To review the mechanisms of action of macrolides in pediatric respiratory diseases and their clinical indications.
SourcesReview in the PubMed database, comprising the following terms in English: “macrolide and asthma”; “macrolide and cystic fibrosis”; “macrolide bronchiolitis and viral acute”; “macrolide and bronchiolitis obliterans” and “macrolide and non-CF bronchiectasis”.
Summary of the findingsThe spectrum of action of macrolides includes production of inflammatory mediators, control of mucus hypersecretion, and modulation of host-defense mechanisms. The potential benefit of macrolide antibiotics has been studied in a variety of lung diseases, such as cystic fibrosis (CF), bronchiectasis, asthma, acute bronchiolitis, and non-CF bronchiectasis. Several studies have evaluated the benefits of macrolides in asthma refractory to therapy, but the results are controversial and indications should be limited to specific phenotypes.
In viral bronchiolitis, there is no consistent benefit in acute conditions, although recent data have shown an effect in recurrent wheezing prevention. In patients with CF results are also contradictory, but the consensus states there is a small clinical benefit, especially for patients infected with P. aeruginosa. There was also no positive action of macrolides in patients with post-infectious bronchiolitis obliterans. Children with non-CF bronchiectasis seem to have clear benefits regarding the use of macrolides, which showed clinical advantages in parenchyma protection and lung function.
ConclusionsThe long-term use of macrolides should be limited to highly selected situations, especially in patients with bronchiectasis. Careful evaluation of the benefits and potential damage are tools for their indication in specific groups.
revisar os mecanismos de ação de macrolídeos em doenças respiratórias pediátricas e as suas indicações clínicas.
Fonte de dadosrevisão na base de dados Pubmed, compreendendo os termos em inglês referente ao tema básico.
Síntese dos dadosO seu espectro de ação estende-se desde a produção de mediadores inflamatórios, o controle da hipersecreção de muco e modulação de mecanismos de defesa do hospedeiro. O potencial benefício dos antibióticos macrolídeos foi estudado em doenças pulmonares como a fibrose cística, as bronquiectasias, a asma, a bronquiolite aguda e as bronquiectasias não ligadas à fibrose cística. Diversos estudos avaliaram os benefícios dos macrolídeos na asma resistente a terapia, porém, os resultados são controversos e as indicações devem ser limitadas a fenótipos específicos. Na bronquiolite viral não há benefícios consistentes nos quadros agudos, embora dados recentes mostrem um efeito na prevenção de sibilância recorrente. Em pacientes com fibrose cística os resultados também são contraditórios, mas o consenso é de que há um pequeno benefício clínico, especialmente para os pacientes infectados por P. aeruginosa. Também não foi observada ação positiva dos macrolídeos em pacientes com bronquiolite obliterante pós-infecciosa. Crianças com bronquiectasias não relacionadas à fibrose cística parecem ter claros benefícios em relação ao uso de macrolídeos, os quais mostraram vantagens clínicas, de proteção ao parênquima e na função pulmonar.
ConclusõesO uso em longo prazo de macrolídeos deve ser limitado a situações altamente selecionadas, especialmente em pacientes com bronquiectasias. Avaliação cuidadosa dos benefícios e potenciais danos são ferramentas para indicação em grupos específicos.
