To compare the biochemical and immunological profiles of pediatric patients with acute myeloid leukemia (AML) with healthy children and adolescents.
MethodsThis was a cross-sectional study in which 21 therapy-naïve patients with AML were compared with a group of 24 healthy individuals. The following data were analyzed: serum proteins, leucocytes and subgroups, erythrocytes, hematocrit, hemoglobin, platelets, cytokines in peripheral blood mononuclear cells cultures under spontaneous and BCG- or PHA-stimulated conditions, immunoglobulin A, and erythrocytic glutathione. Statistical analysis was performed using SPSS software, considering as significant p-values<0.05.
ResultsSerum albumin levels were higher (p<0.0001) in the control group, as well as all the parameters related to red blood cells (p<0.0001). For leucocytes and subgroups, no statistical difference was found between the AML and the control groups. For cytokines, the concentrations were significantly higher under spontaneous and BCG-stimulated conditions for TNF-α, IL-6, IL-10, and IFN-γ in the control group. Under PHA-stimulated conditions, the concentration was higher (p=0.002) only for IL-6. No difference was found between the two groups for the other cytokines and for IgA in the saliva. Erythrocytic glutathione was higher (p<0.0001) in AML patients.
ConclusionsIt was possible to characterize the biochemical and immunological profile of pediatric patients with AML, as well as highlight some significant differences in these parameters when comparing with healthy children and adolescents.
Comparar o perfil bioquímico e imunológico de pacientes pediátricos portadores de Leucemia Mieloide Aguda (LMA) em relação a um grupo de crianças e adolescentes saudáveis.
MétodosEstudo transversal, em que foram avaliados 21 pacientes com LMA virgens de terapia e um grupo de 24 indivíduos saudáveis. Foram analisadas: proteínas séricas, leucócitos e subgrupos, eritrócitos, hematócrito, hemoglobina e plaquetas, citocinas em cultura de células mononucleares do sangue periférico sob condição espontânea e estimulada por BCG ou PHA, imunoglobulina A e glutationa eritrocitária. Análise estatística foi realizada através do software SPSS considerando p<0,05.
ResultadosAlbumina sérica foi superior (p<0,0001) no grupo de controle, bem como, todos os parâmetros relacionados com os glóbulos vermelhos (p<0,0001). Para os leucócitos e subgrupos não houve diferença estatística entre os pacientes com LMA e o grupo controle. As concentrações foram significativamente mais elevadas sob condições espontânea e estimulada por BCG para as citocinas TNF-α, IL-6, IL-10 e IFN-γ no grupo controle. Sob condição estimulada com PHA a concentração foi superior (p=0,002) apenas para a IL-6. Não houve diferença estatística para as demais citocinas e para IgA salivar entre os dois grupos. Glutationa eritrocitária foi superior (p<0,0001) nos pacientes LMA.
ConclusõesDiante do exposto foi possível caracterizar o perfil bioquímico e imunológico de pacientes pediátricos com LMA, bem como, evidenciar diferenças significativas em alguns desses parâmetros ao se comparar os indivíduos doentes e o grupo de crianças e adolescentes saudáveis.
Common types of cancer in the pediatric age range include leukemias, particularly acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML), central nervous system tumor, lymphoma, neuroblastoma, Wilms tumor, osteosarcoma, and Ewing's sarcoma. Children comprise 80% of ALL cases, and only 10% of AML cases. An increasing prevalence of pediatric cancer has been observed in Brazil.1–3
Acute leukemia is a primary neoplasia of the bone medulla, characterized by a heterogeneous group of diseases in which there is a substitution of normal medullary and blood elements by immature cells (blasts) and accumulation of these cells in other tissues.4
According to Casciato,5 leukemic cells replicate slower than the corresponding normal cells. Hematopoiesis is abnormal even before the proportion of cells in the medulla shows a perceptible increase. The precursors of immature leucocytes exhibiting malfunctioning progressively substitute the bone medulla and infiltrate other tissues.
