This study aims to identify the scientific evidence on the risks and effects of exposure to environmental contaminants in children during sensitive developmental periods.
Data sourceThe search was performed in the Bireme database, using the terms: children's health, environmental exposure, health vulnerability, toxicity pathways and developmental disabilities in the LILACS, MEDLINE and SciELO systems.
Data synthesisChildren differ from adults in their unique physiological and behavioral characteristics and the potential exposure to risks caused by several threats in the environment. Exposure to toxic agents is analyzed through toxicokinetic processes in the several systems and organs during the sensitive phases of child development. The caused effects are reflected in the increased prevalence of congenital malformations, diarrhea, asthma, cancer, endocrine and neurological disorders, among others, with negative impacts throughout adult life.
ConclusionTo identify the causes and understand the mechanisms involved in the genesis of these diseases is a challenge for science, as there is still a lack of knowledge on children's susceptibility to many environmental contaminants. Prevention policies and more research on child environmental health, improving the recording and surveillance of environmental risks to children's health, should be an ongoing priority in the public health field.
O presente estudo busca identificar as evidências científicas sobre os riscos e efeitos da exposição de contaminantes ambientais no organismo infantil durante os períodos sensíveis de seu desenvolvimento.
Fonte de dadosAs pesquisas foram realizadas pelo banco de dados da Bireme, com os termos children's health, environmental exposure, health vulnerability, toxicity pathways and developmental disabilities nos sistemas LILACS, MEDLINE e SciELO.
Síntese de dadosA criança difere do adulto por suas características singulares de ordem fisiológica, comportamental e do potencial de exposição a riscos frente às diversas ameaças do ambiente. A exposição a agentes tóxicos é analisada por meio dos processos toxicocinéticos nos diversos sistemas e órgãos durante as janelas sensíveis do desenvolvimento infantil. Os efeitos causados transparecem no aumento da prevalência de malformações congênitas, diarreia, asma, cânceres, distúrbios endócrinos e neurológicos, entre outros, com impactos negativos ao longo da vida adulta.
ConclusãoIdentificar as causas e compreender os mecanismos envolvidos na gênese desses agravos é um desafio que se impõe à ciência, visto que ainda há uma lacuna de conhecimento sobre a suscetibilidade infantil para muitos contaminantes ambientais. Políticas de prevenção e mais pesquisas em saúde ambiental infantil, que impulsionem o registro e a vigilância epidemiológica dos riscos ambientais à saúde da criança, deve ser uma prioridade contínua no campo da saúde pública.
In 1946, the World Health Organization declared, in an open letter to its members, that all children have the right to a healthy development where they can live harmoniously in a diversified environment1 so that, in a near future, they can reach their full potential as world citizens.2 From a broader view, it can be suggested that this concept of environment includes not only the natural world, but also the physical context in which the child interacts with its world: the external environment (air, water, earth, and living beings); the community (social environment, school, and neighborhood where the child lives); and the home environment.3
The interest and the degree of knowledge about the different ways in which the environment can influence children's health in the places where they routinely stay in their daily life has increased considerably in the last two decades.2 They include not only the child as a biological being with a genetic potential, but also the interaction of a multitude of influences (physical, chemical, and biological), as well as factors (psychosocial, cultural, and economic) that have a complex impact on the life of these children.4–7
Children comprise 26% of the world's population and are among the most vulnerable groups to be exposed to environmental risks. It is estimated that they account for over 30% (31–40%) of the global disease burden, especially in children under 5 years of age.8,9 Considering this terrible picture, of a completely imbalanced state between the child and its environment, there is a population of 223 million young citizens who, over the past two decades, died before reaching the age of 5 years.10
Unhealthy environments, unfavorable situations regarding access to clean water, waste disposal (sanitation), limited family income, parents’ low educational level, and early cessation of breastfeeding may contribute to the children's illnesses and mortality.11,12 Worldwide, 10% of all diseases recorded in children could be prevented if governments invested more in access to clean water, hygiene measures, and basic sanitation.13
The fact that more than one million children die each year as a result of acute respiratory diseases, 60% of these deaths being related to environmental pollutants, cannot be ignored.10 Degraded ecosystems, environmental smoke, air pollution, and climate changes are the likely factors that cause changes in the health status of this population.14,15 The pathways through which these agents can act and mutually operate are not always clear and are often indirect, but their consequences are considered a risk to the integrity of the airways, due to changes in the respiratory system balance and their influence on the interactions between the host, pathogen, and environment, increasing the likelihood of infection in that population.16,17
Depending on their age, gender, geographic region, and socioeconomic status, children have peculiar behavior. They are dependent on the developmental stages characterized by physical, psychosocial, and cognitive changes, the potential of risk exposure and parental perceptions regarding the acquisition of skills and capabilities in the presence of different physical threats in the home environment.18,19 The most challenging is that, while low levels of exposure to many chemicals are inevitable, little is known about the risks of such exposures on the morbidity, mortality, and subtle changes in this population.4,20 It is noteworthy that these products exposed in the environment can act synergistically, which means that their combined effect may be more harmful to children.