Macrolides are antibiotics belonging to a chemical compound family characterized by the presence of a macrocyclic lactone ring, for which the reference drug is erythromycin.1 These are drugs that have been used for years to treat respiratory infections, given their excellent tissue penetration and action against many of the common respiratory pathogens, including Mycoplasma species, Chlamydia, and Legionella.2,3 Macrolides exert their antimicrobial effect by binding to bacterial ribosome, specifically the 50S subunit, promoting protein synthesis inhibition. The effect may be bacteriostatic or bactericidal, depending on the concentration and susceptibility of microorganisms.4
In the early 1980s, positive results of erythromycin use in patients with a disease originally described in Japan and known as acute diffuse panbronchiolitis (DPB) aroused the interest of physicians and researchers on the immunomodulatory potential of macrolides.5 Acute DPB is an idiopathic disease characterized by distal airway obstruction, mucoid impaction, and dilation, with extensive inflammatory infiltration of neutrophils and CD8+ lymphocytes. It can be associated with infection by Pseudomonas aeruginosa species in more advanced stages and develop into extensive bronchiectasis.6 The use of erythromycin is recommended for patients with this disease, representing one of the main therapeutic resources; it is believed that its action mechanisms include anti-inflammatory and anti-mucus actions.6,7
In an extensive review of action mechanisms of macrolides as immunomodulators in lung diseases, Kanoh and Rubin4 described the existing evidence regarding the action of these drugs in several areas of pulmonary and systemic physiology, including modulation of inflammatory cytokine synthesis, adhesion molecule expression, activity and survival of inflammatory and respiratory epithelial cells, in addition to effects on airway secretions (Table 1).4
Immunomodulatory effects of macrolides.4
| Target | Action |
|---|---|
| Effects on airway secretion | Interference with ion transport in epithelial cellsInhibition of mucus hypersecretionInterference with mucin gene expression |
| Anti-inflammatory effects | Interference with cytokine production (IL-8, TNF-alpha, and IL-6 reduction)Reduction in adhesion molecule expressionInterference with chemotaxis and the release of inflammatory mediators (including reactive oxygen species) of neutrophils and eosinophils, interference with differentiation, and apoptosis of inflammatory cellsStabilization of respiratory epithelium through tight-junction modificationInterference with the proliferation of fibroblasts and vascular endothelial cells involved in angiogenesis |
| Effects on cell signaling | Interference with intracellular calcium signaling pathwayInterference in the mitogen-activated protein kinase (MAPK) system via action on the extracellular-signal-regulated kinases (ERKs), modulating the expression of transcription factors (especially NF-kB) |
| Effects on bacteria | Interference with bacterial adhesionInhibition of virulence factors (e.g., exotoxin A, elastase, etc.)Biofilm inhibitionQuorum-sensing inhibition |
In recent years, the use of macrolides in a number of respiratory diseases such as asthma, acute viral bronchiolitis, cystic fibrosis (CF), bronchiolitis obliterans (BO), and non-CF bronchiectasis has increased significantly, but there is still much controversy about the unrestricted use of macrolides in these cases,4,5 either due to concerns about the emergence of resistant bacterial strains,8,9 or safety concerns.10 The purpose of this review article was to evaluate the existing data in the literature regarding the use of macrolides in clinically relevant pediatric respiratory diseases.
MethodsA search was performed in the PubMed database, comprising the following terms in English: “macrolide and asthma”; “macrolide and cystic fibrosis”; “macrolide and acute viral bronchiolitis”; “macrolide and bronchiolitis obliterans”; and “macrolide and non-CF bronchiectasis.”
The search was conducted with no limitation regarding specific periods, seeking to include the most relevant publications on the assessed topics. In addition to the descriptive text, a table containing the summary of findings with recommendations for the use of macrolides in the assessed conditions was included, according to the level of scientific evidence (Grading of Recommendations Assessment, Development and Evaluation – GRADE system).11
Use of macrolides in pulmonary diseasesAsthma refractory to basic treatmentAsthma is an airway disease characterized by chronic inflammation, bronchial hyperresponsiveness, and airflow limitation. The disease manifests clinically with recurrent coughing, wheezing, and/or dyspnea. The mechanisms responsible for the maintenance of the inflammatory response, which is characterized by the increased number of activated lymphocytes, eosinophils, neutrophils, and mast cells of variable shape, are only partially recognized, but evidence suggests that chronic or subacute infections with atypical bacteria such as Mycoplasma pneumoniae and Chlamidophila pneumoniae may be important contributors to the pathogenesis and severity of asthma in some patients.3,12
In addition to effective antimicrobial activity for the abovementioned agents, macrolides also exhibit anti-neutrophil immunomodulatory activity, which makes them candidates for asthma treatment. Several clinical trials have been performed to assess the effect of macrolides on different aspects of asthma therapy, such as acute asthma,13 asthma refractory to treatment,14 or as corticosteroid-sparing agents.15
Most of these studies have small samples and are susceptible to random biases due to lack of power. In a systematic review published in 2005, Richeldi et al.16 analyzed seven studies (416 patients) and observed some symptom improvement, but no impact on pulmonary function (forced expiratory volume in the first second [FEV1]), thus not recommending the routine use of this therapy. In a more recent meta-analysis that included a total of 12 randomized controlled trials, no significant improvement in FEV1 was observed after macrolide administration for three weeks or more.17 However, the joint analysis of the data showed significant improvement in symptom scores, quality of life, peak expiratory flow, and bronchial hyperreactivity in asthmatic patients treated with macrolides. In this meta-analysis, adverse events were uncommon, mostly unimportant, and rarely led to treatment withdrawal. There were no severe cardiovascular side effects reported in the patients treated with macrolides.17
Macrolides appear to have a more important role in airway diseases with neutrophilic inflammation, such as CF and bronchiectasis. The inflammatory profile in the patient's airway has been recognized as one of the main determinants of response to treatment with macrolides.