The signs and symptoms of acute leukemia result from the drop of blood cells, which lead to anemia, weakness, general discomfort, paleness, fatigue, palpitation, and dyspnea to exercise. Fever and infections may occur as a consequence of decreased granulocytes.5,6
The characterization of the biochemical and immunological profile of AML patients is important for nutritional and medical interventions and following normal growth and development of children and adolescents, in order to improve the immunological response and tolerance of patients to treatment, as well as their quality of life.
This study aimed to compare the biochemical and immunological profiles of pediatric patients with AML to those of healthy individuals matched in age.
MethodsPopulationThe studied sample comprised patients admitted to the Centro Infantil Boldrini, Campinas-SP, Brazil, for treatment of leukemia, immediately after the diagnostic of AML, and participation of a clinical trial of nutritional intervention.
The inclusion criteria were: a confirmed diagnostic for AML; treatment naivety; age range 0–19 years; having an informed consent signed by the patient or his/her legal guardian, after being informed of the objectives and methods employed in the research and being aware of the procedures and discomforts to which participants would be submitted, being free to accept to participate or not without constraint.
The study also included a control group of healthy children and adolescents in the same age range. The inclusion criteria for this group were individuals without any pathology nor receiving any medication at the time of selection or sample collection who signed an informed consent.
At the end of the investigation, 21 patients with AML had been evaluated: 11 females and 10 males, with median age 6.83 years (0.58–19.83). The control group was composed of 24 individuals among children and adolescents distributed between 17 females and seven males, median age 9.67 (1.5–18.25) years. The age of the groups was not significantly different (p=0.351) according to the Mann–Whitney test.
Biochemical and immunological evaluationSample collectionThe biological samples were collected from the patients by the nursing team of the Instituto Infantil Boldrini, where all patients were diagnosed and recruited. Between 15 and 20mL of blood were collected, carefully avoiding hemolysis.
Blood from healthy individuals was collected in the outpatient clinic of the Centro de Investigação em Pediatria (CIPED) by the nurse in charge. For saliva collection, a plastic 1mL sterile and rejectable Pasteur pipette was used. The participants were instructed not to eat or drink anything except water 1h before collection and to practice oral hygiene by washing the mouth with pure water.
Dosages of blood serum proteinsSerum prealbumin fraction was determined by nephelometry and the serum albumin by the colorimetric reaction with bromocresol green using spectrophotometry. The analyses were performed in the biochemistry laboratory of the Department of Clinical Pathology (DCP), of the Hospital das Clínicas of the Universidade Estadual de Campinas (UNICAMP).
HemogramsThe hemograms were performed at the Instituto Infantil Boldrini as part of a routine in the clinical treatment. For healthy individuals (control group), the hemograms were performed at the Laboratory of Clinical Pathology of the Hospital das Clínicas (UNICAMP).
Reduced erythrocytic glutathioneThe assay followed a minor modification of the method described by Beutler,7 proposed by Penna8 200μL of peripheral blood EDTA were lysed with 1.8mL of distilled water. Then, 2mL of 1.67% metaphosphoric acid solution were added and the mixture was filtered. 4mL of 0.3M Na2HPO4 solution were added to 1mL of the clear filtrate and read at 412nm on a Bechman spectrophotometer. A second optical density reading was taken after the addition of 100mL of dithiobisnitrobenzoic acid (DTNB) solution to the filtrates. Results were expressed in mg/dL.
Stimuli used in culturesLyophilized BCG (Moreau Rio de Janeiro vaccine vials) were freshly reconstituted with a culture medium RPMI 1640 (Sigma–Aldrich, USA) and used at 5×105UFC/mL. Phytohemagglutinin (PHA, Sigma–Aldrich, USA) was used a non-specific positive control at 7.5μg/mL, and medium alone was used as a negative control.