Based on the premise that the pediatric population reacts in a very unique way to the environment, scientific advances have provided hypotheses and even strong arguments about how lifestyle and the social environment where the child lives can change their gene functioning. Drugs, pesticides, air pollutants, chemicals, heavy metals, hormones, and nutrition products, among others, are examples of elements that can result in gene expression alterations that will be inherited by the next generations.21,22
This susceptibility is always focused on agents or specific compounds in specific exposure scenarios, including intrauterine exposure,23 which can interfere during critical and sensitive growth and developmental periods and have a negative impact throughout life, possibly causing structural and functional deficits, temporary or permanent impairment, and even death.24 These occur because children have no control over the environment in the prenatal and postnatal periods, including the quality of the air they breathe, the water they drink, the food they eat,25 and their exposure to disease-transmitting vectors.26
Regarding infection transmitted by vectors, there is a scientific acknowledgment that Brazil with its epicenter in the Northeast region, has been showing since October 2015 an excess number of cases of newborns with microcephaly and severe brain abnormalities,27,28 whose the proven causative agent is the Zika virus, transmitted by the Aedes aegypti mosquito, resulting from maternal exposure during the first months of pregnancy.29–32 Because it also transmits dengue fever and chikungunya, the elimination of this mosquito has been considered by the World Health Organization, since February of 2016, an international public health emergency.32
Still during pregnancy, maternal exposure to chemicals resulting from industrial, agricultural and mining activities may reflect the possible increased prevalence of prematurity,33 hematopoietic cancers,34,35 birth defects, asthma, endocrine, neurological, and behavioral disorders of their children.36–39 As for the biological effects of maternal exposure to physical agents, such as ionizing radiation (dose above 250mGy), they can lead to intrauterine death, fetal malformations, fetal growth disorders, and carcinogenic effects.40
Given these facts, the objective of this article was to explore the scientific evidence regarding the impact of exposure to environmental contaminants in children during sensitive developmental periods, based on their special characteristics.
Children's particular characteristicsDue to the fact that they are going through a phase of growth and development, the pediatric population is a special demographic group concerning biology, physiological, metabolic, and behavioral processes, which are complex and multilayered.8,41 From the embryonic phase to late adolescence, children are often exposed to intrinsic risk factors (genetic, metabolic, and hereditary, very often correlated) and extrinsic or environmental factors (food, socioeconomic, geophysical, and urbanization conditions, as well as mother-child relationship), which may adversely affect the dynamic processes of growth and development.42,43 What determines the nature and severity of the effects of these factors on children's health is the occurrence of adverse environmental exposures at different stages, called “critical exposure windows”, “windows of vulnerability”,44 or “developmental windows”,45 where the functions of cell maturation, differentiation, and growth are occurring at different rates.