However, few studies have specifically assessed their efficacy in the neutrophilic asthma phenotype. Simpson et al.14 reported a significant decrease in Interleukin-8 (IL-8) and neutrophil counts in the sputum. In one of the most recent controlled trials,18 the results were stratified by inflammatory profile, identifying a significant reduction in the number of exacerbations in patients with non-eosinophilic phenotype. In this case, the phenotype was defined by the absence of systemic eosinophilia.18 It is important to recognize these subtypes associated with asthma complexity, as asthma therapy is advancing toward a treatment directed to different phenotypes, considering the great variability and complexity of this pathology.
In addition to the immunomodulating effects, the beneficial effects of azithromycin in severe non-eosinophilic asthma could also be due to its antimicrobial properties. Chronic respiratory infection with atypical bacteria such as Mycoplasma pneumoniae and Chlamydophila pneumoniae may play a role in the pathogenesis of uncontrolled asthma.2
In brief, long-term treatment with macrolides in asthmatic individuals yields controversial results. However, a recent meta-analysis that increased the statistical power of the analyses showed benefits in several asthma outcomes, including quality of life and symptoms, although there was no improvement in FEV1. Currently, there is little evidence to justify the routine use of macrolides in asthma treatment.
However, in some subgroups of patients, such as children with evidence of atypical bacterial infection, non-eosinophilic asthma (or neutrophilic) may benefit from macrolide effects. Therefore, there are no recommendations for the systematic use of macrolides in patients with refractory asthma, but they can be indicated as adjunctive therapy in the treatment of specific asthma cases, particularly in some specific phenotypes (Table 2).
Summary of key scientific evidence on macrolide use in lung diseases, with recommendations for their use (based on the GRADE system).11
| Pathology | Study types and key findings | Recommendation |
|---|---|---|
| Asthma refractory to treatment | Cochrane systematic review (seven studies, 416 patients) – no effect on FEV1, but showing symptom and peak flow improvement.16Meta-analysis (12 studies, 831 patients) – no effect on FEV1, but showing symptom and quality of life improvement.17 | Their systematic use is not recommended, but some patient subgroups may benefit (Grade 2B). |
| Acute viral bronchiolitis | Cochrane systematic review (two studies, 281 patients) – no differences in length of hospital stay, duration of oxygen therapy, and readmissions.26 | Their systematic use is not recommended (Grade 1A). |
| Cystic Fibrosis | Meta-analysis (eight studies, 654 patients) – significant improvement in FEV1 and FVC, particularly in patients chronically infected with P. aeruginosa.36Cochrane systematic review (ten studies, 959 patients) showing significant lung function improvement, reduction of acute pulmonary exacerbations, and weight gain improvement.37 | Use is recommended for patients with chronic infection by P. aeruginosa (Grade 1A). |
| Post-transplant bronchiolitis obliterans (PTBO) | Meta-analysis (ten studies, 140 patients) showing improvement in lung function (about 8% in FEV1) and a trend to reduction in mortality from PTBO.54 | Use is recommended in PTBO (Grade 1A). |
| Post-infectious bronchiolitis obliterans | Review of 42 cases showing unspecified clinical improvement with combination of corticosteroids and azithromycin.57 | Insufficient evidence to recommend their use (Grade 2C). |
| Non-cystic fibrosis bronchiectasis | Meta-analysis of ten studies (601 patients) – reduction in acute exacerbations, FEV1 fall attenuation, reduction in sputum volume, and clinical score improvement, but increased risk of diarrhea and bacterial resistance.64 | Use is recommended for patients with at least three pulmonary exacerbations or two admissions in the last 12 months (Grade 2A). |
Viral bronchiolitis is an acute, potentially severe disease that usually affects young infants. It often occurs in the first year of life and is the most common cause of hospitalization in infants in the 1st year of life.19 The disease responds poorly to treatment, including antiviral drugs. Considering that viruses are potent inducers of production and release of cytokines and pro-inflammatory chemokines, the potential benefit of immunomodulatory and anti-inflammatory actions of macrolides has also been assessed in respiratory viral infections, although in small studies with contradictory results.