Cytokine concentration in culture supernatants of peripheral blood mononuclear cells (PBMC)Cytokine concentration was measured in PBMC cultures, by a modification of the protocol of Gaines et al.9 Fresh PBMC from patients and controls were isolated by density gradient centrifugation over Hystopaque® (Sigma–Aldrich, USA), washed, diluted to 2×106cells/mL in RPMI 1640 medium (Sigma–Aldrich, USA) supplemented with 10% human AB serum (Sigma–Aldrich, USA), 1% glutamine (Sigma–Aldrich, USA), and 0.1% gentamycin and stimulated for 48h with reconstituted BCG, PHA, or medium alone at 37°C with 5% CO2 in round-bottomed 96-well tissue culture plates (Nunc, Thermo Fisher Cientific, USA).
Culture supernatants were collected and stored at −80°C for two-monoclonal antibody sandwich enzyme linked immunosorbent assay (ELISA). Commercial kits (Duoset RD Systems, United States) were used to measure interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-10 concentration, according to the manufacturer's protocol. The levels of the transforming growth factor beta (TGF-β), and IL-8 were measured in blood plasma. All samples were measured in duplicates and the results were expressed in pg/mL.
Immunoglobulin A in salivaThe collected saliva was centrifuged at 1000×g for 7min and then stored at −80°C until time for analysis; the results were expressed in mg/mL. The concentration of immunoglobulin A (IgA) was performed by nephelometry.
Ethical aspectsAll ethical aspects were observed as recommended for biomedical research involving human beings, according to the National Health Council resolution No. 196 of 1996. The research protocol was approved by the National Committee for Ethics in Research (CONEP) and registered under No. 14097.
Statistical analysisAll results were analyzed using SPSS® for Windows (Inc. Released 2007. SPSS for Windows, version 16.0, USA). Descriptive analysis of the variables were presented as median (minimum and maximum values). The comparison of biochemical evaluation among patients and control group was performed using the Mann–Whitney non-parametric test at p<0.05.
ResultsThe present study included 21 patients with AML and 24 healthy subjects paired in age, with no statistical differences.
Using the French American British (FAB) classification, nine patients were classified as M5; four, as M2; one, as M1; and one, as M7. Six patients did not have the sub-type specified in their medical records.
Albumin and prealbumin serum proteinsTable 1 presents the comparative results of serum albumin and prealbumin levels for AML patients and controls. It was observed that both serum albumin and prealbumin were significantly higher (p<0.0001) in the healthy individuals when compared with the AML patients.
Levels of serum albumin and prealbumin in acute myeloid leukemia (AML) patients and in healthy individuals (control) in the same age range.
Table 2 presents the comparative results for the hematological parameters between AML patients and healthy individuals.
Hematological parameters determined in pediatric acute myeloid leukemia (AML) patients and in healthy individuals (control) in the same age range.
| Parameters | AML (n=21) | Control (n=24) | pa |
|---|---|---|---|
| Leucocytes (cell×109/L) | 13.1b (1.1–494.0) | 6.51b (1.79–15.11) | 0.219 |
| Lymphocytes (cell×109/L) | 2.3 (0.6–58.4) | 2.8 (1.83–8.64) | 0.368 |
| Monocytes (cell×109/L) | 1.3 (0.1–10.6) | 0.45 (0.24–0.91) | 0.308 |
| Granulocytes (cell×109/L) | 4.3 (0.1–48.6) | 3.64 (1.91–5.57) | 0.825 |
| Erythrocytes (cell×1012/L) | 2.55 (1.78–4.36) | 4.82 (4.17–5.4) | <0.0001 |
| Hematocrit (%) | 23.4 (16.6–36.6) | 40.7 (34.1–47.2) | <0.0001 |
| Hemoglobin (g/dL) | 7.7 (5.6–12.5) | 13.55 (11.1–15.5) | <0.0001 |
| Plalelets (cell×109/L) | 38.0 (16–220) | 307.0 (162–461) | <0.0001 |
There was no statistical difference in the median levels of total leucocytes and subgroups (lymphocytes, monocytes and granulocytes) between the two groups. However, the higher values of erythrocytes, hematocrit, hemoglobin and platelets (p<0.0001) in the control group when compared with the AML patients are noteworthy.