Since conception, there is a close association between fetal growth and the environment, to the point that, at a certain period, growth is limited by the space of the uterine cavity.41 After 9 months of intrauterine growth, the child's organs and systems become relatively mature, enough to safely adapt to life.46 The physical growth and functional maturation of the body will continue, and may vary from system to system, from organ to organ and from tissue to tissue, because each child is different in their structure and function at any age.45,47
As an example of this differentiated rate of child growth, studies suggest that in the second month of intrauterine life, the fetal head should correspond to half of the body, whereas at birth it is 25%, and in adulthood, it should correspond to 10% of the whole physical structure.42,48 At 6 months of life, the brain must reach 50% of its adult weight, and at 3 years, it should have reached approximately 90% of this weight.39,45 Approximately 50% of the size of an adult is reached at 2 years of age.42,48 In contrast, 50% of the weight of the liver, heart, and kidneys of an adult is not reached until the child is approximately 9 years old. The same points in growth of skeletal muscle and total body weight are not reached until near the 11th year of life.46 These periods of child development are especially sensitive to exposure to certain biological, chemical agents, and physical factors found in the environment.46
In children younger than 5 years of age, the influence of environmental factors is much more important than genetic factors. The younger the child, the more dependent and vulnerable he/she is in relation to the environment that surrounds him/her. And if that environment is not adequate, there is the possibility of failure regarding some aspect of child development.42,49 In this context, the embryonic development taking place inside the mother's uterus (environment) would not be protected from the embryotoxic effects of harmful environmental agents.50 As an example, the tragedy of the drug thalidomide, used during pregnancy in the 1950s, led to the special attention of researchers on the occurrence of teratogenic effects of the drug on the fetus’ health, causing phocomelia with irreversible damages.5 In 2010, Japanese scientists identified how thalidomide interferes with fetal development. The drug binds to a protein called cereblon (CRBN), inactivating it, resulting in limb malformation.51
The precocity and the persistence of adverse conditions in children's systems or organs before their complete maturation can cause temporary or permanent damage to the normal physical maturation and, consequently, to their current and future health.52
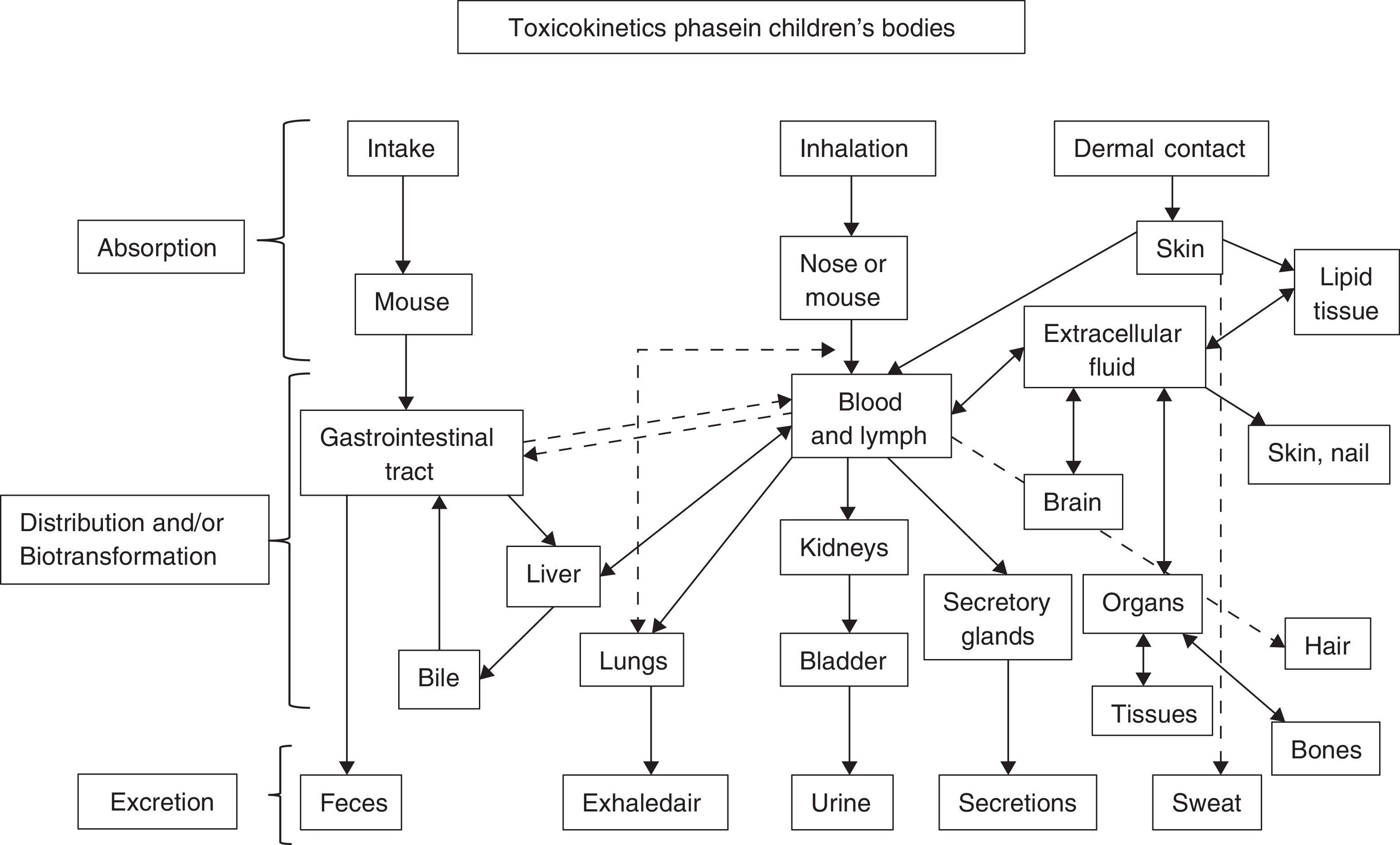
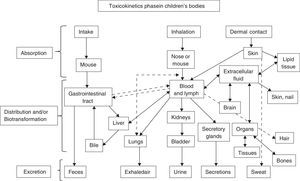
Exposure pathways of environmental toxicants in target systems and organs during children's developmental stagesContamination occurs through an exposure pathway, as shown in Fig. 1, between the agent in the physical environment (in utero, breast milk, oral, parenteral) and the developmental period when the child was exposed (fetal, neonatal, childhood, puberty).53 It is extremely important to know the magnitude of the adverse substance, the duration and frequency of exposure and the child's individual susceptibility, and the introduction pathways of the pathological agents.54,55
Adapted from Ruppenthal.104
