To describe aspects of the microcephaly epidemic in the state of Piauí.
MethodsAll cases of congenital microcephaly confirmed in the state between 2015 and 2016 were included (n=100). Investigation forms of the Regional Reference Center for Microcephaly were reviewed. Discarded cases (n=63) were used as a comparison group.
ResultsIn October, November, and December 2015 incidence rates reached 4.46, 6.33 and 3.86/1000 live births, respectively; 44 cases were reported in the state capital. Among the mothers of confirmed and discarded cases, the frequency of skin rash during pregnancy was 50/97 (51.5%) and 8/51 (15.7%), respectively (p<0.001); 33 confirmed cases (35.9%) had a head circumference z-score between −2 and −3, 23 (25%) between −3 and −4, and 8 (8.7%) had a z-score of less than −4. Head computer tomography scans revealed calcifications in 78/95 (82.1%) cases. Lissencephaly, hydrocephalus and agenesis of the corpus callosum were also frequently observed. Ophthalmic findings included retinal pigment epithelium rarefaction and atrophy. Absence of otoacoustic emissions was observed in 21/70 cases. One newborn also presented lower limb muscle atrophy. There were no significant differences in vaccination rates for influenza, diphtheria-tetanus-acellular pertussis, and hepatitis B in either group.
ConclusionsThe state of Piauí, like others in the northeastern region, faced an epidemic of congenital microcephaly between 2015 and 2016, presumably related to congenital Zika virus infection, more intense in the capital. Current challenges include the improvement of vector control, basic research, scaling-up of diagnostic tools for pre-natal screening of Zika virus, vaccines, and health care for affected children.
Descrever os aspectos da epidemia de microcefalia no Estado do Piauí.
MétodosForam incluídos todos os casos de microcefalia congênita confirmados no estado entre 2015-2016 (n = 100). Os formulários de investigação do Centro Regional de Referência em Microcefalia foram analisados. Os casos descartados (n = 63) foram usados como grupo de comparação.
ResultadosEm outubro, novembro e dezembro de 2015, as taxas de incidência atingiram 4,46, 6,33 e 3,86/1.000 nascidos vivos, respectivamente; 44 casos foram relatados na capital do estado. Entre as mães de casos confirmados e descartados, a frequência de erupção cutânea durante a gravidez foi 50/97 (51,5%) e 8/51 (15,7%), respectivamente (p < 0,001); 33 casos confirmados (35,9%) apresentaram um escore z de perímetro cefálico entre -2 e -3, 23 (25%) entre -3 e -4 e 8 (8,7%) apresentaram escore z inferior a -4. As tomografias computadorizadas cerebrais revelaram calcificações em 78/95 (82,1%) dos casos. Lisencefalia, hidrocefalia e agenesia do corpo caloso também foram observadas com mais frequência. Os achados oftalmológicos incluíram rarefação e atrofia do epitélio pigmentar da retina. Foram observadas ausência de emissões otoacústicas em 21/70 casos. Um recém-nascido também apresentou atrofia muscular dos membros inferiores. Não houve diferenças significativas nas taxas de vacinação para gripe, vacina difteria tétano e coqueluche acelular e hepatite B em qualquer grupo.
ConclusõesO Estado do Piauí, como outros na região Nordeste, enfrentou, entre 2015 e 2016, uma epidemia de microcefalia congênita, supostamente relacionada à infecção congênita pelo vírus Zika, mais intensa na capital. Os desafios atuais incluem melhora do controle de vetores, pesquisa básica, ampliação de ferramentas de diagnóstico para exame pré-natal do vírus Zika, vacinas e cuidados de saúde para crianças afetadas.
From mid-2015, a significant increase in the incidence of microcephaly and other central nervous system (CNS) malformations was observed in Brazil, mainly in the northeast region.1
In 2015, Zika virus (ZIKV) was identified by PCR in patients with rash, fever, conjunctivitis, and arthralgia in northeastern Brazil, during the epidemic of an exanthematic disease.2 An association between congenital infection with ZIKV and microcephaly was proposed. The causality was elucidated in several steps: (i) PCR-identification of ZIKV in the amniotic fluid of pregnant women who gave birth to babies with microcephaly3; (ii) the identification of ZIKV nucleic acids by PCR and arbovirus-like particles by electron microscopy in the brain of an aborted microcephalic fetus4; (iii) a case-control study in the state of Pernambuco showing a higher proportion of ZIKV-infection among children with microcephaly when compared to the controls.5
Microcephaly is defined by a head circumference (HC) more than two standard deviations (a z-score<−2) below the mean for gestational age, and severe microcephaly is defined as a HC z-score<−3.6 Microcephaly is a clinical manifestation representing disruption in neurogenesis and death of neuronal progenitors.7
The most well recognized causes of microcephaly include genetic alterations, congenital infections (such as cytomegalovirus [CMV], herpes simplex virus [HSV], rubella virus, Toxoplasma gondii, and syphilis), or embryonic exposure to teratogenic substances.8–10 Congenital Zika is a syndrome characterized by severe microcephaly, decreased brain tissue with a specific pattern of damage, including subcortical calcifications, damage to the back of the eye, congenital contractures, and hypertonia.11 From November 2015 to December 2016, 10,867 cases of congenital microcephaly were investigated in Brazil. Of those, 2366 were confirmed, 49 were classified as probable, 5,269 were found to be non-microcephalic (discarded), and, as of 31 December 2016, 3183 cases remained under investigation. The criteria for confirmation included typical findings of congenital infection, such as cerebral calcifications or ventricular and posterior fossa alterations, among other clinical signs observed by any imaging method or ZIKV-positivity in laboratory tests.12
Of the total number of confirmed cases, 100 were reported in Piauí, northeastern Brazil. The present study aims to describe the clinical, radiological, and epidemiological characteristics of microcephaly cases in the state of Piauí during the 2015–2016 epidemic.
