To evaluate the prognosis factors of children with sepsis and acute kidney injury.
MethodsThis was a retrospective study of children with sepsis and acute kidney injury that were admitted to the pediatric intensive care unit (PICU) of a tertiary hospital. A multivariate analysis was performed to compare risk factors for mortality.
ResultsSeventy-seven children (47 males) were retrospectively studied, median age of 4 months. Mean length of hospital stay was 7.33±0.16 days, 68.9% of patients received mechanical ventilation, 25.9% had oligo-anuria, and peritoneal dialysis was performed in 42.8%. The pRIFLE criteria were: injury (5.2%) and failure (94.8%), and the staging system criteria were: stage 1 (14.3%), stage 2 (29.9%), and stage 3 (55.8%). The mortality rate was 33.7%. In the multivariate analysis, the risk factors for mortality were PICU length of stay (OR=0.615, SE=0.1377, 95% CI=0.469–0.805, p=0.0004); invasive mechanical ventilation (OR=14.599, SE=1.1178, 95% CI=1.673–133.7564, p=0.0155); need for dialysis (OR=9.714, SE=0.8088, 95% CI=1.990–47.410, p=0.0049), and hypoalbuminemia (OR=10.484, SE=1.1147, 95% CI=1.179–93.200, p=0.035).
ConclusionsThe risk factors for mortality in children with acute kidney injury were associated with sepsis severity.
Avaliar os fatores prognósticos de crianças com sepse e lesão renal aguda.
MétodosEstudo retrospectivo de crianças internadas com sepse e lesão renal aguda em unidade de terapia intensiva pediátrica de serviço terciário. Usou-se a análise multivariada na comparação dos fatores de risco para mortalidade.
ResultadosForam avaliados 77 pacientes (47 masculinos) com mediana de 4 meses de idade. A média do tempo de internação foi de 7,33±0,16 dias, 68,9% necessitaram de ventilação mecânica, 25,9% eram oligoanúricos e 42,8% necessitaram de diálise. A classificação da lesão renal aguda foi pRIFLE I em 5,2% e F em 94,8% e estágio 1 (14,3%), estágio 2 (29,9%) e estágio 3 (55,8%). A taxa de mortalidade foi de 33,7%. Na análise multivariada os fatores de risco foram tempo de internação (OR=0,615 erro padrão=0,1377, 95% CI=0,469-0,805, p=0,0004), ventilação mecânica (OR=14,599, erro padrão=1,1178, 95% CI=1,673-133,7564, p=0,0155), necessidade de diálise (OR=9,714, erro padrão=0,8088, 95% CI=1.990-47,410, p=0,0049) e hipoalbuminemia (OR=10,484, erro padrão=1,1147, 95% CI=1,179-93,200, p=0,035). No modelo de Cox a sobrevida foi influenciada pela necessidade de diálise (HR=2,952, erro padrão=0,44862, 95% CI=1,225-7,112, p=0,016) e hipoalbuminemia (HR=3,326, erro padrão=0,59474, 95% CI=1,037-10,670, p=0,043).
ConclusõesOs fatores de risco para mortalidade nas crianças com lesão renal aguda foram associados à gravidade da sepse.
Acute kidney injury (AKI) is a significant factor that contributes to the morbidity and mortality of children and newborns admitted to intensive care units.1 The admission of patients with AKI to the pediatric intensive care unit (PICU) ranges between 48% and 68%.1,2 An association between sepsis and AKI was observed in 71.03% of the patients admitted to this PICU.
Multiple factors may be implicated in the etiology of AKI in children with sepsis.1 The frequency of sepsis-associated AKI has increased3–5; between 10% and over 30% of cases of AKI were due to sepsis and infection.2,6–8 Pediatric patients with sepsis and multiple systemic organ dysfunction had lower survival rates than those with ischemia.4 Sepsis was associated with death in 62% of patients with AKI and was a risk factor for mortality.8–10 Children with sepsis had a ten-fold higher risk of death.8 There is a scarcity of publications about the risk factors for mortality in pediatric patients with AKI and sepsis. Most studies reported data on neonates and children with AKI after heart surgery.3,11,12
In pediatric patients admitted to the PICU with AKI associated with hemolytic uremic syndrome or cancer, or post-heart surgery, the factors related to mortality were thrombocytopenia, age >12 years, and presence of hypoxemia and/or hypotension and/or coagulopathy.5 In this study, the mortality of patients with AKI was higher (29.6%) when compared with that of patients without it (2.3%).5 The determination of predictive factors of mortality in pediatric patients with sepsis-associated AKI may contribute to the identification of these patients, as well as the implementation of early therapeutic measures to reduce mortality.