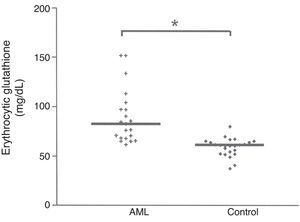
Erythrocytic reduced glutathione (GSH) concentrationThe median GSH concentration in the erythrocyte of AML patients was significantly higher (p<0.0001) than the values measured in healthy subjects, as illustrated in Fig. 1. GSH concentration was 82.88 (61.94–151.77) mg/dL in the AML group, compared to 61.72mg/dL in the control group.
Cytokine concentration in supernatants of PBMC culturesComparative production of cytokines was determined in supernatants of peripheral blood mononuclear cells culture (PBMC) under three different conditions for the AML and control group: spontaneous (culture medium without stimulation); culture medium plus the vaccine BCG; and culture medium plus phytohemagglutinin (PHA). The results, expressed as medium values and the spread (min-max), are shown in Table 3.
Cytokines concentration (pg/mL) determined in supernatants of peripheral blood mononuclear cells cultures of acute myeloid leukemia (AML) patients and healthy subjects (control) in the same age range.
| Cytokines | Group | Conditions | ||
|---|---|---|---|---|
| Spontaneous | BCG | PHA | ||
| TNF-α | AML | 40.491a (0.0–418.9) | 831.47a (41.01–15,825.3) | 683.17a (38.6–1977.2) |
| Control | 22.42 (0.0–316.7) | 4800.42 (1408.7–13,101.1) | 986.61 (87.7–27,882.1) | |
| pb | 0.011 | <0.0001 | 0.052 | |
| IL-6 | AML | 455.641 (0.0–10,328.2) | 4807.2 (41.0–31,186.7) | 3010.4 (54.2–28,250) |
| Control | 736.17 (19.8–5044.4) | 28,961.67 (25,176.7–293,344.6) | 9654.65 (1904–29,051.1) | |
| pb | 0.197 | <0.0001 | 0.002 | |
| IL-10 | AML | 0.0 (0.0–1796.8) | 124.54 (0.0–1783.05) | 393.80 (63.8–1127.9) |
| Control | 0.0 (0.0–119.7) | 615.46 (118.6–2204.9) | 627.52 (61.5–1465.2) | |
| pb | 0.065 | 0.003 | 0.284 | |
| IFN-γ | AML | 23.17 (0.0–70.6) | 98.71 (0.0–1665) | 150.23 (0.0–3913.8) |
| Control | 0.0 (0.0–48.9) | 676.77 (0.0–1935.3) | 87.96 (0.0–630.5) | |
| pb | 0.012 | 0.003 | 0.273 | |
TNF, tumor necrosis factor; IL, interleukin; IFN, interferon.
Under spontaneous condition, TNF-α and IFN-γ concentrations were significantly higher in the AML patients than in healthy subjects. No difference was found between the two groups for IL-6 and IL-10, under this condition.
Under BCG-stimulated condition, all cytokines concentrations (TNF-α, IL-6, IL10, IFN-γ) were significantly higher than in the control group.
In the presence of PHA, only IL-6 concentration was significantly higher (p<0.002) in the control, when compared with the AML group. TNF-α was also higher the control than in the AML group; however, it did not reach statistical significance. No difference was found between the two groups for IL-10 and IFN-γ under this condition.
The concentration of TGF-β and IL-8, which was measured in the blood plasma in both groups, and only in the presence of BCG, did not show statistical difference between the groups (data not shown).
Determination of IgA in salivaImmunoglobulin A (IgA) was determined in the saliva of the subjects in both groups of study.
The concentration of IgA was 6.5 (1–17.4) mg/dL for the AML group and 9.05 (1.8–25.1) mg/dL for the control group, showing no difference (p=0.693) between the groups.
DiscussionSerum proteins have been used as a sensitive indicator for undernutrition. However, other factors not related to nutritional condition may affect the level of serum protein, such asstate of hydration, reduction of hepatic protein synthesis, altered capillary permeability due to infections or zinc deficiency catabolism in periods of stress, and hypermetabolism in certain neoplasias.10,11
The present albumin (4.8g/dL) and prealbumin (21.45mg/dL) results in the control group were statistically higher (p<0.0001) than the values found in the AML patients. These results reflect differences expected in the nutritional state of healthy children and adolescents when compared with age-matched AML patients.