Patients and methodsThe state of Piauí has an area of 251,577.738km2. It has a population of 3,194,718 inhabitants; 847,430 of which live in the state's capital, Teresina. Piauí is the second poorest state in Brazil, with a per capita gross domestic product of 3777.60 USD, and it has the fourth lowest human development index in the country (0.646).
A case series study was performed. All cases of congenital microcephaly confirmed in the state of Piauí between 8 November 2015 and 31 December 2016 were included. Inclusion was contingent on the newborn being submitted to the investigation protocol of the Regional Reference Center for Microcephaly (RRCM) established during the epidemic. At the end of the mentioned period, RRCM investigated 188 newborns; 88 cases were discarded. The RRCM's criteria for case confirmation were the presence of clinical, laboratory, and radiological findings compatible with CNS malformation.12 Monthly incidence rates were calculated as the number of confirmed microcephaly cases×1000/number of live births in the month. Sixty-three of the discarded cases had available data. Discarded cases were used as a comparison group to evaluate some associations between clinical and epidemiological variables and the presence of microcephaly. HC z-scores were calculated for gestational ages with the software Intergrowth-21st, available in http://intergrowth21.ndog.ox.ac/pt/ManualEntry. Fisher's test was employed to compare the frequencies of the categorical variables, and the Kruskal–Wallis test used to compare the medians of the continuous variables. For each comparison, the numbers of confirmed and discarded cases varied, according to the existence of information for each variable.
For geospatial analyses, the base map was acquired from the Brazilian Institute of Geography and Statistics (IBGE). Google Earth® was used to determine the address coordinates of all confirmed and discarded cases. Coordinates were recorded in the WGS 84 Datum (World Geodetic System 1984) geodetic coordinate system. Spatial data were analyzed in a GIS platform using ArcGis 9.3 software (Environmental Systems Research Institute, Redlands, CA, USA). With this analysis, the goal was to describe the geographical distribution of microcephaly cases and show areas with high concentrations of cases or high intensity of cases per unit area. The kernel density technique was applied in order to assess the intensity of incidence per unit area. This technique produces a non-topographical surface displaying the distribution of the disease.
The Research Ethics Committee of the Instituto Oswaldo Cruz/Fundação Oswaldo Cruz approved this study (protocol # 2.121.367).
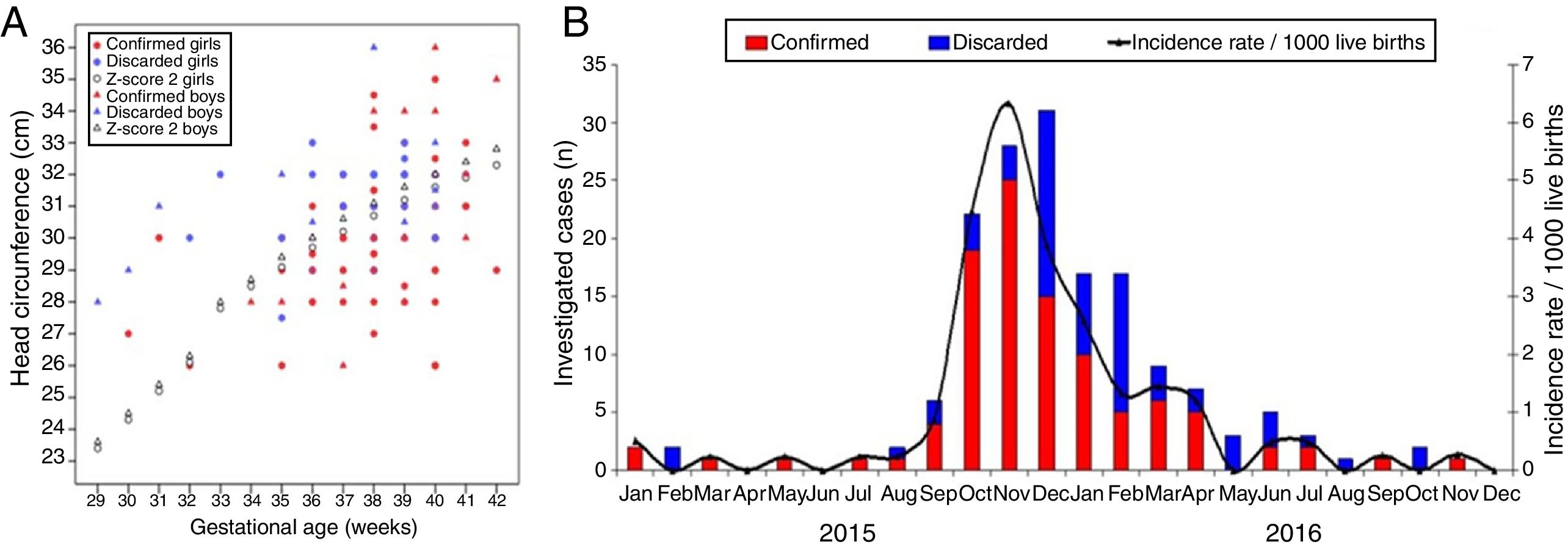
ResultsSpatiotemporal distribution of microcephaly casesFig. 1A demonstrates the epidemic curve of microcephaly, including the initially suspected and subsequently discarded cases. The number of cases increased from September 2015, with 4 cases confirmed. The epidemic peak was observed in November 2015, when 25 infants with confirmed microcephaly were born in the state of Piauí (1/4 of the total confirmations). In 2015, in October, November, and December, almost 60% of the confirmed cases were born. The epidemic has declined since April 2016. During the pre-epidemic period (January to August 2015), the average monthly incidence rate of congenital microcephaly in the state of Piauí was 0.18 cases/1000 live births. In September 2015, the monthly incidence increased five-fold compared to the pre-epidemic period, reaching 0.89/1000 live births. In October, November, and December 2015 there was a highly significant increase, with monthly incidence rates of 4.46, 6.33, and 3.86/1000 live births respectively. During the peak of the epidemic, in November 2015, confirmation rate reached 89%.