For years, the definition of AKI was heterogeneous. The proposal to standardize the definition of AKI using the pediatric RIFLE (pRIFLE) criteria13–17 and the staging system14,16,18,19 favors the comparison of results between different studies. A prospective study showed that the injury and failure classification of the pRIFLE was a predictive factor of mortality in children admitted to the PICU.17
This study assessed independent predictive factors of mortality in a pediatric patient cohort with sepsis-associated AKI.
MethodsPatients, definitions, and assessed parametersThis was a retrospective observational study of a pediatric patient cohort admitted to the PICU of Faculdade de Medicina of Botucatu, UNESP-Universidade Estadual Paulista, which is a tertiary hospital. Data were obtained from a database of patients admitted to the PICU from January 1990 to December 1994. The study included children aged 1 to 132 months of age, of both genders, admitted to the PICU with a diagnosis of sepsis and AKI.
Patients with chronic kidney disease before PICU admission and those without serum creatinine values prior to admission were excluded. Chronic kidney disease was ruled out by taking into account the clinical history, physical examination, and imaging results with normal renal ultrasonography in all patients. The creatinine level prior to PICU admission was considered as the patient's serum creatinine level up to six months before admission. AKI was diagnosed when the serum creatinine value was higher than the normal value for age and height according to the reports of Guignard and Santos.20 All patients included in the study had a diagnosis of sepsis defined by the International Pediatric Sepsis Consensus Conference criteria.21,22
The analyzed data were: (a) demographic data (age, gender), (b) days in the PICU; (c) clinical data (oliguria or anuria, hypotension or hypertension, number of vasoactive drugs, use and duration of mechanical ventilation, need for dialysis, AKI classification criteria, and evolution during PICU stay); (d) laboratory data: serum albumin (hypoalbuminemia if value <2.5g/dL), serum bicarbonate (metabolic acidosis if value <15mEq/L), electrolytes (hypocalcemia if serum calcium value <7.9mg/dL; hyperkalemia if potassium >5.5mEq/L, or hypokalemia if potassium <2.5mEq/L, hyponatremia if sodium value <125mEq/L), and serum glucose (hypoglycemia if value <50mg% and hyperglycemia if value >150mg%); hematocrit, platelet count, and proteinuria (dipstick method +1 or +2 on urinalysis).
Oliguria was defined as diuresis <500mL/24h in older children or <1mL/kg/h in smaller children and infants.23,24 Anuria was defined as complete cessation of diuresis for a period of 24h.23,24 Hypotension was defined as a systolic blood pressure lower than the fifth percentile of the normal level for age.25 Hypertension was defined as systolic and/or diastolic blood pressure equal to or above the 95th percentile for age, gender, and height.26 Thrombocytopenia was defined as platelet level <50,000/mm3. This level is considered as moderate thrombocytopenia, and platelet count >50,000/mcL do not require routine treatment. The classification criterion was applied on the first day of AKI diagnosis.
The pRIFLE criteria defined AKI as: pRIFLE R, or Risk (25% decrease in the estimated creatinine clearance [CrCl] or diuresis <0.5mL/kg/h for 8h); pRIFLE I, or injury (50% decrease in CrCl or diuresis <0.5mL/kg/h for 16h); pRIFLE F, or failure (75% decrease in CrCl, or CrCl <35mL/min/1.73m, or diuresis <0.3mL/kg/h for 24h, or anuria for 12h); pRIFLE L, or loss (persistent failure>four weeks); and pRIFLE E, or end-stage (end-stage kidney disease-persistent failure>three months).18,27 Only the R, I, and F classifications for AKI were used in the present study. The Schwartz equation was used to calculate CrCl.18,27 Basal CrCl was calculated through the Schwartz equation using the serum creatinine value up to three months before PICU admission.