Breastfeeding: the ideal form of infant nutrition, it has ensured the survival of the human species, providing nutritional, immunological, cognitive, psycho-affective, economic, and social advantages,56,57 significantly reducing the child's risk of developing several diseases. Influenced by factors of the nursing mother, infant, and/or toxicant, breastfeeding can introduce into the baby's circulation several toxic substances, such as dioxins, polychlorinated biphenyls (PCBs), mercury and chlorinated pesticides,58 nicotine,59 lead,60 and medications,61 among others. While in the maternal toxicokinetics the breast milk/plasma ratio for PCB is high, ranging from 4 to 10, for organic and inorganic mercury it is 0.9.60 Nicotine may be present in breast milk at the same maternal plasma concentration, and its half-life is also similar, with 60–90min of elimination in both.59 The lead excreted in breast milk has a concentration between 10% to 30% of maternal blood lead values; as lead is not lipophilic, it is not concentrated in the milk.60 The recommendation to discontinue breast-feeding due to the presence of chemicals in breast milk is not supported in the literature, as long as the finding of these substances does not offer toxicity to the infant.53
Respiratory system – at birth, a full-term baby has approximately 10 million alveoli, whereas at 8 years old, he/she will have 300 million alveoli.62 The impact of passive smoking since the intrauterine phase can result in reduced weight, length, and cephalic perimeter in the newborn,63 in addition to the increase in airway resistance, with a mean reduction of 20% in forced expiratory flow.64 The continuity of postnatal parental smoking can have adverse effects on the child's immune system against pathogens, as well as on lung growth and development, causing a four-fold increased risk of wheezing during the first year of life.64–66
A child breathes more air than an adult at rest, even though the adult has a greater lung capacity, which corresponds to about 6.5l, whereas in a child this capacity is about 2l.62 Considering the body weight, the air volume passing through the airways of the child at rest is twice of that in adults, under similar conditions.6 An infant has three times the ventilation per minute (volume of air passing through the lungs at every minute) than an adult, and a 6-year-old child has twice this volume.45
As children breathe more air and tend to be more physically active than adults, inhaling toxic gases may compromise their pulmonary function67 or exacerbate pre-existing conditions, increasing the incidence of acute respiratory infections in this population, expressed as increased hospitalizations68 and school absenteeism.69 These substances include: particulate matter in suspension (dust, smoke and aerosols)70; pollutants of public health concern, such as O3,71 NO2,72 CO, and SO273; lead, mercury and volatile organic compounds14; as well as biomass burning residue45 and tobacco smoke.74–76
Endocrine-reproductive system: since embryonic life, events related to the child's formation, growth and development, require hormonal interactions at specific moments.77,78 These hormones play a critical role in coordinating multiple cell activities, through the regulation of biological functions such as the hypothalamic-pituitary axis, the thyroid and sexual organs, keeping in homeostasis the metabolism, electrolyte balance, sleep and mood.77,79 However, these activities can be negatively impacted when susceptible to agents of exogenous origin to the body. Among these agents are substances known as endocrine disruptors, also known as eco-hormones, substances with hormonal activity or xenohormones.77,78,80