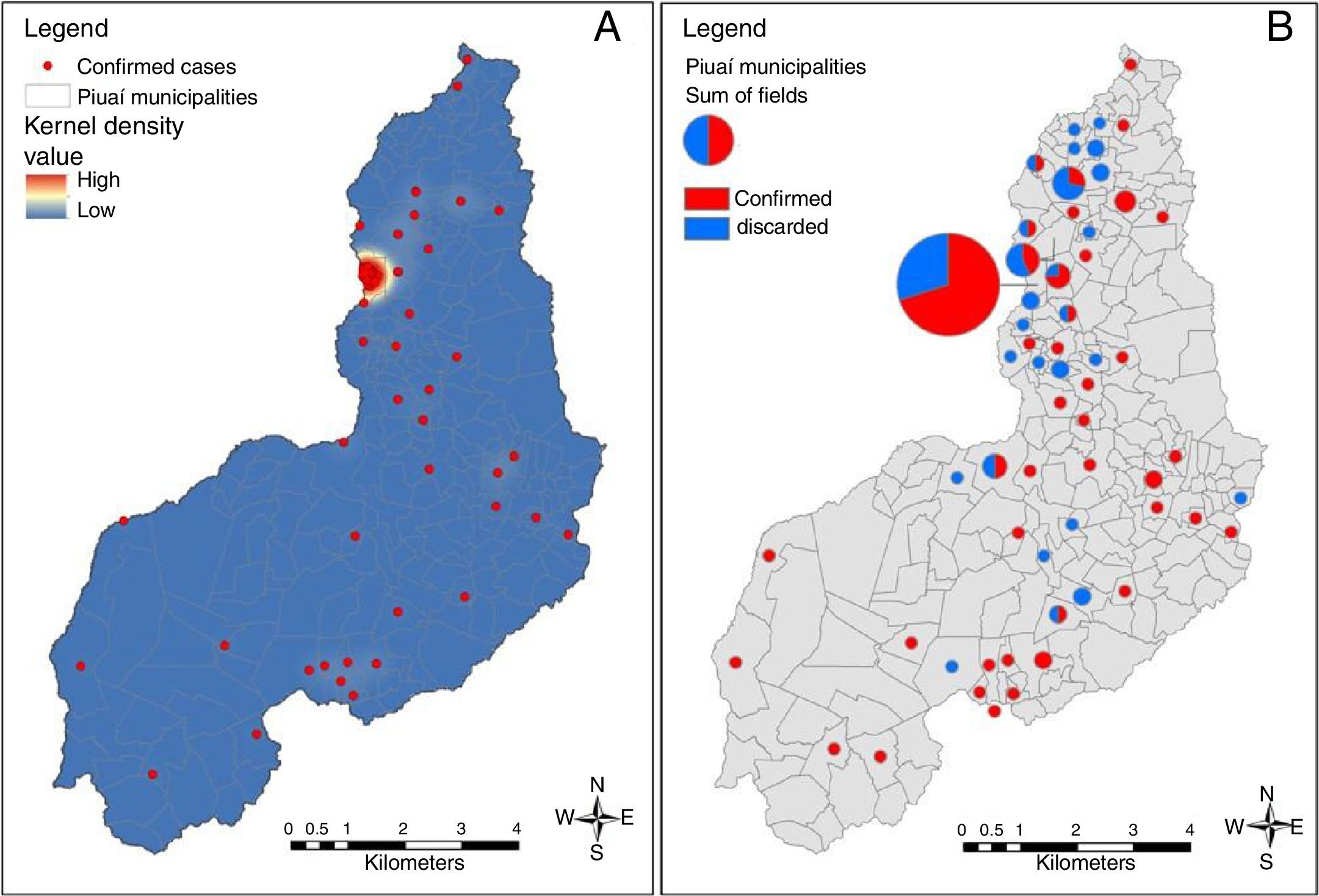
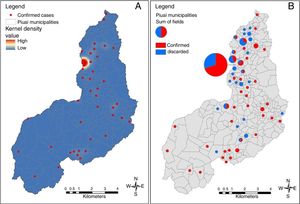
The map in Fig. 2 depicts the spatial distribution of microcephaly cases. Almost half of the cases (44/100 [44%]) were reported in the state capital, the city of Teresina; 46 (20.5%) of the 224 municipalities in the state of Piauí registered at least one confirmed case. Except for Teresina, the maximum number of cases per municipality was 3. Teresina thus represented the most intense area of epidemic development in the state of Piauí. Georeferencing of the confirmed and discarded cases and the kernel density analysis revealed a clustered pattern of confirmed cases in the municipality of Teresina.