The staging system defined AKI as: stage 1 – serum creatinine increase >0.3mg/dL (>26.4mmoL/L) or ≥150–200% increase (1.5- to two-fold) from baseline or urine output <0.5mL/kg/h for >6h); stage 2 – serum creatinine increase >200–300% (two- to three-fold) from baseline or urine output <0.5mL/kg/h for >12h); stage 3 – serum creatinine increase >300% (> three-fold) >24h from baseline or serum creatinine ≥4.0mg/dL [354μmoL/L] or with a sharp increase of at least 0.5mg/dL [44μmoL/L]) or diuresis <0.3mL/kg/h or anuria for 12h).18
In this study, the use of the pRIFLE criterion was carried out according to CrCl alteration and the staging system according to alterations in serum creatinine levels, as 74.1% of the patients had non-oliguric AKI.
The study was approved by the Local Ethics Committee.
Statistical analysisThe results were analyzed using the SAS System for Windows (SAS Institute Inc.®, USA). Descriptive data are presented as percentage. Continuous variables (age, PICU length of stay, days of mechanical ventilation, urinary volume, and laboratory test values) were expressed as mean±SD or median. Categorical variables (gender, presence of oliguria, hypotension or hypertension, vasoactive drugs, mechanical ventilation, need for dialysis, hypocalcemia, hyperkalemia or hypokalemia, hyponatremia, thrombocytopenia, and/or presence of proteinuria) were expressed as frequency of occurrence. When comparing the survivor and deceased groups, Mann–Whitney's U test was used to analyze the continuous variables and Fisher's exact test for categorical variables. Multiple logistic regression analysis was used to define the risk factors and the evolution of variables that showed p<0.20 at the univariate analysis; odds ratio were adjusted and confidence intervals were calculated.
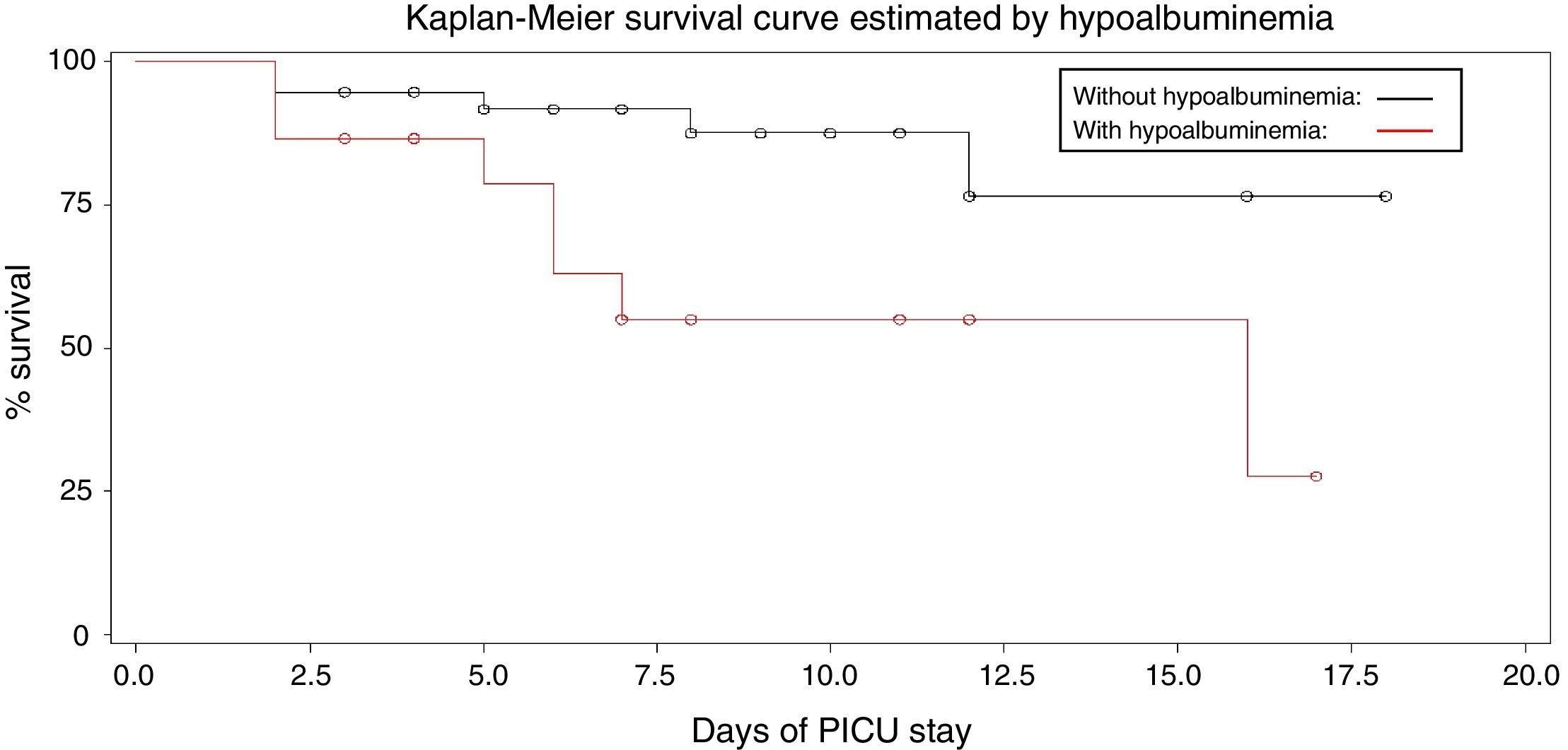
Kaplan–Meier survival curves were generated and the log-rank analysis was used to compare survival rates. Cox regression analysis was performed to assess the risk factors for mortality during PICU stay. p-Values <0.05 were considered significant.