In a study performed on 226 adult patients with cancer, Marín Caro et al.12 found that 32% of the studied population presented serum albumin concentration between 3.0 and 3.5g/dL (mild undernutrition), demonstrating a negative correlation between serum protein levels and feeding problem.
Severely ill patients normally present metabolic alterations in carbohydrates, lipids, and proteins. Such alterations result from increased need of energy and protein catabolism, contributing to immunological and gastrointestinal alterations.13
Regarding the hematological parameters (Table 2), no statistical differences were found between AML patients and healthy subjects for leucocytes, lymphocytes, monocytes, and granulocytes, although the median values tended to be higher in the AML group. This lack of statistical significance might be explained as a function of two factors: (a) the relatively small number of subjects in the present samples; (b) the great variability normally found for white blood cells (leucocytes) in patients with leukemia.5
Viana et al.14 conducted a study on 83 children with AML and also found a wide variation in the number of leucocytes/mL of blood.
In the present study, the total number of leucocytes (13.1×109cells/L) for the AML group can be considered slightly above normal and was normal for the control (6.5×109cells/L), since the reference values range from 4.5 to 10.5×109cells/L.
Conversely, erythrocytes, hematocrit, hemoglobin, and platelets were all significantly higher (p<0.0001) in the control group when compared with the AML group (Table 2). These data appear to explain the strong tendency for anemia in AML patients.
The cell-mediated immunity against tumors can increase by expression of cytokines and co-stimulators in tumoral cells and by treatment of tumors carrying individuals with cytokines, which stimulate the proliferation and differentiation of T lymphocytes and natural killer (NK) cells. IFN-γ and TNF-α are considered efficient antitumoral agents in animal models, but their use in patients is limited due to serious toxic collateral effects. Growth factors, such as granulocytes–macrophages colonies stimulating factor (GM-CSF) and granulocytes colonies stimulating factor (G-CSF) are used in cancer treatment protocols to decrease the periods of neutropenia and thrombocytopenia after chemotherapy or bone medulla autologous transplant.15
One study compared the levels of pro-inflammatory cytokines in adult patients with cancer with a group of healthy subjects (control). It was found that the blood serum levels of IL-1β and TNF-α were higher (p<0.0001) in patients with cancer, when compared with the control group.16
In the present study, the spontaneous production of TNF-α and IFN-γ in supernatants of PBMC was significantly higher in AML patients, suggesting that oxidative stress and inflammation are involved in AML. Conversely, there was no significant difference between groups in production of IL-6 and IL-10, which act as regulatory cytokines.
There is increasing evidence in the literature that the ROS-induced generation of oxidative stress plays a role in leukemia. A strong association between oxidative stress and the incidence of disease relapse was demonstrated by Zhou et al.17 These investigators showed that the activities of adenosine deaminase and xanthine oxidase were higher in AML relapse condition, whereas those of glutathione peroxidase, monoamine oxidase, superoxide dismutase, and the total antioxidant capacity (T-AOC) were lower in the primary condition than in controls. According to these authors, oxidative stress is a crucial feature of AML and probably affects its development and relapses.
In the present study (Table 3), all cytokines investigated showed significantly higher concentrations in the control group when compared with the AML group in the presence of BCG vaccine, a specific antigenic factor, which suggests a lower capacity of AML patients to positively react to antigens. Conversely, in the presence of PHA, a non-specific mitogen stimulator, only IL-6 showed significantly higher concentration in the control (p<0.002) when compared with the AML group. TNF-α, IL-10, and IFN-γ did not show difference between the two groups.