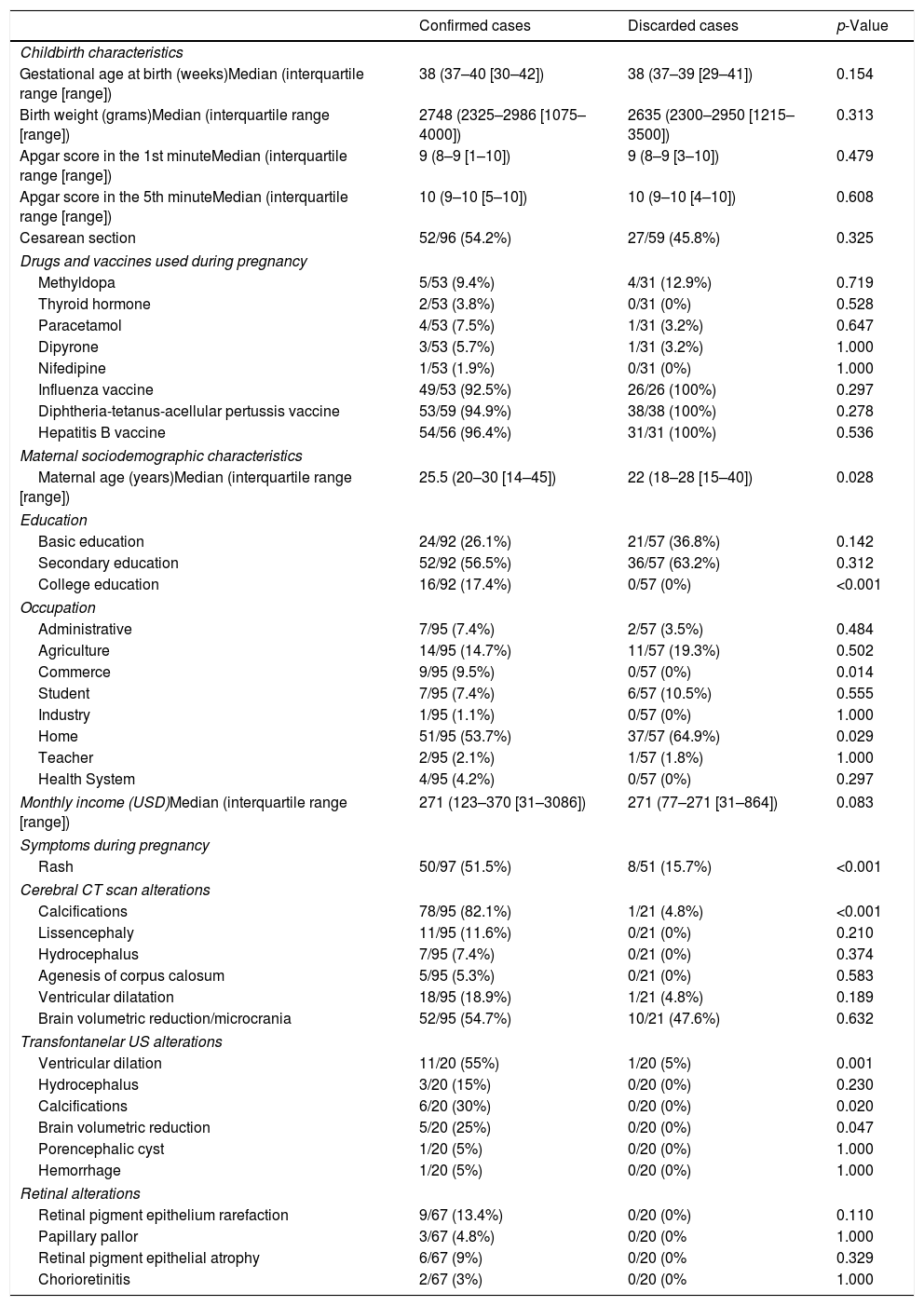
Obstetric and childbirth dataTable 1 presents obstetric and childbirth data. Maternal age was slightly, but significantly, higher in the group of confirmed cases. The median gestational age of 38 weeks at birth shows that, in both groups, term pregnancies predominated, with a small proportion of preterm deliveries. In addition, the birth weight medians were similar between confirmed and discarded cases, with a similar low birth weight frequency and a small proportion of very low birth weight. Perinatal asphyxia was a rare condition among both infants born with microcephaly and discarded cases, as demonstrated by high Apgar scores in the 1st and 5th minutes after childbirth in both groups.
Childbirth, pregnancy and maternal characteristics, radiological findings and retinal (fundoscopic) alterations in confirmed and discarded microcephaly cases in the state of Piauí, Brazil, 2015–2016.