ResultsThe study included 77 children with sepsis and AKI. Diarrhea and/or pneumonia were the most common causes of sepsis (84%).
The mean age of patients was 12.79±23.45 months (median 4 months); 77% were ≤10 months; 61.03% were boys. The mean number of days in the PICU was 7.33±0.16 days (range 1–18 days); 66 patients (85.7%) had a diagnosis of AKI on the first day of PICU admission; 11 patients (14.3%) were hospitalized for a median of 2 days (range 1–43 days) before AKI diagnosis. Diuresis ranged from 0 to 11mL/kg/h; 57 patients (74.1%) had non-oliguric AKI and ten (12.9%) were anuric. Three patients (0.04%) had hypotension and eight (10.3%) had hypertension; 98.7% required vasopressor drugs and 92.2% received more than two vasoactive drugs. Fifty-four patients (70.1%) required mechanical ventilation and 42.8% required acute renal replacement therapy; peritoneal dialysis was carried out in these patients. Indications for dialysis included oliguria, increase in serum urea, hyperkalemia (K>5.5mEq/L), pulmonary edema, and insufficient diuresis to allow administration of medications and/or nutrition.
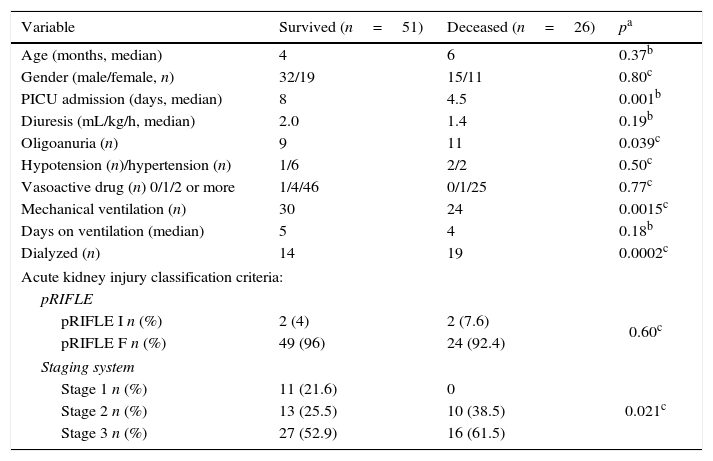
The pRIFLE classification of AKI patients was: pRIFLE I in four (5.2%) and pRIFLE F in 73 (94.8%). CrCl ranged from 4.19 to 56.66mL/min/1.73m2. As for the classification according to staging system, 11 patients (14.3%) had stage 1; 23 (29.9%) stage 2, and 43 (55.8%) had stage 3. Among the four patients with pRIFLE I, two had stage 1 and two had stage 2. Among the 73 patients with pRIFLE F, nine had stage 1; 21, stage 2; and 43, stage 3. There was an association between both classification criteria; none of the patients with stage 3 had pRIFLE I and all patients with stage 3 had pRIFLE F (p<0.025, Fisher's exact test). Table 1 shows the clinical characteristics and evolution of patients.
Clinical features and evolution of patients with sepsis and acute kidney injury.
| Variable | Survived (n=51) | Deceased (n=26) | pa |
|---|---|---|---|
| Age (months, median) | 4 | 6 | 0.37b |
| Gender (male/female, n) | 32/19 | 15/11 | 0.80c |
| PICU admission (days, median) | 8 | 4.5 | 0.001b |
| Diuresis (mL/kg/h, median) | 2.0 | 1.4 | 0.19b |
| Oligoanuria (n) | 9 | 11 | 0.039c |
| Hypotension (n)/hypertension (n) | 1/6 | 2/2 | 0.50c |
| Vasoactive drug (n) 0/1/2 or more | 1/4/46 | 0/1/25 | 0.77c |
| Mechanical ventilation (n) | 30 | 24 | 0.0015c |
| Days on ventilation (median) | 5 | 4 | 0.18b |
| Dialyzed (n) | 14 | 19 | 0.0002c |
| Acute kidney injury classification criteria: | |||
| pRIFLE | |||
| pRIFLE I n (%) | 2 (4) | 2 (7.6) | 0.60c |
| pRIFLE F n (%) | 49 (96) | 24 (92.4) | |
| Staging system | |||
| Stage 1 n (%) | 11 (21.6) | 0 | 0.021c |
| Stage 2 n (%) | 13 (25.5) | 10 (38.5) | |
| Stage 3 n (%) | 27 (52.9) | 16 (61.5) | |
n, number of patients; PICU, pediatric intensive care unit; pRIFLE, pediatric risk, injury, failure, loss, end stage renal disease.
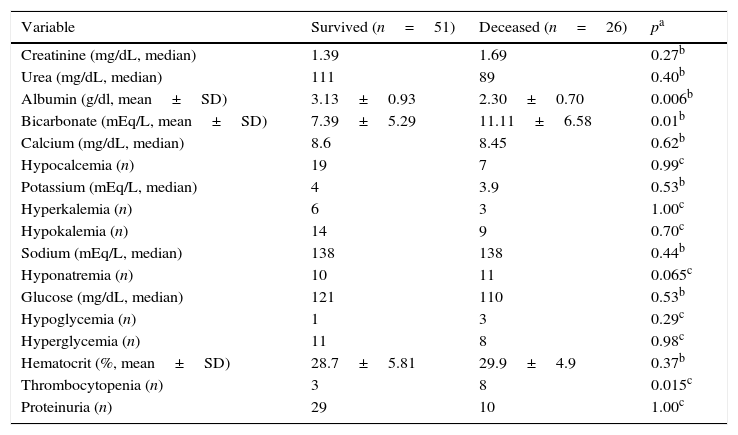
Patients’ laboratory data and evolution are shown in Table 2. Hypoalbuminemia was observed in 28.3% and metabolic acidosis in 90.7% (68/75) of patients.
Laboratory features and evolution of patients with sepsis and acute kidney injury.
| Variable | Survived (n=51) | Deceased (n=26) | pa |
|---|---|---|---|
| Creatinine (mg/dL, median) | 1.39 | 1.69 | 0.27b |
| Urea (mg/dL, median) | 111 | 89 | 0.40b |
| Albumin (g/dl, mean±SD) | 3.13±0.93 | 2.30±0.70 | 0.006b |
| Bicarbonate (mEq/L, mean±SD) | 7.39±5.29 | 11.11±6.58 | 0.01b |
| Calcium (mg/dL, median) | 8.6 | 8.45 | 0.62b |
| Hypocalcemia (n) | 19 | 7 | 0.99c |
| Potassium (mEq/L, median) | 4 | 3.9 | 0.53b |
| Hyperkalemia (n) | 6 | 3 | 1.00c |
| Hypokalemia (n) | 14 | 9 | 0.70c |
| Sodium (mEq/L, median) | 138 | 138 | 0.44b |
| Hyponatremia (n) | 10 | 11 | 0.065c |
| Glucose (mg/dL, median) | 121 | 110 | 0.53b |
| Hypoglycemia (n) | 1 | 3 | 0.29c |
| Hyperglycemia (n) | 11 | 8 | 0.98c |
| Hematocrit (%, mean±SD) | 28.7±5.81 | 29.9±4.9 | 0.37b |
| Thrombocytopenia (n) | 3 | 8 | 0.015c |
| Proteinuria (n) | 29 | 10 | 1.00c |
n, number of patients.