In a double-blinded, randomized, placebo-controlled trial, Tahan et al.20 assessed the efficacy of clarithromycin administered daily for three weeks in children younger than 7 months hospitalized for respiratory syncytial virus (RSV) bronchiolitis. Nine subjects were excluded from analysis due to corticosteroid use, which left 12 individuals in the group that received clarithromycin and nine in the placebo group. The use of clarithromycin was associated with a statistically significant reduction in hospital length of stay, oxygen use time, need for β2-agonists, and hospital readmission within six months.20 This study received harsh criticism in letters sent to the journal due to errors in the statistical analysis, questionable methodology, and small number of patients.21,22
However, the doubts regarding the effect of macrolides on acute viral bronchiolitis were even more pronounced, suggesting the need for further research in the area. Three subsequent randomized and placebo-controlled trials with larger samples (n=71/184/97) were published, carried out in infants aged <24 months, hospitalized for clinical picture of viral bronchiolitis. The macrolide assessed in these studies was azithromycin, and its use was not superior to placebo regarding the length of hospital stay (primary endpoint), days of symptoms, or need for supplemental oxygen.23–25 Therefore, the use of macrolides is not recommended for the treatment of infants with acute viral bronchiolitis26 (Table 2).
Recently, a pilot study in hospitalized children with bronchiolitis due to RSV showed that treatment with azithromycin for two weeks, when added to the routine care of bronchiolitis, resulted in a reduction of an airway inflammation marker (neutrophilic inflammation) and IL-8 in nasal lavage samples. Additionally, the participants treated with azithromycin took longer to develop recurrent wheezing (third episode of wheezing after acute bronchiolitis) and had significantly fewer days with respiratory symptoms during the following year.27
IL-8 has a potent neutrophil chemotactic effect and activates immune system cells in response to infection by RSV. An increase in IL-8 in the upper airways has been reported as a marker of acute bronchiolitis severity. The subsequent neutrophil degranulation may result in epithelial cell damage. Therefore, an intervention to reduce IL-8 levels in the airways could attenuate the damage due to the effect on neutrophils and, subsequently, prevent respiratory sequelae of recurrent wheezing caused by RSV.28
Nonetheless, the relatively small sample size studied to date is an important limitation to make a definitive assessment of the usefulness of this intervention for recurrent wheezing prevention. Therefore, although the general trend toward better clinical outcomes is encouraging, it cannot be concluded that treatment with azithromycin for acute bronchiolitis reduces the occurrence of recurrent wheezing. These potential beneficial effects must be evaluated in larger and more definitive clinical trials with a longer follow-up duration.
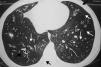
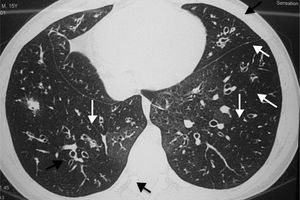
Cystic fibrosisCF is a genetic disease that leads to chronic bronchitis, mucoid impaction, and bronchiectasis29 (Fig. 1), showing similar characteristics to those of acute diffuse panbronchiolitis. Many CF patients develop chronic P. aeruginosa infections, with worsening of the inflammatory process and progressive decline in pulmonary function.30 Possible action mechanisms of macrolides in CF include P. aeruginosa virulence reduction, decreased bacterial adherence to the respiratory epithelium, reduced bacterial motility, and interference with biofilm production.31,32 Among the immunomodulatory actions of macrolides in the host with CF, factors include the interference with elastase production by neutrophils (main effectors of the inflammatory response in CF), inhibition of inflammatory cytokine production by alveolar macrophages, and decrease in mucus hypersecretion.31,32
The macrolide most often used in CF patients has been azithromycin, and the first encouraging clinical trial was published in 2002, evaluating 41 patients with CF in a cross-over, double-blind, randomized, placebo-controlled trial for 15 months.33 The primary endpoint was the change in FEV1, and the drug dose was administered at two weight ranges (250mg/day, if weight ≤40kg or 500mg/day, if weight >40kg). The authors observed significant pulmonary function improvement (5.4%, 95% CI=0.8–10.5%) in the group receiving azithromycin when compared to the placebo group, and no significant difference was observed regarding bacteria concentration in sputum, exercise tolerance, and quality of life. The treatment was also well tolerated, with no significant adverse events.33
Subsequently, a new multicenter, double-blind, randomized, placebo-controlled trial was conducted in the United States with a sample of 185 patients chronically infected with P. aeruginosa and older than 6 years.34 The azithromycin dose was equal to that of the abovementioned study, but use was limited to three times per week. The primary endpoint was also FEV1, and the authors showed a significant difference between the treatment and placebo groups (6.2%, 95% CI=2.6–9.8%). Other encouraging results were a 35% reduction in the risk of acute pulmonary exacerbation and significant weight gain in patients that received azithromycin.34
This same group evaluated the effect of azithromycin in CF patients not infected with P. aeruginosa35; this time, the use of azithromycin for 24 weeks did not result in significant FEV1 improvement when compared with the placebo group, but there was a significant reduction in the occurrence of acute pulmonary exacerbations in the group treated with azithromycin.35
A recent meta-analysis assessing the use of macrolides in CF patients included six randomized placebo-controlled trials (654 patients).36 Treatment with azithromycin resulted in significant FEV1 and FVC improvement, particularly in patients chronically infected with P. aeruginosa. The incidence of side effects was not significantly different between the placebo group and the group treated with azithromycin.36
In a systematic review of macrolide use in CF, a total of 10 studies were included (959 patients).37 Four clinical trials (549 patients) showed significant improvement in pulmonary function when comparing azithromycin with placebo (mean difference in a six-month period was 3.97%, 95% CI=1.74–6.19%).