| Confirmed cases | Discarded cases | p-Value | |
|---|---|---|---|
| Childbirth characteristics | |||
| Gestational age at birth (weeks)Median (interquartile range [range]) | 38 (37–40 [30–42]) | 38 (37–39 [29–41]) | 0.154 |
| Birth weight (grams)Median (interquartile range [range]) | 2748 (2325–2986 [1075–4000]) | 2635 (2300–2950 [1215–3500]) | 0.313 |
| Apgar score in the 1st minuteMedian (interquartile range [range]) | 9 (8–9 [1–10]) | 9 (8–9 [3–10]) | 0.479 |
| Apgar score in the 5th minuteMedian (interquartile range [range]) | 10 (9–10 [5–10]) | 10 (9–10 [4–10]) | 0.608 |
| Cesarean section | 52/96 (54.2%) | 27/59 (45.8%) | 0.325 |
| Drugs and vaccines used during pregnancy | |||
| Methyldopa | 5/53 (9.4%) | 4/31 (12.9%) | 0.719 |
| Thyroid hormone | 2/53 (3.8%) | 0/31 (0%) | 0.528 |
| Paracetamol | 4/53 (7.5%) | 1/31 (3.2%) | 0.647 |
| Dipyrone | 3/53 (5.7%) | 1/31 (3.2%) | 1.000 |
| Nifedipine | 1/53 (1.9%) | 0/31 (0%) | 1.000 |
| Influenza vaccine | 49/53 (92.5%) | 26/26 (100%) | 0.297 |
| Diphtheria-tetanus-acellular pertussis vaccine | 53/59 (94.9%) | 38/38 (100%) | 0.278 |
| Hepatitis B vaccine | 54/56 (96.4%) | 31/31 (100%) | 0.536 |
| Maternal sociodemographic characteristics | |||
| Maternal age (years)Median (interquartile range [range]) | 25.5 (20–30 [14–45]) | 22 (18–28 [15–40]) | 0.028 |
| Education | |||
| Basic education | 24/92 (26.1%) | 21/57 (36.8%) | 0.142 |
| Secondary education | 52/92 (56.5%) | 36/57 (63.2%) | 0.312 |
| College education | 16/92 (17.4%) | 0/57 (0%) | <0.001 |
| Occupation | |||
| Administrative | 7/95 (7.4%) | 2/57 (3.5%) | 0.484 |
| Agriculture | 14/95 (14.7%) | 11/57 (19.3%) | 0.502 |
| Commerce | 9/95 (9.5%) | 0/57 (0%) | 0.014 |
| Student | 7/95 (7.4%) | 6/57 (10.5%) | 0.555 |
| Industry | 1/95 (1.1%) | 0/57 (0%) | 1.000 |
| Home | 51/95 (53.7%) | 37/57 (64.9%) | 0.029 |
| Teacher | 2/95 (2.1%) | 1/57 (1.8%) | 1.000 |
| Health System | 4/95 (4.2%) | 0/57 (0%) | 0.297 |
| Monthly income (USD)Median (interquartile range [range]) | 271 (123–370 [31–3086]) | 271 (77–271 [31–864]) | 0.083 |
| Symptoms during pregnancy | |||
| Rash | 50/97 (51.5%) | 8/51 (15.7%) | <0.001 |
| Cerebral CT scan alterations | |||
| Calcifications | 78/95 (82.1%) | 1/21 (4.8%) | <0.001 |
| Lissencephaly | 11/95 (11.6%) | 0/21 (0%) | 0.210 |
| Hydrocephalus | 7/95 (7.4%) | 0/21 (0%) | 0.374 |
| Agenesis of corpus calosum | 5/95 (5.3%) | 0/21 (0%) | 0.583 |
| Ventricular dilatation | 18/95 (18.9%) | 1/21 (4.8%) | 0.189 |
| Brain volumetric reduction/microcrania | 52/95 (54.7%) | 10/21 (47.6%) | 0.632 |
| Transfontanelar US alterations | |||
| Ventricular dilation | 11/20 (55%) | 1/20 (5%) | 0.001 |
| Hydrocephalus | 3/20 (15%) | 0/20 (0%) | 0.230 |
| Calcifications | 6/20 (30%) | 0/20 (0%) | 0.020 |
| Brain volumetric reduction | 5/20 (25%) | 0/20 (0%) | 0.047 |
| Porencephalic cyst | 1/20 (5%) | 0/20 (0%) | 1.000 |
| Hemorrhage | 1/20 (5%) | 0/20 (0%) | 1.000 |
| Retinal alterations | |||
| Retinal pigment epithelium rarefaction | 9/67 (13.4%) | 0/20 (0%) | 0.110 |
| Papillary pallor | 3/67 (4.8%) | 0/20 (0% | 1.000 |
| Retinal pigment epithelial atrophy | 6/67 (9%) | 0/20 (0% | 0.329 |
| Chorioretinitis | 2/67 (3%) | 0/20 (0% | 1.000 |
Exposure to vaccines routinely offered to pregnant women was evaluated. There were no significant differences in vaccination rates for influenza, diphtheria-tetanus-acellular pertussis (DTaP) and hepatitis B in either group. Indeed, the rate of immunization coverage was high both in mothers who gave birth to infants with microcephaly and those of discarded cases.
Among mothers of infants with confirmed microcephaly, 50/97 (51.5%) reported the presence of a skin rash during pregnancy. This number is significantly higher than that observed among mothers of discarded cases (8/51 [15.7%]; p<0.001).
Clinical and radiological characteristics of confirmed and discarded microcephaly casesAmong the confirmed cases in which it was possible to recover the exact values of HC (n=92), it was observed that 33 infants (35.9%) had a HC z-score between −2 and −3, 23 (25%) had z-scores between −3 and −4, and 8 (8.7%) had z-scores less than −4. Fifteen children were born with normal HC (z-score>−2). The median HC among confirmed children was 30cm (interquartile range [IQR]=29–31cm; range [R]=26–36cm), while among discarded cases the median was 31cm (IQR=30.5–32cm; R=27.5–36cm), p<0.001 (Kruskal–Wallis test). Fig. 1B presents the correlation between gestational age and HC of confirmed and discarded cases, discriminating the sex of the newborn. A large proportion of confirmed cases had a HC z-score below −2 for the gestational age. Median HC z-scores among confirmed and discarded cases were −2.51 (IQR=−3.19 to −1.63; R=−5.22 to 1.43) and −1.49 (IQR=−2.01 to −0.91; R=−3.45 to 2), p<0.001 (Kruskal–Wallis test), respectively.
