breastfed for similar periods of times, children with immediate and late CMPA had a higher percentage of deficiency when compared with healthy children (20.3% vs. 8.2%; p=0.049). Although vitamin D levels were normal in both groups, a lower mean was observed in allergic children (30.93 vs. 35.29; p=0.041), reinforcing the tendency of allergic children to have lower vitamin levels. Some studies have already investigated the association between vitamin D deficiency and food allergy, but most of them were performed in children with the immediate form of CMPA, IgE-mediated.4–7 This study also raises the question about the late form, non-IgE-mediated, which usually manifests through gastrointestinal symptoms. There is an empirical basis for believing that insufficient levels of vitamin D in the early stages of life may interfere with the development of immunity, contributing to the onset of allergic diseases, including food allergy.16 The main mechanism through which it would act in immune regulation would be to favor a greater differentiation of naive T lymphocytes into T regulatory cells (Tregs), which would inhibit both TH1 and TH2 harmful immune responses.16,17 It should be considered that, although deficient levels of vitamin D were observed in infants with CMPA, the cross-sectional design of the study did not allow to establish a causal association between vitamin D deficiency and the occurrence of allergy. It can be speculated that the association may be the opposite, considering that the pathogenesis of CMPA itself may have favored low vitamin D levels, both because it allows malabsorption and because it can be associated with the inflammatory response.18
The systemic inflammatory response, which might be generated during the allergic process, may be associated with low concentrations of fat-soluble vitamins, including vitamin D.18,19 The low levels could also be associated to the fact that the mothers of children with CMPA in EBF adopted a diet that restricted cow's milk and dairy products, without vitamin D supplementation, a fact already associated with deficiencies of vitamin D and other micronutrients.20
The main sources of vitamin D for infants are dietary intake, sun exposure, and vitamin supplements.21 Breast milk can provide a protective effect against the development of several types of allergies due to the presence of immunological factors and functional nutrients that favor a healthy microenvironment for immune system development and intestinal maturation.22 However, as it has low concentrations of vitamin D,23 children receiving BF, especially those in EBF and without adequate sun exposure, may represent a group at higher risk for vitamin D deficiency.24
Low levels of vitamin D were observed in allergic children who were receiving BF, and a higher frequency of deficient/insufficient levels of vitamin D was observed among children with CMPA who were submitted to EBF/predominant BF (25.9%). Evidence suggests that children who develop CMPA, even those in EBF, have genetic factors that predispose them to this outcome.24 It is possible that, in an infant receiving EBF who already has a genetic predisposition to CMPA, the occurrence of vitamin D deficiency acts as a trigger for the development of the disease. In this sense, it was observed that of the eight infants who were in EBF/predominant BF and developed CMPA (most of them allergic colitis), seven were found to have inadequate levels of vitamin D.
Studies carried out in different countries suggest the need for supplementation of infants receiving BF, and sometimes of the mother, even when they live in a sunny region.25 None of the children in this study received vitamin supplementation, and only nine mothers of children with CMPA (15.3%) and four mothers of healthy children (6.6%) were taking supplementation. It was observed that in Brazil, the indication of vitamin D supplementation in the prenatal and breastfeeding periods is not yet a common practice, and this perhaps needs to be considered.
Sun exposure is considered one of the most important determinants of vitamin D status. Infants’ vitamin D levels tend to decline over the first few months of life if they are not exposed to sunlight or if they do not receive an adequate dietary intake of vitamin D.26 In the Northeast of Brazil, abundant solar irradiation occurs during most of the year. However, living in a sunny region may not protect infants from this vitamin deficiency. When analyzing the association between sun exposure and vitamin D levels in this study, it was observed that adequate sun exposure did not ensure sufficient levels of vitamin D.
In a recent study carried out in this same region, insufficient levels of vitamin D were also observed in the mother/newborn binomial,27 corroborating other data that indicate that even in cities with high solar irradiation, high frequencies of vitamin D deficiency may occur. The fact that sun exposure was not associated with adequate levels of vitamin D in this study may have been due to the influence of other factors, such as the time of day when the child was exposed, skin color, and the use of sunscreen, which were not possible to be analyzed. It is probable that fear of the sun's harmful effects on the skin hindered adequate sun exposure; in fact, dermatologists recommend that babies aged up to 6 months should not be exposed to direct sunlight.28
When analyzing the results of this study, its limitations should be considered. The complexity of the problem to be assessed was one of the main difficulties found, and other factors that could be related to vitamin D levels were not controlled, such as the restriction of milk and dairy products in the maternal diet, the use of vitamin D supplementation during pregnancy, and adherence to these recommendations, as well as the type of delivery and other aspects associated with the baby's intestinal microbiota. Sample heterogeneity was considered one of the problems due to individual differences of the disease. Social class bias may also have occurred, as families with better socioeconomic status might have sought the pediatric gastroenterology outpatient clinic, aiming at obtaining a medical report to receive the specialized formulas provided by the public healthcare services. Difficulties inherent to the questionnaire application may also have occurred, since the questionnaire was applied by two different researchers; the reliability of the information provided by the mothers should also be considered. The sample size was small, perhaps insufficient to subsidize changes in guidelines previously established by regulatory societies; however, they are sufficient to serve as a warning and to trigger complementary randomized and prospective studies on the subject. A larger sample size could allow CMPA infants to be grouped and analyzed according to the clinical manifestations (immediate and late), considering the different immunological mechanisms involved in the pathogenesis of CMPA.
The lack of a consensus regarding the cutoff points that define vitamin D status was also considered to be a bias, making it difficult to understand which values would have a pathogenic effect and compromising the comparison with studies that used other reference values. While the American Endocrinology Society states that deficient levels are those below 20ng/mL and sufficient values are those equal to or greater than 30ng/mL,29 the US Institute of Medicine (2010) considers levels above 20ng/mL as appropriate.30 In this study, sufficient levels were those equal to or greater than 30ng/mL.
The findings of the present study suggest a higher frequency of inadequate levels of vitamin D in infants with different clinical manifestations of CMPA. Despite the several limitations of the study, the data may indicate the need to investigate vitamin D levels in infants with CMPA. The practice of EBF/predominant BF was related to inadequate levels, mainly in children diagnosed with CMPA, whereas sun exposure did not appear to confer a protective effect to this group. The fact that allergic infants did not use vitamin D supplementation may have contributed to the study findings. Therefore, it is probably not safe to rely on sun exposure as a source of vitamin D, especially in infants in EBF, and routine supplementation should be evaluated. It is suggested that other studies be carried out to demonstrate the association and better elucidate the factors associated with inadequate vitamin D levels in infants with CMPA.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), through financial aid from the universal notice of 2012/2013 under registration number 488488/2013-3.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Silva CM, da Silva SA, Antunes MM, Silva GA, Sarinho ES, Brandt KG. Do infants with cow's milk protein allergy have inadequate levels of vitamin D? J Pediatr (Rio J). 2017;93:632–8.