Patients on azithromycin showed a decrease in the occurrence of acute pulmonary exacerbations, required oral antibiotics less frequently, and had higher weight gain and lower rate of identification of S. aureus in respiratory secretion cultures. Adverse effects were unusual, although an increase in macrolide resistance was observed. The authors concluded that azithromycin has a small beneficial effect in the treatment of CF patients, with a dose administration schedule of three times per week, for six-month periods; therefore, its use is recommended for patients with chronic infection by P. aeruginosa (Table 2).
However, considering the scarce long-term data and the concern about the development of bacterial resistance to macrolides, the current evidence is not strong enough to indicate azithromycin for all CF patients.37
Other macrolides were assessed on a less systematic basis in CF patients (studies with small samples, published as congress abstracts), or showed to be ineffective in this group of patients. Clarithromycin was assessed in a double-blind crossover study in 63 CF patients for 12 months, and no beneficial effect was observed on pulmonary function (primary endpoint), or the frequency of acute pulmonary exacerbations, quality of life, and inflammatory cytokine profile in sputum.38
Bronchiolitis obliteransBO is a rare disease that manifests as a chronic obstructive pulmonary disease, and can affect healthy individuals or patients submitted to bone marrow (BMT) or solid organ transplantation.39 In case of patients that underwent lung transplantation, the likelihood of developing bronchiolitis obliterans over time is so high that it affects 70% of survivors ten years after the transplantation.40
The process involves the obstruction of small airways, and two types of histopathological patterns were originally described: a pattern of lymphoid tissue proliferation, constituting intraluminal polyps (initially known as bronchiolitis obliterans organizing pneumonia [BOOP]) and a pattern of concentric airway fibrosis, known as constrictive bronchiolitis.41 This constrictive pattern was the one most often found in biopsies of children with post-infectious bronchiolitis obliterans in the state of São Paulo, Brazil.42
The physiopathological mechanism of BO is not fully known, but there is evidence that severe epithelial injury of the distal airways triggers an intense process of uncontrolled fibroproliferation.39 Among the triggering insults of bronchiolitis obliterans are the following: toxic gas inhalation,43 severe viral respiratory infections, especially by adenovirus,44 autoimmune diseases,45 and graft vs. host reactions.46
Clinically, patients with BO exhibit progressive respiratory difficulty, initially dry cough (which may progress to suppurative), and obstructive patterns of varying degrees at the spirometry, usually with significant air trapping and lack of response to bronchodilator therapy.39
Radiologically, a pattern of regional hyperinflation interspersed with normal areas can be observed, characterizing a mosaic pattern on computed tomography of the chest. This pattern is frequently seen in many obstructive diseases and is not specific for bronchiolitis obliterans, particularly in infants. In post-infectious BO, the tomographic finding with the highest diagnostic sensitivity is bronchiectasis.47
The treatment of BO in patients undergoing transplantation (BMT or lung) usually involves immunosuppressive drug adjustments, including steroids, cyclosporine, and calcineurin inhibitors,39 but there is evidence that azithromycin can benefit many patients, probably due to its effect on neutrophilic airway inflammation and IL-8.48
Initial studies in patients with bronchiolitis obliterans post-BMT were encouraging, showing improvement in lung function and disease stabilization,49 but in a more recent randomized, double-blind trial, Lam et al.50 did not identify any effect in patients with post-BMT bronchiolitis obliterans, even though they were relatively advanced disease cases.50
However, experience in patients submitted to lung transplantation has been more encouraging, and some studies have shown improvement in lung function and inflammatory parameters in bronchoalveolar lavage,51,52 also with an impact on these patients’ survival.53 In a recent meta-analysis, comprising data from ten studies and 140 patients, Kingah et al.54 reported positive effects on lung function (about 8% in FEV1) after macrolide use in patients with post-transplant bronchiolitis obliterans, in addition to a trend in reduced mortality from BO.54 Thus, macrolide use is recommended for patients undergoing bone marrow or solid organ transplantation who manifest symptoms or functional abnormalities suggestive of BO (Table 2).
In cases of post-infectious BO, the approach includes the use of systemic and inhaled corticosteroids, usually associated with the use of bronchodilators or anticholinergics.55,56 It is noteworthy that there have been no controlled intervention studies with these drugs or even with azithromycin, but in a recent review study of 42 cases in China, Li et al.57 reported successful treatment in 84% of cases using a combination of corticosteroids and azithromycin. There is no evidence to recommend the systematic use of macrolides in patients with post-infectious bronchiolitis obliterans (Table 2).
Non-CF bronchiectasisBronchiectasis is the pathological bronchial dilatation, usually associated with mucosal thickening and impaired secretion clearance, resulting in accumulation of secretion, chronic suppurative cough, and pulmonary function loss.58 Considered a nearly universal phenomenon in patients with CF, it can also be observed in several other clinical situations, such as immunodeficiencies, ciliary dyskinesia, and as a sequela to severe pulmonary infections.58 They are usually associated with a chronic process of infection and predominantly neutrophilic inflammation and, in this context, the use of macrolides could be indicated to alleviate symptoms, reduce the frequency of exacerbations, and provide some functional improvement over time.59
Although the causes can be multiple and the prevalence varies significantly in different populations, there is evidence that specific groups, such as indigenous populations in developed countries and children from low-income families, are more susceptible to develop non-CF bronchiectasis.9,60,61
There are some recent randomized studies on the use of macrolides in adult patients with non-CF bronchiectasis using azithromycin and erythromycin, all demonstrating a reduction in exacerbation frequency, with no impact on patients’ lung function.62 Only one randomized study evaluating the use of macrolides in children or adolescents with non-CF bronchiectasis was published in a specific population of aboriginal origin in New Zealand and Australia.9 A total of 44 children were assessed in the placebo group and 45 in the study group, which received azithromycin 30mg/kg in a weekly administration. A significant reduction in frequency of acute exacerbations was observed in the group receiving the drug, but there was a significant increase in the isolation of bacterial strains resistant to azithromycin in nasal swab samples from the study group at the end of the study.9
In a recent meta-analysis, the compilation of data from ten studies (601 patients) showed that macrolide use resulted in a reduction in acute exacerbations, FEV1 fall attenuation, reduction in sputum volume, and clinical score improvement, but also in an increased risk of diarrhea and bacterial resistance.63,64 Although most of the evidence of this therapeutic approach comes from studies carried out in adult patients, the effects in children and adolescents appear to be similar63,64 (Table 2). The criteria for this therapeutic indication, however, must include lack of clinical control with the usual treatment, such as at least three acute pulmonary exacerbations or two hospitalizations in the last 12 months.65 Additionally, the treatment period must be limited (12–24 months) to assess whether there is definitely a response.65
ConclusionsAlthough the in vitro effect and studies in specific populations have demonstrated the anti-inflammatory action of macrolides in pediatric respiratory diseases, the magnitude of the impact on clinical responses is still debatable. Further studies are necessary to define populations with clear benefits. Perhaps the greatest appeal for the use of macrolides it is the lack of new and affordable options for high-impact diseases such as asthma, viral bronchiolitis, and CF.
A major concern regarding the overuse of macrolides, in general, is bacterial resistance induction. A number of studies have demonstrated their overuse induces resistance, especially in pneumococcal strains.66,67 Therefore, further studies to determine their actual importance in the treatment of diseases mentioned in this review, as well as dose and frequency-of-use assessments, are extremely important so that medical practice does not lead to more problems than benefits for the population.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: da Silva Filho LV, Pinto LA, Stein RT. Use of macrolides in lung diseases: recent literature controversies. J Pediatr (Rio J). 2015;91:S52–60.