Adequate concentration of glutathione is necessary for normal cell proliferation including lymphocytes and epithelial cells in the intestine.18 Glutathione (GSH) is also essential for the activation of T lymphocytes and polymorphonuclear leucocytes and also for the production of cytokines; therefore it is indispensable for the expression of the immune response in situations of immunological challenges.19 The authors cited above mentioned that the cellular concentrations of GSH are drastically reduced in situations such as protein undernutrition and oxidative stress, and in various pathological conditions, such as protein-energy undernutrition, acquired immunological deficiency (AIDS), and advanced neoplasias.
A study by Russo et al.20 demonstrated that the levels of GSH in cancer cells may be several folds higher than in corresponding normal cells. According to Engin,21 the levels of erythrocytic glutathione were 31% higher in patients with localized carcinoma and 78% higher in patients with metastatic cancer compared with healthy controls. These results may reflect the high levels of GSH in tumors and also that these high levels may be associated with resistance of tumoral cells to chemotherapeutic treatment.
The results of the present are in accordance with those of Russo et al.20 and Engin,21 revealing a higher concentration of GSH in the AML patients (p<0.0001) when compared with the healthy subject group (control), as illustrated in Fig. 1.
Recent published study22 indicated that CD34+ AML cells have elevated expression of multiple glutathione pathway regulatory proteins, presumably as a mechanism to compensate to increased oxidative stress in leukemic cells. Consistent with this observation CD34+ AML cells have lower levels of reduced (GSH) and increased levels of oxidized (GSSG) glutathione. These findings suggest the intrinsic balance and homeostasis and the GSH to GSSG ration is altered (aberrant) in the CD34+ AML cells.
The above cited authors propose that the decreased GSH level is due to higher consumption of GSH in several processes required for cancer cell survival including: (1) reduction of reactive oxygen species, such as H2O2; (2) proper S-glutathionylation of the proteome in response to oxidative stress; and (3) detoxification of increased production of lipid peroxides.
These same authors also presented new agents such as parthenolide (PTL) and piperlongumine (PLM) having a dramatic inhibitory effect on the leukemic GSH system, whereas only a limited and transient perturbation in normal cells. The same group of researchers had previously shown,23 the PTL effectively eradicated AML stem and progenitor cells that are typically resistant/refractory to conventional chemotherapy.
Secretory salivary immunoglobulin A (sIgA) is an important parameter for evaluating the immunological status of the gastrointestinal mucosa, with the advantage of using noninvasive procedure and essentially without discomfort to the patient. It is considered the most important humoral mediator for the mucosa immunity, assisting in a variety of protective mechanisms. It presents higher resistance to proteolytic degradation than any other class of immunoglobulin and can be found in the entire digestive and respiratory tracts, impeding the absorption of a large quantity of antigens.24,25
According to Souza et al.,26 in patients with cancer, the synthesis of antibodies may be decreased or exacerbated depending on the immunological mechanisms involved in the proliferation of tumoral cells, determining an elevation or reduction in the concentrations of immunoglobulin fractions.
In the present study, no statistical difference was observed in the concentration of salivary IgA (p=0.693) between the AML patients and the healthy controls, in spite of detecting higher levels in the patients.
In the comparison of pediatric patients with AML with normal subjects matched for age some conclusions could be drawn: a) the AML group presented significantly lower concentrations of serum albumin and prealbumin, suggesting imminent danger of protein undernutrition: b) the AML patients showed lower values (p<0.0001) for all red blood cells parameters, suggesting a state of anemia in these patients; (c) the statistic lower production of cytokines of AML group under spontaneous and BCG-stimulated conditions appears to indicate a drop in the immunological response of patients compared to the normal subjects; (d) the significantly higher GSH concentration in the erythrocytes of AML patients, compared to controls, may reflect the aberrant glutathione metabolism and homeostasis described in the reference.22
FundingThis study received funding from CNPq.
Conflicts of interestThe authors declare no conflicts of interest.
We thank National Research Council (CNPq), for financial support and for granting a scholarship to the first author.
Please cite this article as: Sanches FL, Nitsch TM, Vilela MM, Sgarbieri VC. Comparison of biochemical and immunological profile of pediatric patients with acute myeloid leukemia in relation to healthy individuals. J Pediatr (Rio J). 2015;91:478–84.
Study conducted at Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil.