To summarize the main clinical entities associated with fever without source (FWS) in infants, as well as the clinical management of children with occult bacteremia, emphasizing laboratory tests and empirical antibiotics.
SourcesA non-systematic review was conducted in the following databases – PubMed, EMBASE, and SciELO, between 2006 and 2015.
Summary of the findingsThe prevalence of occult bacteremia has been decreasing dramatically in the past few years, due to conjugated vaccination against Streptococcus pneumoniae and Neisseria meningitidis. Additionally, fewer requests for complete blood count and blood cultures have been made for children older than 3 months presenting with FWS. Urinary tract infection is the most prevalent bacterial infection in children with FWS. Some known algorithms, such as Boston and Rochester, can guide the initial risk stratification for occult bacteremia in febrile infants younger than 3 months.
ConclusionsThere is no single algorithm to estimate the risk of occult bacteremia in febrile infants, but pediatricians should strongly consider outpatient management in fully vaccinated infants older than 3 months with FWS and good general status. Updated data about the incidence of occult bacteremia in this environment after conjugated vaccination are needed.
Listar as principais entidades clínicas associadas a quadros de febre sem sinais localizatórios (FSSL) em lactentes, bem como o manejo dos casos de bacteremia oculta com ênfase na avaliação laboratorial e na antibioticoterapia empírica.
Fonte dos dadosFoi realizada revisão não sistemática da literatura nas bases de dados PubMed, EMBASE e Scielo no período de 2006 a 2015.
Síntese dos dadosA ocorrência de bacteremia oculta vem diminuindo sensivelmente em lactentes com FSSL, principalmente devido à introdução da vacinação conjugada contra Streptococcus pneumoniae e Neisseria meningitidis nos últimos anos. Juntamente disso, uma redução constante na solicitação de hemogramas e hemoculturas em lactentes febris acima de 3 meses vem sendo observada. A infecção do trato urinário é a infecção bacteriana mais prevalente no paciente febril. Algoritmos consagrados, como o de Boston e Rochester, podem guiar a decisão clínica inicial para estimar o risco de bacteremia em lactentes entre um e 3 meses de vida.
ConclusõesNão há esquema padronizado para a estimativa do risco de bacteremia oculta em lactentes febris, porém deve-se considerar fortemente o manejo ambulatorial de lactentes acima de 3 meses com FSSL em bom estado geral e com esquema vacinal completo. São necessários dados atualizados sobre a incidência de bacteremia oculta em crianças vacinadas em nosso meio.
Fever without source (FWS) is one of the major diagnostic challenges for the emergency service pediatrician. Approximately 20% of febrile children do not have an initially-established etiological diagnosis, and around 20% of emergency consultations in children between 2 and 24 months of age are due to fever.1,2 In this scenario, it is essential to perform a rapid diagnosis of children with possible severe bacterial infection (SBI), who require immediate antibiotic therapy. SBIs include sepsis/septic shock, occult bacteremia (OB), bacterial meningitis, pneumonia, urinary tract infection, bacterial gastroenteritis, osteomyelitis, and septic arthritis.3
The evaluation of febrile infants is even more of a concern considering the relative immaturity of the immune system in the first 3 months of life. Factors that may increase the risk of SBI in infants include reduced macrophage and opsonization activity, in addition to lower neutrophil activity.4 While it is a consensus that early introduction of antibiotics leads to a favorable outcome in children with FWS in poor general status and with toxemia, the management of children in good general status is still a matter of much discussion, in whom the risk of SBI, particularly OB, is very low.5
Although several authors have studied the combination of clinical and laboratory parameters for risk stratification of SBI in febrile infants, to date there is no single test or set of tests that is able to detect infants with SBI with optimal sensitivity.6 This article aimed to describe the main clinical conditions associated with FWS in infants and to analyze the methods of laboratory evaluation of fever in this age group.
Main entities associated with FWSViral infectionsViral infections are common causes of FWS in infants, and many patients are treated with antibiotics in this situation, despite the lack of evidence for bacterial infections. Viruses and bacteria interact with different pattern-recognition receptors in circulating leukocytes, triggering different specific immune responses.7 In a study involving 75 children with FWS,8 one or more viruses were detected by polymerase chain reaction in 76% of the cases; among children with fever and a probable defined infection source, this percentage was 40%, and among the afebrile children in the control group, in 35% of cases. The most often identified viruses were adenovirus, herpes virus type 6, enteroviruses, and parechovirus.