The mortality rate was 33.7% (26/77). Eleven patients had disseminated intravascular coagulation, and ten died (one had acute respiratory syndrome and one had multiple organ failure).
At the univariate analysis, the factors PICU length of stay, oligo-anuria, invasive mechanical ventilation, need for dialysis, AKI stage 2 and 3 criteria, hypoalbuminemia, serum bicarbonate, and thrombocytopenia were associated with mortality.
In the multiple logistic regression analysis, the independent risk factors associated with mortality were PICU length of stay (OR=0.615 SE=0.1377, 95% CI=0.469–0.805, p=0.0004), invasive mechanical ventilation (OR=14.599, SE=1.1178, 95% CI=1.673–133.7564, p=0.0155), need for dialysis (OR=9.714, SE=0.8088, 95% CI=1.990–47.410, p=0.0049), hypoalbuminemia (OR=10.484 SE=1.1147, 95% CI=1.179–93.200, p=0.035), and serum bicarbonate (OR=1.231, SE=0.0735, 95% CI=1.066–1.422, p=0.0046).
Other variables were not associated with mortality: oliguria, anuria (OR=1.13, SE=0.1302 95% CI=0.881–1.467, p=0.32), thrombocytopenia (OR=1.469, SE=1.0595, 95% CI=0.184–11.718, p=0.72), and AKI stage (OR=0.060, SE=2.2017, 95% CI ≤0.001–4.557, p=0.20).
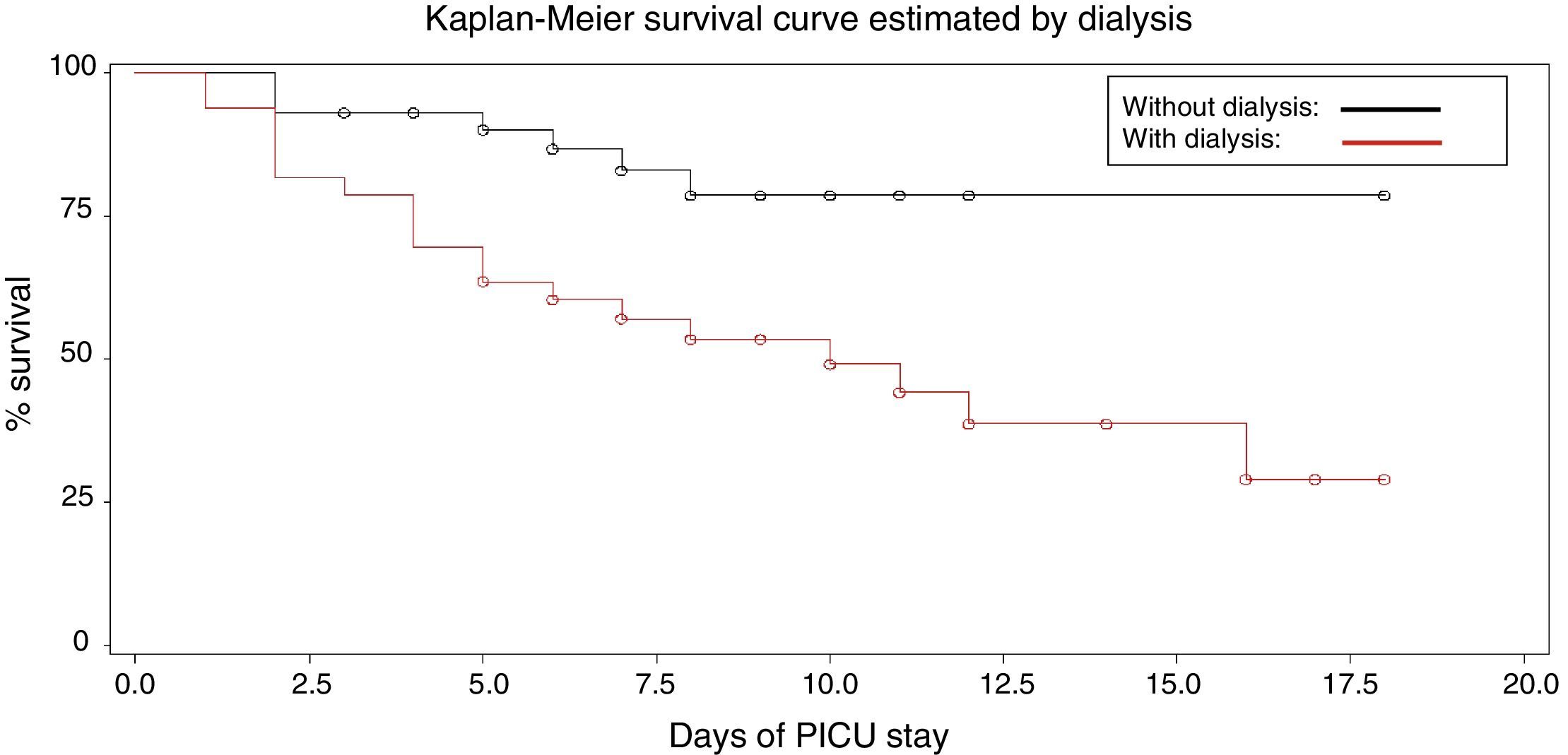
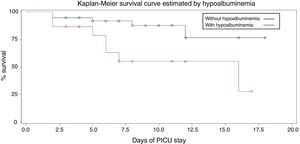
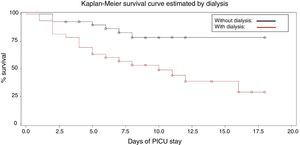
The estimated probabilities of survival during the PICU stay were 25% in hypoalbuminemic patients, when compared with 75% in patients with normal albumin (log rank=5.88, p=0.015) and 24% in patients requiring dialysis compared to 76% of patients who did not need dialysis (log rank=8.513, p=0.0035; Figs. 1 and 2).
In the survival analysis using Cox model, the parameters that influenced survival were: need for dialysis (HR=2.952, SE=0.44862, 95% CI=1.225–7.112, p=0.016), hypoalbuminemia (HR=3.326, SE=0.59474, 95% CI=1.037–10.670, p=0.043), and serum bicarbonate (HR=1.097, SE=0.04098, 95% CI=1.042–1.198, p=0.024). Need for invasive mechanical ventilation did not influence survival (HR=2.424, SE=1.07710, 95% CI=0.294–20.016, p=0.411).
The median of days to AKI improvement was five days (range 2–21 days) in patients with AKI stage 1; seven days (3–19 days variation) in patients with stage 2; and eight days (3–21 days variation) in patients with stage 3. AKI recovery in the first week occurred in 72.7% (8/11) of patients with AKI stage 1, 61.5% (8/13) of patients with stage 2, and 48.1% (13/27) of patients with stage 3.
DiscussionAn association between sepsis and AKI was observed in 71.03% of the patients admitted to this PICU; these patients had a high mortality rate (33.7%), similar to that described in other studies.4,8,13,28–31
There is a high incidence of sepsis-associated AKI in critically-ill patients.31 The identification of severe AKI predictive factors has the potential to optimize treatment.31,32
Among the factors associated with mortality, the presence of oliguria or anuria, need for dialysis, need for mechanical ventilation, and hypoalbuminemia should be emphasized.
Oliguria and anuria increased by 2.24 and 1.9 times the risk of mortality, respectively29; the latter is associated with a high mortality rate.10,16 In the present study, oliguria was a risk factor for mortality in the univariate analysis, but did not remain as such in the multivariate analysis. The lower proportion of oligo-anuric patients must have influenced the statistical analysis. Most of the present patients were non-oliguric, different from what was observed in the aforementioned studies. The presence of oliguria makes the disease more severe, hindering fluid administration, thus facilitating the development of hypervolemia, which complicates the underlying disease.31
The need for dialysis increased by 3.76 times the risk of mortality in children with AKI,29 especially in patients undergoing dialysis for longer periods.9 The present data are consistent with the aforementioned studies.9,29
Need for mechanical ventilation was associated with mortality in patients with AKI unrelated to primary urinary tract disorder9,23 and in those with prerenal and non-prerenal AKI11,29; the present findings are consistent with these studies.9,11,23 Patients with sepsis-associated AKI are more likely to require mechanical ventilation and hemodynamic support with vasoactive therapy, and to receive large volumes of fluid for resuscitation.31 This fact contributes to longer hospital stay in the PICU and increased mortality.33
In the present study, patients with decreased serum bicarbonate levels had higher survival. In the literature, the presence of acidosis was not an independent factor for the mortality of patients with AKI.24 The present study was conducted in a tertiary institution that is a reference center for medical or surgical critically-ill patients, who had probably been treated for acidosis with bicarbonate solutions before arrival at this service.