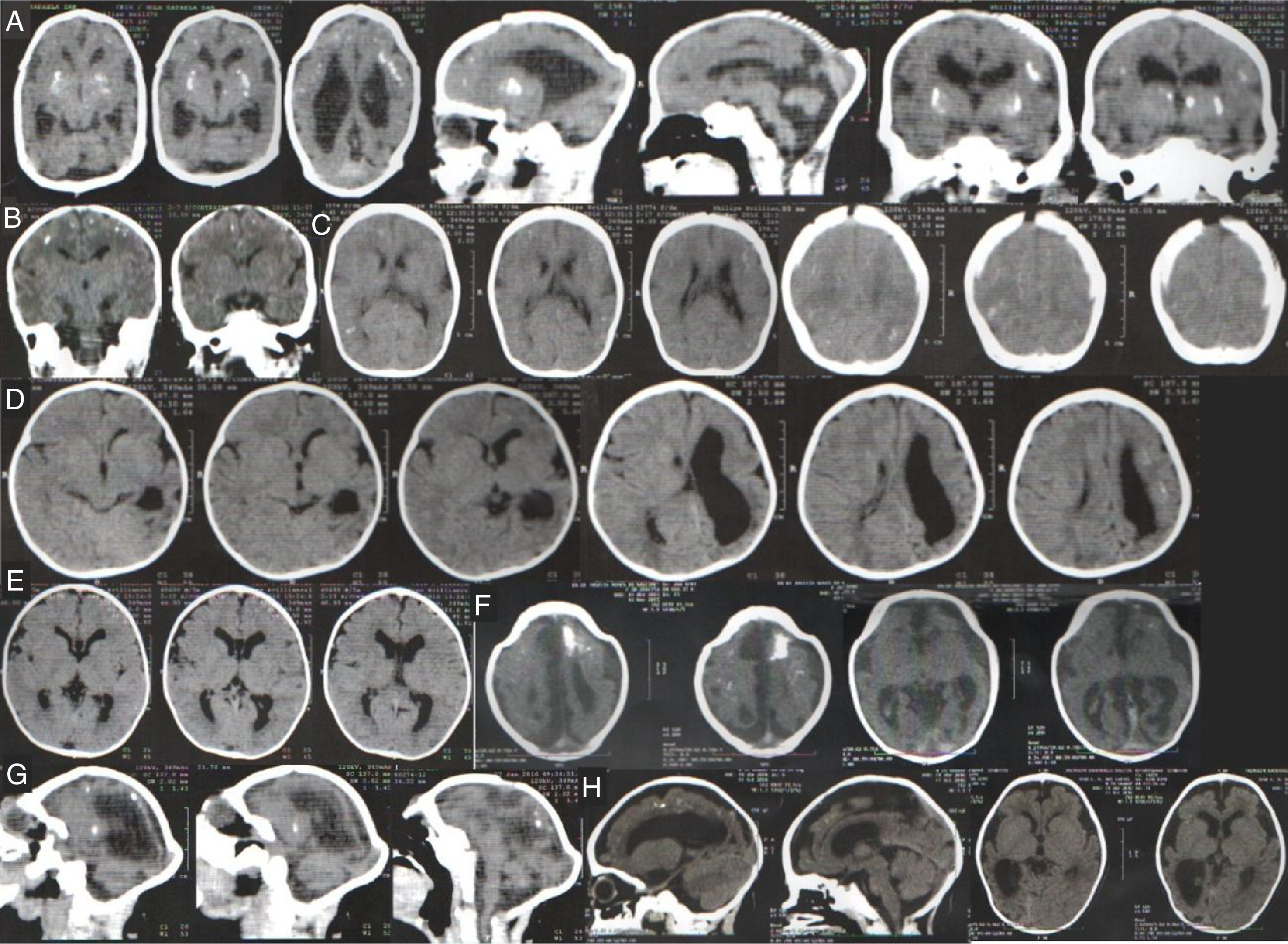
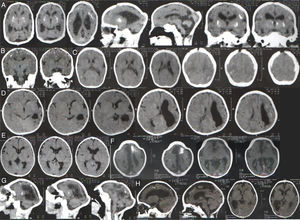
Table 1 summarizes clinical and radiological findings. It was possible to access head computer tomography (CT) scans of 95 infants confirmed with microcephaly and 21 discarded cases. Some radiological findings are presented in Fig. 3. CT scans revealed intracranial calcifications in 78/95 (82.1%) confirmed microcephaly cases and in 1/25 (4.8%) of discarded cases (p<0.001). Among confirmed cases with calcifications, two children had calcifications on basal nuclei (not shown). Ten (10.5%) confirmed cases presented lissencephaly-pachygyria spectrum alterations, 7 (7.4%) presented hydrocephalus, and 5 (5.3%) presented agenesis of the corpus callosum. In addition, 40 infants underwent transfontanelar ultrasound (US) (20 confirmed and 20 discarded cases). Among infants with confirmed microcephaly, 11 (55%) presented dilated cerebral ventricles and 3 (15%) presented a sonographic diagnosis of hydrocephalus. Transfontanelar US detected 6 (30%) children with calcifications.
Head computer tomography scans of confirmed microcephaly cases in the state of Piauí, Brazil, 2015–2016.
(A) Calcifications at the interface between white and gray matter and in the core-capsular regions. Moderate ventricular ectasia associated with parallelism of the lateral ventricles, suggesting dysgenesis of the corpus callosum. Deformity of the skull and volume reduction of the cerebellum due to atrophy, with enlargement of the cerebrospinal fluid space of the posterior fossa.
(B) Small calcifications at the interface between white and gray matter. Scarcity of cortical sulci, configuring an alteration of the spectrum of lissencephaly.
(C) Small calcifications at the interface between white and gray matter, linear and punctiform. Scarcity of cortical sulci, configuring an alteration of the spectrum of lissencephaly.
(D) Small calcifications at the interface between white and gray substances, linear and punctiform. Asymmetry of the cerebral hemispheres with accentuation of the cortical sulci and volumetric reduction of the left hemisphere due to atrophy, with consequent compensatory ectasia of the lateral ventricle.
(E) Slight parallelism of the lateral ventricles, suggesting dysgenesis of the corpus callosum.
(F) Small punctate calcifications at the interface between white and gray matter, and gross calcification in the left frontal lobe. Scarcity of cortical sulci with few shallow sulci, configuring an alteration of the spectrum of lissencephaly. Slight parallelism of the lateral ventricles, suggesting dysgenesis of the corpus callosum. Deformity of the skull in high frontal region.
(G) Small punctate calcifications at the interface between white and gray matter and periventricular region. Scarcity of cortical sulci, configuring an alteration of the spectrum of lissencephaly. Volumetric reduction of the cerebellum. Deformity of the skull.
(H) Small punctate calcifications at the interface between white and gray matter in the right frontal lobe. Scarcity of cortical sulci, in addition to shallow sulci, configuring an alteration of the spectrum of lissencephaly. Expansion of the extra-axial fluidic spaces, more evident in the temporal fossae. Reduction of right hemisphere volume with compensatory ectasia of the right lateral ventricle.
In this series, 87 newborns were submitted to ophthalmologic evaluation (67 confirmed cases and 20 discarded cases). The most frequent fundoscopic findings in confirmed cases were retinal pigment epithelium rarefaction (9/67 [13.4%]), retinal pigment epithelial atrophy (6/67 [9%]), and papillary pallor (3/67 [4.8%]). All examinations of discarded cases were normal. With the objective of neonatal screening for deafness, RRCM evaluated otoacoustic emissions in 91 newborns in the first week of life (70 confirmed and 21 discarded cases). The frequency of absence of otoacoustic emissions was 21/70 (30%) among the confirmed cases and 2/21 (9.5%) among the discarded cases (p=0.051).
Two newborns with microcephaly also presented musculoskeletal involvement and alterations in extremities, including one with lower limb muscle atrophy associated with hip dislocation and one with overlapping fingers. Echocardiography detected cardiac malformations in 2 infants; 1 with patent foramen ovale and ventricular septal defect and 1 with patent foramen ovale and mild pulmonary hypertension.
Serological data related to other congenital infectionsEleven confirmed cases had serological evidence (IgM-positivity) of infection with other teratogenic pathogens, including CMV, herpes and syphilis. Frequencies of IgM-reactivity for rubella, cytomegalovirus, toxoplasmosis, herpes, dengue and chikungunya among confirmed cases were 0/74 (0%), 5/89 (5.6%), 0/81 (0%), 5/79 (6.3%), 8/76 (10.5%), and 6/62 (9.7%), respectively. Among discarded cases, these frequencies were 0/29 (0%), 1/30 (3.3%), 0/31 (0%), 0/22 (0%), 3/23 (13%), and 1/14 (7.1%), respectively. VDRL-positivity among confirmed and discarded cases was 3/84 (3.6%) and 0/28 (0%), respectively. Among CMV-positive microcephaly cases, two had hepatosplenomegaly, jaundice, anemia, and thrombocytopenia.
DiscussionThe present study describes the putative ZIKV-related microcephaly epidemic in the state of Piauí. The epidemic curve shows a concentration of cases during the 8-month period from September 2015 to April 2016. The epidemic peaked in November 2015 when the incidence rate increased almost 15-fold in relation to the pre-epidemic average incidence. This period also corresponds to the highest number of reported cases for all northeastern states. None of the reported cases presented laboratory confirmation for ZIKV infection. It is important to note that, at the time of the epidemic, there were no specific serological tests for ZIKV that could be used to detect IgG or IgM immunoglobulins in newborns or in mothers. Therefore, during the epidemic, laboratory confirmations were based on molecular tests (PCR), which, however, depend heavily on the presence of viremia (i.e. viral nucleic acids), and are therefore not very useful for the diagnosis of infection in the infant after birth, considering that viral infection and replication occur during pregnancy. Among the confirmed cases in the state of Piauí, 68 had blood samples sent for PCR to ZIKV, but all showed negative results. No cerebrospinal fluid sample from affected infants was sent for laboratory testing of viral infections.
It is noteworthy that presumed Zika-related microcephaly exhibited an epidemic behavior pattern, which stands in contrast to the endemic agents with which microcephaly is associated, such as CMV, herpes, and Toxoplasma gondii, which exist in a non-epidemic relationship with the human population. Brazil has a high rubella-vaccine coverage for women, which has thus substantially reduced the incidence of congenital rubella.13 Currently, Brazil experiences an increasing incidence of congenital syphilis.14
The kernel density map shows that the municipality most affected by the Zika outbreak in Piauí throughout the study period was Teresina. Teresina concentrates more than 1/4 of the population of the state and presents serious problems of sanitation, infrastructure, waste management and, consequently, vector control. Another arthropod-borne disease, visceral leishmaniasis, is endemic in the city. From 2007 to 2012, an annual average of 3745 cases of dengue (approximately 50% of cases in the state) has been reported in Teresina, which highlights the local difficulties with mosquito control. On the other hand, the occurrence of microcephaly cases in other municipalities of Piauí illustrates the spread of the microcephaly epidemic in the Brazilian semiarid region, as observed in other states. In northeastern Brazil, the prolonged drought in the 4 years prior to the microcephaly epidemic has led to an increase in sub-standard potable water storage systems, the use of which may have contributed to an increase in mosquito density.
Regarding maternal and obstetric data, full term children predominated among the cases, but with relatively low birth weight for gestational ages. In general, they were children without perinatal asphyxia and therefore unexposed to hypoxia. Consequently, hypoxia cannot serve to explain the neurological impairment of the infants. The vaccination schedule for pregnant women in Brazil includes two doses of the diphtheria-tetanus vaccine (dT), one dose of diphtheria-tetanus-acellular pertussis vaccine (dTaP), two doses of the influenza vaccine, and three doses of the hepatitis B vaccine. Our data demonstrate that the frequency of maternal vaccination with these vaccines was similar among confirmed and discarded cases.
Regarding the clinical presentation, it was observed that the majority of the children had a HC z-score<−2, with about a quarter of cases exhibiting severe microcephaly (HC z-score<−3). Some affected infants had normal HC. The most frequent clinical-radiological picture was microcrania associated with cerebral calcifications, with frequent presentation of ex-vacuo ventricular dilatation, sometimes constituting hydrocephalus and lissencephaly. A more detailed assessment of CNS images of infants with presumed Zika-related microcephaly demonstrated poor gyral development with irregular “beaded” cortex, more consistent with polymicrogyria.15 A small proportion of cases presented musculoskeletal changes, but arthrogryposis did not occur, as observed in other states of the Brazilian northeast.16 The frequency of associated cardiac malformations was also low.
Regarding ocular impairment, more than a quarter of affected newborns presented alterations in the fundoscopic examination consistent with retinal epithelial lesions, which is approximately the same proportion reported in a case series in the state of Bahia, Brazil.17 It was recently demonstrated that congenital ZIKV infection is associated with central retinal degeneration with loss of ganglion cell layer, inner nuclear layer thinning, and photoreceptor loss.18 Screening for hearing loss through otoacoustic emissions has shown that almost 1/3 of affected infants are potentially hearing-impaired. A more detailed audiological evaluation in microcephalic babies born during the epidemic in the state of Pernambuco demonstrated that almost 1/4 failed the first screening test in at least one ear.19
Other congenital infections that could explain the clinical findings were identified in a small number of cases, which were IgM-positive in serological testing for CMV, herpes, and syphilis. In this sense, it is possible that a small proportion of the cases were not caused by congenital infection with ZIKV.
It is interesting to note the explosive nature of the microcephaly epidemic in Brazil and in the state of Piauí. The number of confirmed cases has significantly reduced as of the first half of 2016. The number of cases of Zika fever have also declined throughout Brazil. Thus, the great question as of now is whether congenital Zika syndrome will assume the same epidemiological pattern of other teratogenic infections (such as CMV, toxoplasmosis, and herpes), which produce cases in a more or less stable and endemic way. The probability of Zika fever (and consequently of congenital infections by ZIKV) becoming endemic in Latin America was assessed through mathematical modeling, which has proposed that there is indeed risk that the infection will establish an endemic profile.20
However, the transmission dynamics – and consequently the basic reproduction number – of ZIKV infections are very different from those observed in other arboviruses, due to some biological characteristics: (i) ZIKV can be transmitted directly, person-to-person, sexually and perhaps through saliva21,22; (ii) It is possible that other species of mosquito, such as Culex quinquefasciatus (which has a very high density in practically all urban areas of Brazil), transmit ZIKV23; (iii) ZIKV has only one serotype24; and (iv) The majority of ZIKV infections is asymptomatic.25 These characteristics can lead to a faster induction of herd immunization by natural infection and reduction of the susceptible pool. Thus, the renewal of the population susceptible to ZIKV would require more time, so that the disease would not behave in an endemic manner in the coming years.
In Brazil, ZIKV infections dropped from 170,535 cases in 2016 to 7,911 in 2017. In May 2017, the Brazilian Ministry of Health declared the end of the national emergency for Zika. Currently, the lack of availability of a specific serological test impairs the massive screening of pregnant women in primary health care within the Unified Health System for the congenital infection by Zika. Thus, only pregnant women with a suggestive clinical presentation of ZIKV infection are submitted to molecular testing by PCR.
Current challenges in Brazil include the improvement of vector control (including definition of the role of other mosquito species in transmission), intensification of research to characterize mosquito-independent transmission pathways, development and scaling-up of effective serological diagnostic tools for pre-natal screening, development of vaccines, and improving health care for the affected children.
FundingThis research was supported by funds from the Fundação Oswaldo Cruz (Fiocruz).
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank all of those involved in the care of mothers and children affected by the microcephaly epidemic in the state of Piauí.
Please cite this article as: Almeida IM, Ramos CV, Rodrigues DC, Sousa AC, Nascimento ML, Silva MV, et al. Clinical and epidemiological aspects of microcephaly in the state of Piauí, northeastern Brazil, 2015–2016. J Pediatr (Rio J). 2019;95:466–74.