Occult bacteremiaOB is defined as a positive blood culture in a patient with FWS.6 The prevalence of OB has dramatically decreased in recent years, on account of conjugate bacterial vaccines. In the pre-vaccine era, the prevalence of OB was 2.4–11.6% in children with FWS, and pneumococcus was the main disease-causing agent (50–90% of cases).9,10 At the age of 1 week to 3 months of age, Escherichia coli represents 56% of bacteremia cases, with group B Streptococcus as the second most prevalent agent, representing 21% of cases.11
A recent retrospective cohort study12 evaluated 201 episodes of pneumococcal OB in infants with a median age of 20.5 months. The cases due to PCV7 serotype decreased from 82.2% to 19.5% after vaccination, with most cases occurring after the vaccination period due to PCV19A serotype. The recent introduction of the pneumococcal 13-valent vaccine will certainly reduce these findings.
A blood culture sample is often requested in febrile infants with suspected OB. In a recent multicenter prospective study,13 bacterial growth was found in 1.5% of 65,169 blood cultures, with pneumococcus being the most frequently isolated microorganism (27.3% of cases); in this study, 47.1% of the children were younger than 1 year old.
Studies have also confirmed that there is a significant reduction in requests for blood count in febrile infants when the immunization schedule is complete. Zeretzke et al.14 assessed infants between 6 and 24 months with FWS>39°C and found that, prior to protocol implementation to verify the infants’ immunization status, 100% were submitted to blood collection for complete blood count, with this rate reduced by 58% when the pneumococcal and meningococcal vaccination status was complete.
Urinary tract infection (UTI)This is the most prevalent bacterial infection in febrile infants, corresponding to 5–7% of the cases of FWS.15 In recent years, several authors have studied the presence of factors that increase the risk of UTI in certain patient groups. However, despite these figures, UTI in infants with FWS is probably underdiagnosed, since the majority of patients present with nonspecific symptoms that are common to several other acute infections. The occurrence of UTI in childhood is associated with several long-term kidney complications, such as hypertension, pre-eclampsia, and renal failure; renal scarring may be present in approximately 15% of children after a first episode of UTI.16,17
In 2011, the American Academy of Pediatrics published updated guidelines concerning the diagnosis, treatment, and complementary investigation of patients with UTI in order to reduce the chances of renal scarring and future renal injury.18 This article described an initial investigation algorithm to estimate the risk of UTI in febrile children aged 2–24 months, based on clinical and demographic characteristics.19 The main risk factor in febrile boys was the absence of prior circumcision. In boys, it is recommended to screen for UTI if one or more of the following risk factors are present (UTI risk 1–2%) – white ethnicity, temperature above 39°C, fever for more than 24h, and absence of other infection sources. In girls, the factors include white ethnicity, age <12 months, temperature >39°C, fever for more than 48h, and no apparent source of infection.
UTI diagnosis requires the presence of leukocyturia and at least 50,000colonies/mL of a single uropathogen in an adequately collected urine sample. It is recommended to collect the sample only by catheterization in children without sphincter control, due to the high risk of contamination and false positive results in children from which urine was collected through sample collection bags. Suprapubic aspiration should be reserved for exceptional cases, and has been less and less used in clinical practice. However, the analysis of urinary sediment (urine test I) and/or urinary strip is widely used in emergency for rapid diagnosis of UTI. A recent systematic review found no significant differences between the information obtained by both methods, recommending the initial treatment of children at risk for UTI if there is leukocyturia or alterations in urinary strip (positive leukocyte esterase and/or positive nitrite) until the confirmation can be obtained by urine culture test.20
Laboratory diagnosisIt is a consensus that the likelihood of occult bacteremia and SBI decreased sharply after the introduction of the conjugate vaccine in the immunization schedule. Thus, the outpatient management of children with FWS can be a safe and less costly alternative in those at low risk for SBI.6
After the introduction of the conjugate vaccine against Haemophilus influenzae type B, the risk of OB decreased to 1.5–3%; after the introduction of the 7-valent pneumococcal vaccination, this risk further decreased to 0.5–1%.21 The higher immunization coverage to other serotypes of Streptococcus pneumoniae with the 13-valent vaccine can further reduce these figures, so that infants in good general status probably will not require laboratory tests in order to estimate OB risk.5,22,23 In a recent analysis of 591 children between 3 and 36 months with FWS who were in good general status on admission and were submitted to laboratory evaluation with blood cell count (CBC), C-reactive protein (CRP), or procalcitonin, only 1% had OB, with half of the cases due to OB caused by S. pneumoniae, and none of these children had been immunized against pneumococcus.1 Based on the abovementioned findings, there have been changes in recent years regarding the management of infants with FWS.24
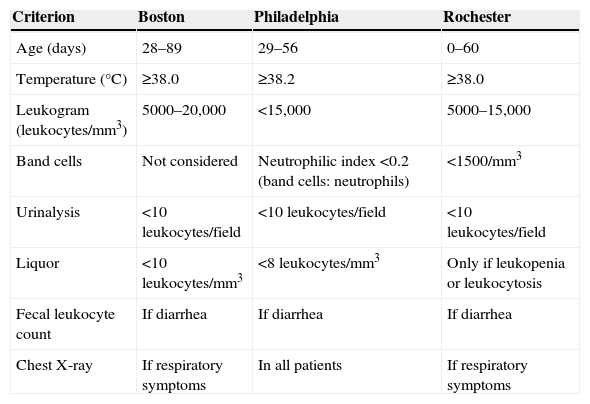
In 1993, Baraff et al.25 published guidelines for evaluation of infants with FWS between 3 and 36 months of age; these recommendations were revised in 2008 with the inclusion of urinalysis for infants at risk of UTI (e.g., uncircumcised boys), questioning the usefulness of laboratory assessment with blood count in fully immunized children in good general status.23 Neutropenia (neutrophil count <1500/mm3), considered a risk factor for OB in infants with FWS according to the criteria of Rochester (Table 1), has recently been studied in non-cancer patients. In a recent case-control study with patients older than 3 months with FWS, neutropenia was not a risk factor for OB, which puts in doubt the prompt indication of antibiotics for neutropenic infants with FWS.26 In infants between 4 and 12 weeks old, classically used algorithms, such as Boston, Baraff, and Rochester, help in the initial decision-making regarding antibiotic therapy and conduct, as described in Table 1. Patients with the laboratory criteria described below can be considered as low risk for OB and undergo outpatient management, returning after 24h for reassessment.
Main algorithms used in risk stratification of infants with FWS up to 90 days of life.
| Criterion | Boston | Philadelphia | Rochester |
|---|---|---|---|
| Age (days) | 28–89 | 29–56 | 0–60 |
| Temperature (°C) | ≥38.0 | ≥38.2 | ≥38.0 |
| Leukogram (leukocytes/mm3) | 5000–20,000 | <15,000 | 5000–15,000 |
| Band cells | Not considered | Neutrophilic index <0.2 (band cells: neutrophils) | <1500/mm3 |
| Urinalysis | <10leukocytes/field | <10leukocytes/field | <10leukocytes/field |
| Liquor | <10leukocytes/mm3 | <8leukocytes/mm3 | Only if leukopenia or leukocytosis |
| Fecal leukocyte count | If diarrhea | If diarrhea | If diarrhea |
| Chest X-ray | If respiratory symptoms | In all patients | If respiratory symptoms |
Over the years, particularly after the introduction of conjugate bacterial vaccines, blood count requests in the evaluation of febrile infants have decreased systematically. A recent study revealed that 58.6% of patients with FWS evaluated in the emergency services, up to 3 years old and in good general status, did not have the test requested; in fact, blood count was performed only in 20% of patients.27
Blood culture request is limited by the low test positivity and the time it takes until the results are known. Infants with FWS and bacteremia have greater risk for the development of focal infections such as meningitis (approximately 10%); thus, identifying a positive blood culture at an early stage can prevent severe bacterial complications.28 The acceptable number of contaminated blood cultures (for instance, with coagulase-negative Staphylococcus species) is 3–4%, which may result in hospitalizations, additional costs, and unnecessary antibiotic therapy.29
Several authors have attempted to develop scores for SBI diagnosis and thus avoid the unnecessary use of empirical antibiotic therapy for children with FWS. The recently described Lab-score uses independently associated parameters with the occurrence of SBIs, with different weights according to the odds ratio obtained for each variable in the univariate analysis of the original study. Based on the combined determination of procalcitonin and C-reactive protein, in addition to the results of the urinary tape analysis, the score can have results from 0 to 9; a cutoff of 3 showed 94% sensitivity and 78% of specificity for the diagnosis of SBIs in children between 7 days and 36 months of life.30 The adequate use of this tool can reduce up to 26.5% the prescription of antibiotics in children with FWS, as shown in a recent clinical trial.3
Traditionally, according to the Baraff criteria, infants with FWS and more than 15,000 leukocytes/mm3 in the blood count had an increased risk for OB. However, recent evidence demonstrates the limited diagnostic value of the leukogram to demonstrate the presence of SBI in febrile infants.31 CRP has slightly better sensitivity than the leukogram for this purpose, but also has limitations to indicate antibiotic therapy in febrile patients. Recently, procalcitonin (PCT) has been studied and identified as a reliable SBI marker in febrile children, which was verified at the differentiation between bacterial and viral meningitis and between pyelonephritis and cystitis.32,33 PCT levels correlate with the bacterial infection invasiveness and have more favorable kinetics, showing an increase within the first six hours of fever when compared to the CRP. This finding may have particular importance in febrile young infants, as they are more likely to develop SBI and are usually taken to the emergency room after just a few hours of fever.34
In a study of 1112 infants with FWS, SBI was diagnosed in 2.1% of cases. PCT values >0.5ng/mL were the only independent risk factors for SBI, with an odds ratio of 21.69. In another study with 868 infants with FWS, in good status at admission, the sensitivity of the PCT for the same cutoff value of 0.9ng/mL was 86.7%, with a specificity of 90.5% when compared to CRP, whose cutoff value of 91mg/L had a sensitivity of 33.3% and specificity of 95.9%.35
Treatment of OB in febrile infantsAccording to the data presented above, the recommendation of a single protocol for the evaluation and treatment of febrile infants is not possible.6 The management of fever in newborns is outside the scope of this article, but it involves aggressive conduct, with the collection of all cultures, including cerebrospinal fluid (CSF), as well as hospital admission and parenteral antibiotic therapy until the final results are obtained.
For infants between 4 and 12 weeks of age with FWS, the collection of blood count, blood culture, and inflammatory markers such as CRP and PCT are recommended, as well as urinalysis with sample obtained by catheterization or suprapubic aspiration, for cases of exception. Hospitalization is recommended in cases of UTI, with cefuroxime (150mg/kg/day) or ceftriaxone (50–100mg/kg/day) prescription; in febrile infants at risk for OB, according to the criteria shown in Table 1, CSF collection is recommended, as well as hospital admission with ceftriaxone (50–100mg/kg/day) until the culture results are obtained, if there is no meningitis. It is essential to reassess patients with FWS, who should be treated on an outpatient basis within 24h, if they are not at risk for OB.
In infants aged between 3 and 36 months of life, the impact of conjugate vaccination, as previously described, makes it unnecessary to collect blood count and blood cultures in patients with good general status. Urine collection should be considered according to the abovementioned factors, especially in febrile girls younger than 24 months and uncircumcised boys younger than 12 months with FWS.18 Routine chest X-rays are not recommended in patients at this age group with no respiratory signs or symptoms, even attenuated ones. Infants in poor general status and/or with toxemia should undergo, in addition to blood count, blood culture, CRP or PCT, CSF collection, and hospital admission with ceftriaxone, at the same aforementioned doses, until culture results are obtained and/or fever resolution.
ConclusionsFever remains an important cause of consultation in emergency services in children up to 3 years old, and the request of multiple laboratory tests for initial assessment is still frequent, as well as antibiotic therapy, even in children at no risk for OB and with no presumed bacterial infection. Over the past few years, mainly due to the introduction of conjugate vaccines in the Brazilian vaccination schedule, it is possible that, as in countries where this vaccination has been applied for a longer period of time, a drastic reduction has occurred in the prevalence of OB in febrile infants. Given the current recommendations, blood count and blood culture collection should not be routinely performed in infants older than 3 months with fever and in good general status. Studies are necessary in Brazil country to confirm the decrease in cases of OB in infants in the post-vaccine era.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Mekitarian Filho E, de Carvalho WB. Current management of occult bacteremia in infants. J Pediatr (Rio J). 2015;91:S61–6.