Hypoalbuminemia was associated with higher mortality in pediatric patients.12 In 228 pediatric patients with AKI, serum albumin was higher among survivors (3.3±0.9g/dL) than in non-survivors (2.6±0.7g/dL).12 Hypoalbuminemia may result from accelerated protein catabolism of metabolic alterations in AKI.30 Moreover, albumin synthesis is suppressed in response to inflammatory conditions,34 and inflammatory mediators are involved in children with sepsis.21,31 In children with severe disease, hypoalbuminemia was associated with high mortality and longer time of mechanical ventilation.35
The classification of renal injury severity has been associated with increased hospital length of stay and increased in-hospital relative mortality risk.31 According to the pRIFLE criteria, the patients in the present study had severe AKI (most were classified as pRIFLE F). In contrast, the staging criterion showed an even distribution between stages 1, 2, and 3. This fact must be associated with differences in the use of parameters to define AKI. The pRIFLE criteria used CrCl and the staging criteria used the increase in serum creatinine levels. Evidence suggests that small changes in serum creatinine are associated with increased patient mortality.18 Other authors have found significant differences in AKI severity distribution when serum creatinine increase was used instead of CrCl to define AKI; the latter showed greater sensitivity.14,16,18
Patients with pRIFLE I or F at admission showed a two-fold higher risk of mortality, when compared to patients with pRIFLE R.13,17 No association was observed between the strata of pRIFLE criteria and mortality, similar to the data reported by Plötz et al.15
When the AKI staging criterion was applied to the present patients, results similar to those reported by Ozçakar et al.19 were observed. The mortality rate was 33%, and it was higher in patients with AKI stage 2 and 3, similar to the present results. In sepsis-associated AKI, the probability of death was higher in patients classified as stage 3 AKI, when compared with those classified as stage 1.31
Studies have shown an association between the degree of kidney disease severity and improvement in renal function: 46% of patients showed renal function recovery within 48h, predominating in 62.5% patients with pRIFLE R at admission, when compared to those with pRIFLE I (38.1%) or pRIFLE F (26.7%).13 The recovery from AKI in the first week, according to the staging criterion, occurred in all patients with AKI stage 1, in half of the patients with stage 2, and in one-third of patients with stage 3.19 The present results were consistent with the aforementioned studies.
The assessed predictive factors of mortality in children with sepsis-associated AKI comprised clinical, laboratory, and AKI classification parameters. There is no single consensus on which parameter is ideal for early diagnosis of AKI, which would imply in early-onset therapy aimed at reducing mortality.
The constant monitoring of critically-ill patients, assessing the presence of oliguria, hypertension or hypotension, and hypoalbuminemia, as well as the evolution to the need for mechanical ventilation and/or dialysis, should raise the early suspicion of AKI development. At the same time, the AKI classification should be routinely carried out since the first day of hospitalization, and on the third day for the early detection of AKI, according to parameter evolution.31
Authors have validated the Renal Angina Index (RAI) using the product of the risk factors (admission to PICU – score 1, stem cell transplantation – score 3, ventilation and inotropics – score 5) and the injury factors (death rate evolution associated with fluid overload percentage, decrease in the estimated creatinine clearance, percentage of fluid overload–score ranging from 1, 2, 4, and 8), whose RAI value ranges from 1 to 40.32 The RAI cutoff value ≥8 is indicative of high risk for AKI.
The present study has limitations, such as the observational retrospective design with a small number of patients studied in a single center; however, this cohort included the evaluation of AKI in pediatric patients with a specific condition, i.e., sepsis. The small number of patients with the pRIFLE I classification may have influenced the power of statistics.
It can be concluded that the mortality rate is high in patients with sepsis and AKI; the predictive factors related to mortality were easy to apply, as well as the AKI classification by the staging system or pRIFLE and reflected the evolution of patients with sepsis and AKI.
Conflicts of interestThe authors declare no conflicts of interest.