Endocrine disruptors can reach the environment mainly through industrial and urban waste, agricultural runoff, and waste release; children's exposure to these toxicants can occur through ingestion of food, dust, and water, inhalation of gases and particles in the air, and skin contact.81 Some are natural substances, such as estrogens and phytoestrogens, whereas synthetic varieties can be found in pesticides, alkylphenols, polychlorinated biphenyls (PCBs), bisphenol A, food additives, toiletries, and cosmetics.82
During the prenatal period, endocrine disruptors can have consequences on fetal development with a greater risk of sensitizing the future reproductive capability.77,78 These substances act by mimicking estrogen action or antagonizing androgen action; they may result in early puberty83 and have adverse effects on sexual differentiation, gonadal development, fecundity, and fertility, as well as sexual behavior.78,79,84 According to Alves et al.,53 the literature suggests that sexual prematurity can also be related to accidental exposure to cosmetics containing estrogen or placental extracts, such as shampoo, conditioners, and body creams, among others, resulting in breast size increase and transitional impotence, with regression of the condition after the use of these products was discontinued.
Nervous system – from embryogenesis onwards, the nervous system continues to shape its structure not only in the beginning, but throughout the entire period of child development until the end of adolescence, in response to genetically programmed events and environmental influences, in a series of complex processes that occur at specific periods in time and space.25,39
At birth, the brain of a child reaches approximately 24% of its adult weight. The brain weight at birth is approximately between 300 and 330g. At 1 year, the brain mass will be tripled, growing at a much slower pace until the 1500g of the adult phase is reached.39,85 The population of nerve cells is completed around the age of 2 years, but total neuronal tissue myelination will not be completed until 18 years of age.39 Because the brain is the main organ of stress and adaptation, it is at the same time both vulnerable and adaptable. It interprets and regulates behavioral, neuroendocrine, autonomic, and immunological responses.85
Vulnerable periods during the nervous system development are sensitive to environmental injuries, because they depend on the regional and temporal onset of critical developmental processes, such as proliferation, migration, differentiation, synaptogenesis, myelination, and apoptosis.39 In this sense, a wide range of chemical categories (benzene, ethanol, nicotine, methylmercury, polychlorinated biphenyl (PCBs), arsenic, lead, manganese, dichlorodiphenyltrichloroethane, tetrachloroethylene, and organophosphate insecticides)36 may interfere with one or more of these processes, leading to developmental neurotoxicity39 through ingestion of contaminated food and liquids, gas inhalation, or skin contact.81 The result of such interference in the normal ontogeny of developmental processes in the nervous system will be children who may present clinical disorders, such as cognitive impairment, attention deficit/hyperactivity disorder, mental retardation, autism, and cerebral palsy, among others, that will persist throughout their life.36 The development and maturation of the nervous system, the dose and duration of exposure, as well as the child's nutritional status, all influence this toxicity.86
In addition to the exposure to toxic chemicals, biological agents can also have a negative effect on the infant's brain development. As mentioned before, there is an association between prenatal infection by the Zika virus and cases of microcephaly and other disorders of the nervous system in fetuses and newborns, called “congenital Zika syndrome”.29 Fetal autopsies have shown several changes in the brain, such as hydrops,87,88 hydrocephalus and multifocal dystrophic calcifications in the cortex,87 intracranial calcifications in the periventricular parenchyma and parts of the thalamus,89 cell degeneration, and necrosis.90 The detection of viral RNA and antigens in the analyzed brain tissues was also confirmed.87,90 Moreover, an ophthalmological study in infants with microcephaly caused by the virus demonstrated the risk of visual impairment in these children, due to the presence of bilateral macular and perimacular lesions and optic nerve abnormalities.91
In certain cases, the child's vulnerability to neurotoxins may be related not only to the stages of neurodevelopment, but also to the immaturity or failure of other protective barriers, as the blood–brain barrier92,93 and the placental barrier.54,55,94
Blood–brain barrier – its primary function is to maintain a safe and constant chemical environment for proper functioning of the brain and protect it from xenobiotics, bacteria, fungi, parasites, viruses, and autoimmune reactions.46 This protection is due to its lower permeability, having exclusion mechanisms that prevent the diffusion of adverse polar substances with low molecular weight, such as drugs, food additives, pesticides, and metals, such as nickel, chromium, and mercury.54,93 This barrier is not fully developed at birth; this may be one of the reasons for the higher toxicity of chemicals in newborns.93 An example is the vulnerability of the neonatal brain when exposed to bilirubin, which may result in encephalopathy (kernicterus) in the first weeks of life, with irreversible neurological damage. While bilirubin concentrations of 40mg/dL are not tolerated in neonates, these do not appear to cause any adverse effects on adults.46
Placental barrier – in addition to protecting the fetus against the passage of harmful substances from the mother's body, it performs the exchange of vital nutrients for fetal development.46 In fact, the placenta is not an effective protective barrier against the inflow of foreign substances into the circulation, at a time of extreme fetal vulnerability. Although there are some biotransformation mechanisms (or metabolization) that can prevent the placental passage of these substances through a deactivating mechanism, making the resulting product less toxic than its precursor,54,55,94 more than 200 chemical products, foreign to the organism, were detected in the umbilical cord blood.36
Recent studies have reported that the placenta is not immune to the Zika virus. A group of researchers from Fiocruz26 identified traces of viral DNA in the placental tissue of a pregnant woman who had the pregnancy interrupted. The study disclosed the immunopositivity in Hofbauer cells, present in the placenta and which, in pregnant women, should act as a fetal protection factor. The researchers suspect that the virus may be using the migratory ability of these cells to reach the fetal vessels. Martines et al.90 also showed in their study the detection of Zika viral RNA in placental tissue of early miscarriages.
Prenatal exposure to substances such as nicotine, lead, anesthetic gases, carbon monoxide, antineoplastic agents, and solvents, among others, can have negative effects on fetal development54,94 and cause damage that cannot be predicted until their effects are observed in adulthood.95 Methylmercury, when crossing the placenta, can easily reach high levels in the umbilical cord blood and produce a variety of congenital abnormalities, including microcephaly, mental retardation, and motor deficit.96 As for lead, in addition to crossing the brain and the placental barriers and being secreted in milk, it is quickly and easily distributed to all tissues. The toxicity of this substance when ingested by infants and young children may reach an absorption rate of 50%, with effects on the central and peripheral nervous system.97
As observed, the effects of environmental toxicants in the complex process of the nervous system development can lead to an abnormal neuropsychomotor development, with transient or persistent deficits, with possible and more insidious consequences in adulthood.39,46 The development, the maturation of the nervous system and detoxification mechanisms, the dose, exposure duration, and the nutritional status of the child influence the toxicity.86
Metabolism and digestion: the gastrointestinal (GI) tract, as well as the skin and the respiratory system, is in constant interaction with the environment. Depending on the age of the child, the GI function as a protective barrier is as important as its digestion and absorption functions.96 Young children have a faster metabolic rate and digest their meals faster than adults. They ingest more food and water per body weight unit.98 A newborn consumes a greater amount of water (equivalent to 10–15% of body weight) than an adult (2–4% of body weight).99 Infants who are formula-fed may have a mean daily consumption of 140–180mL/kg/day of water, which would be equivalent to consuming an average of 35 cans (360mL) of non-alcoholic beverages for an average adult male.99 Preschool children (1–5 years) ingest three to four times more food per kilogram of body weight than the average adult.98
Children, due to their little varied diet, containing more fruits and vegetables, have a greater chance of exposure to contaminated foods and liquids.96 Orally ingested environmental toxins can be modified in the GI tract by gastric pH, digestive enzymes or bacteria living in the intestine. When they are absorbed by the skin or by inhalation (through sinus drainage into the pharynx and esophagus), they can be secreted into the intestinal lumen through the biliary system and lead to gastrointestinal toxicity.96 As the digestive system is not capable of easily eliminating all the toxins, children may still be exposed to these agents for a longer period of time.82,100 Common childhood disorders, such as intestinal constipation, can also significantly increase toxin absorption by delaying the intestinal transit time.96
In this context, it is noteworthy that the rate of calcium transport in the body of newborns and infants is approximately five times that of adults.101 If the child's diet is deficient in iron or calcium and exposure to lead occurs, the small intestine, still developing, will absorb this harmful agent, which will compete with calcium for cell transportation at a high pace. Thus, the rate of lead absorption children may also be five times higher than that by adults.96,101 These lead concentrations in children's blood, especially in early childhood, may also be correlated to the intake of dust containing the metal, due to the oral exploration occurring in this phase of development, from sources within the household and the contaminated soil.44,102
Acute exposures to toxic substances absorbed in the GI tract are sometimes difficult to diagnose. Clinical pictures of nausea, vomiting, diarrhea, and GI bleeding may suggest an infectious etiology contamination through ingestion of non-potable water and contaminated food, due to the lack of hand hygiene during meal preparation.13 However, if there are other signs involving other organs, such as drowsiness, exposure to environmental toxins must be suspected, particularly heavy metals such as nickel, cadmium, lead, and mercury.96 In cases of ethanol intoxication, the limited glycogen reserves in infants and preschool children may increase the risk of hypoglycemia.103
Excretory system: differences between children and adults, regarding the susceptibility to intoxications, can result from the combination of toxicokinetics, toxicodynamics, and exposure factors.104 Kinetic factors are especially important in the postnatal period, largely as a result of the immaturity of the excretory system, either due to its reduced enzymatic metabolism and/or renal excretion.105 During fetal life, the kidneys have little excretory function due to the placenta, which also has this function.106 The maturation and nephrogenic induction usually occur after the 36th week of pregnancy.107 In this sense, preterm infants appear to be particularly at risk for kidney disease for a long period of time after the birth, because an increased number of these infants survive, including many who were born well before nephrogenesis is complete. Data indicate that during the process of preterm newborn treatment in the neonatal ICUs, the smaller children often receive nephrotoxic drugs, having as consequence larger glomeruli, but in smaller numbers.108
The glomerular filtration rate of a child is a third of the rate found in adults, which allows harmful chemicals to remain for longer periods in the child's body, among them lead and mercury.41 According to Capitani,97 children have a retention rate in the lead excretion process of around 30%, while in adults it is around 1–4%. These heavy metals can make the kidneys incapable to filter the waste, salts, and liquids in the blood, which may result in the loss hydro-electrolyte homeostasis, leading to acute renal failure.109
Dermal exposure: the skin is the organ derived from embryonic mesoderm and ectoderm and the most extensive organ of the human body.110 The development and growth of fetal skin is characterized by a series of sequential and strictly controlled steps, which depend on a variety of cell interactions that constitute the organ. Its presence is vital for the functions of mechanical protection, thermoregulation, immunosurveillance, and maintenance of a barrier that prevents the insensitive loss of body fluid.111
While the permeability of the skin barrier is still under development in preterm infants, resulting in a higher percutaneous absorption of chemicals,9 a full-term newborn has a more mature skin, whose barrier properties are similar to those of older children and adults.112 Infants have a surface area relative to volume three times that of adults, and in children this proportion is two-fold higher per unit of body weight when compared to adults.45,99 As they are highly active, they tend to have more skin cuts, abrasions, and rashes, thus increasing the potential for skin absorption of xenobiotics and toxicants, such as dust contaminated with heavy metals.45,111,112 Concentrated ammonia can produce, in contact with the child's skin, tissue necrosis and deep burns. These burns, depending on the size of the affected area, can secondarily cause acute renal failure.109
Behavioral development: child behavioral development can include changes in maturation depending on the child's relationship with the environment. This development can have four interrelated aspects, such as: motor skills (gross and fine); cognitive ability; emotional development; and social development. Any alteration in one of these areas can affect the development of the other three.46
Small children are powerless and helpless. With their peculiar behaviors and habits, they live in a playful world, without fearing their limits of exposure to any mechanical energy. With their extraordinary curiosity, they approach objects with sources of thermal and electrical energy, put inside their mouths or breathe in agents containing chemical energy,19 which they find in their environment, left within their reach, effectively contributing to increase the number of case reports of injuries caused by chemical, physical, and biological agents, mostly unintentional, whose results are often destructive to the entire family.113
In brief, the following points are noteworthy:
- 1.
A child is not a small adult. They differ regarding their physiology, metabolism, growth, development, and behavior. From the intrauterine stage until the end of adolescence, the physical growth and functional maturation of their bodies are at a differentiated and constant rhythm. Exposure to environmental threats at these sensitive stages of the child's life may negatively influence these dynamic processes, and may cause irreversible damage.
- 2.
Worldwide, children are exposed to environmental contaminants, acknowledged or not, which silently deteriorate their health, damaging their future achievements, with physical, emotional, and social consequences, in addition to the burden for the family, society, and the state as a whole.
- 3.
To identify the causes and understand the mechanisms involved in the genesis of environmental harm in a pediatric patient is very difficult and a challenge to science. Epidemiological data generally focus on assessing exposure to individual contaminants at relatively high doses. However, many situations in the real world, involve low-dose, long-term exposure to single or multiple agents. With the exception of some chemicals, there are relatively few data on the mechanisms involved in the genesis of these damages in the development of childhood diseases.
- 4.
The occurrence of these damages and their determinants in childhood has, as key factors, poverty and lack of access to information on inherent health practices. The impacts on children's health caused by the combination of classic risks (home environmental pollution and non-potable water) and modern risks (toxic chemicals, hazardous waste, air pollution and climate changes), vary greatly between and within developing countries.
- 5.
The child's rights to a safe environment to grow, develop, play, and learn are decisive arguments for the state to define effective strategies to promote healthy environments, which will protect children from events that are harmful to their health, that will monitor these trends over time and promote interventions where they are most needed. Articulated intra and inter-sectorial actions that promote the recording and surveillance of environmental risks to children's health should be a continued priority in public healthcare.
- 6.
The relationship between research and politics are rarely direct – from politics reporting to the research, or vice versa. Research and politics appear to work as parallel groups, sometimes interconnected by interests, occasionally feeding on each other. Relevant academic discussions in different fields of knowledge and an active state on the main issues that affect the lives of children are strategic actions for the survival of future generations. Studies on economic and social impacts and children's relationship with their physical environment in different fields of knowledge are essential for the survival of future generations.
- 7.
The children's environmental health needs to become an adult science in the academic world and in society.
The authors declare no conflicts of interest.
Please cite this article as: Perlroth NH, Branco CW. Current knowledge of environmental exposure in children during the sensitive developmental periods. J Pediatr (Rio J). 2017;93:17–27.