This study aimed to investigate the relationship between circulating endothelial progenitor cell count and endothelial activation in a pediatric population with obesity.
MethodsObservational and transversal study, including 120 children and adolescents with primary obesity of both sexes, aged 6–17 years, who were recruited at this Cardiovascular Risk Clinic. The control group was made up of 41 children and adolescents with normal body mass index. The variables analyzed were: age, gender, body mass index, systolic and diastolic blood pressure, high-sensitivity C-reactive protein, lipid profile, leptin, adiponectin, homeostasis model assessment-insulin resistance, monocyte chemoattractant protein-1, E-selectin, asymmetric dimethylarginine and circulating progenitor endothelial cell count.
ResultsInsulin resistance was correlated to asymmetric dimethylarginine (ρ=0.340; p=0.003), which was directly, but weakly correlated to E-selectin (ρ=0.252; p=0.046). High sensitivity C-reactive protein was not found to be correlated to markers of endothelial activation. Systolic blood pressure was directly correlated to body mass index (ρ=0.471; p<0.001) and the homeostasis model assessment-insulin resistance (ρ=0.230; p=0.012), and inversely correlated to adiponectin (ρ=−0.331; p<0.001) and high-density lipoprotein cholesterol (ρ=−0.319; p<0.001). Circulating endothelial progenitor cell count was directly, but weakly correlated, to body mass index (r=0.211; p=0.016), leptin (ρ=0.245; p=0.006), triglyceride levels (r=0.241; p=0.031), and E-selectin (ρ=0.297; p=0.004).
ConclusionCirculating endothelial progenitor cell count is elevated in obese children and adolescents with evidence of endothelial activation, suggesting that, during infancy, endothelial repairing mechanisms are present in the context of endothelial activation.
O objetivo deste estudo foi investigar a relação entre os números de células progenitoras endoteliais circulantes e a ativação endotelial em uma população pediátrica com obesidade.
MétodosEstudo observacional e transversal, incluindo 120 crianças e adolescentes com obesidade primária de ambos de sexos, com idades entre 6 e 17 anos, recrutados de nossa Clínica de Riscos Cardiovasculares. O grupo de controle contou com 41 crianças e adolescentes com índice de massa corporal normal. As variáveis analisadas foram: idade, sexo, índice de massa corporal, pressão arterial sistólica e diastólica, proteína C reativa de alta sensibilidade, perfil lipídico, leptina, adiponectina, resistência à insulina para avaliação do modelo de homeostase, proteína quimiotática de monócitos-1, E-seleticna, dimetilarginina assimétrica e números de células endoteliais progenitoras circulantes.
ResultadosA resistência à insulina foi correlacionada a dimetilarginina assimétrica (p=0,340; p=0,003), que foi diretamente correlacionada, porém de forma muita amena à E-seleticna (ρ=0,252; p=0,046). Não constatamos que a proteína C reativa de alta sensibilidade está correlacionada a marcadores de ativação endotelial. A pressão arterial sistólica foi diretamente correlacionada ao índice de massa corporal ρ=0,471; p<0,001) e à resistência à insulina para avaliação do modelo de homeostase (ρ=0,230; p=0,012) e inversamente correlacionada a adiponectina (ρ=-0,331; p<0,001) e lipoproteína de alta densidade-colesterol ρ=-0,319; p<0,001). Os números de células progenitoras endoteliais circulantes foram diretamente correlacionados, porém de forma muito amena ao índice de massa corporal (r=0,211; p=0,016), à leptina (ρ=0,245; p=0,006), aos níveis de triglicerídeos (r=0,241; p=0,031) e à E-seleticna ρ=0,297; p=0,004).
ConclusãoOs números de células progenitoras endoteliais circulantes são elevados em crianças e adolescentes obesos com comprovação de ativação endotelial, sugerindo que, na infância, os mecanismos de reparação endotelial estão presentes no contexto da ativação endotelial.
In obesity, various inflammatory agents, such as C-reactive protein1–3 and leptin,4 disturb the production of nitric oxide via the inhibition of its rate-limiting enzyme, endothelial nitric oxide synthase (eNOS).5,6 Other endogenous substances, such as asymmetric dimethylarginine (ADMA), a competitive antagonist of eNOS, also play a role in further compromising nitric oxide bioavailability. Its production is stimulated by inflammatory agents, such as C-reactive protein.7 Unlike nitric oxide, it can be easily assayed and used as a surrogate for nitric oxide bioavailability.
Damaged endothelium is repaired through the proliferation of adjacent endothelial cells, whose regenerative capacity is limited, and through the migration of progenitor cells, which originate in the bone marrow, known as circulating endothelial progenitor cells (EPCs). They express a variety of cell markers similar to those expressed by vascular endothelium, which display vascular regenerative properties and, as such, participate in new vessel formation.8
In patients with cardiovascular risk factors, such as arterial hypertension and insulin resistance, the count and function of EPCs is reduced. In these, the risk of cardiovascular events is increased.9,10
Inflammatory markers also appears to reduce the number of EPCs, implying a possible role in obesity.11,12
The aims of the present study were two fold. Firstly, to demonstrate that endothelial activation is present in childhood obesity; and secondly, that repairing mechanisms, through EPC activation, are not, at this early stage, compromised.
MethodsSubjectsThe authors conducted an observational and transversal analysis, in a cohort of obese children and adolescents, recruited randomly, and followed-up at a Cardiovascular Risk Clinic. All parents gave their informed consent for the children to participate in the study, which had been approved by the local Ethics Committee.
The inclusion criteria for the study group were primary obesity (body mass index [BMI] above the 95th percentile for sex and age) in children and adolescents aged 6–17 years, without recent or chronic illnesses. The exclusion criteria included secondary causes of obesity, acute infectious or inflammatory disorders (within a month of sampling), and chronic disorders.
The control group included healthy children and adolescents, unrelated to the study group, within the same age range with a normal BMI (percentil 5–85), recruited from the Cardiology Clinic where they were sent for evaluation of murmurs, but proved to have no subjacent cardiac anomalies. All had undergone a 12-h fast prior to the clinical evaluation and blood sampling.
The study group comprised 120 and the control group 41 children and adolescents, of both sexes. Based on this sample size, a significance level (p-value) of 0.05, a power of 0.80, and an effect size of 0.52 were obtained. These values were calculated using the G*Power 3.1.5 program.
The variables analyzed were: age, gender, body mass index, systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, high-sensitivity C-reactive protein (hsCRP), monocyte chemoattractant protein-1 (MCP-1), lipid profile, leptin, adiponectin, homeostasis model assessment-insulin resistance (HOMA-IR), E-selectin, asymmetric dimethylarginine, and circulating EPC count.
Clinical and anthropometric evaluationWeight (in kilograms to the nearest 100g) was determined using a SECA 220® digital weight scale (Medical Scales and Measuring Systems, Hamburg, Germany) and for standing height (in centimeters to the nearest 0.1cm) a stadiometer included in the same equipment was used, with the children wearing only undergarments. Body mass index was calculated based on the formula: BMI=(weight/height2).13 The World Health Organization (WHO) BMI percentile charts were use to define obesity (if BMI >percentil 95; normal weight: BMI between percentil 5–85).
The criteria for metabolic syndrome in children and adolescents were defined according to the International Diabetes Federation concensus.14
Blood collection and biochemical analysisFasting venous blood samples (15mL) were obtained to estimate the hematological parameters. Blood specimens were collected in vacutainer tubes with or without ethylenediaminetetraacetic acid (EDTA) as needed. Serum and plasma were prepared and then frozen (−80°C) for storage until analysis. High-sensitivity C-reactive protein was determined by immunonephelometry from serum samples and processed in the BN ProSpec® System (Siemens Healthcare Diagnostics Inc, Munich, Germany) analyzer (undetected if <0.02mg/dL).
Monocyte chemoattractant protein-1, asymmetric dimethylarginine and E-selectin were determined by ELISA using commercially available enzyme-linked immunosorbent assay kits OptEIA™ (BD Biosciences Pharmingen, CA, USA).
Leptin and adiponectin levels were determined using commercially available enzyme-linked immunosorbent assay kits (eBioscience – San Diego, CA, United States and BioVendor – Brno, Czech Republic, respectively).
Insulin levels were determined by chemiluminescence from serum samples and processed in the IMMULITE 2000® (Siemens Healthcare Diagnostics Inc, Munich, Germany) analyzer and glucose levels were determined from plasma samples analyzed in the VITROS 5.1 FS® (Ortho Clinical Diagnostics, Johnson & Johnson, NY, USA) system by microslide technology.
The HOMA-IR was calculated based on the formula; HOMA-IR=insulin (mU/L)×glucose (mmol/L)/22.5, considering 3 as the cut-off value for the diagnosis of insulin resistance.15
Total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) concentrations were measured using an automated biochemical analyzer VITROS 5.1 FS® (Ortho Clinical Diagnostics, Johnson & Johnson, NY, USA).
For determination of circulating EPC count, peripheral blood (PB) was collected in EDTA tubes, stored on ice, and processed within 2h. Identification and characterization of circulating EPC was performed using an anti-CD146 conjugated with flourescein isothiocyanate (clone: P1H12, Becton Dickinson (BD), CA, USA), anti-KDR conjugated with phycoeritrin (clone: 89106, R&D System, Headquarters, Minneapolis, USA), anti-CD34 peridinin chlorophyll protein cyanine 5.5 (clone: 8G12, BD Bioscience, CA, USA), anti-CD133 allophycocyanin (clone: 293C3, Miltenyi Biotec, Bergisch Gladbach, Germany), and CD45 krome orange (clone: J.33, Immunotech – Beckman Coulter, Marseille, France) combination of monoclonal antibodies (mAb).
For sample staining, a direct immunofluorescence technique was used. Data acquisition was performed in a FACSCanto II® flow cytometer (BD Biosciences Pharmingen, CA, USA) using the FACSDiva® software (BD Biosciences Pharmingen, CA, USA). For EPC identification, the modified International Society for Hematotherapy and Graft Engineering (ISHAGE) sequential gating strategy proposed by Schmidt–Lucke was followed.
Circulating EPCS were identified according to a minimal antigenic profile that includes at least one marker of stemness/immaturity (CD34 and CD133), plus at least one marker of endothelial commitment (KDR and CD146). CD45 staining was used to exclude leukocytes.
For data analysis, the Infinicyt™ software, V.1.5 (Cytognos SL, Salamanca, Spain) was used.
Statistical analysisThe data was analyzed using the IBM SPSS 20 software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, NY, USA). The descriptive analysis of the parametric variables was done by calculating the mean±standard error of the mean. Student's t-test and Mann–Whitney U test were used to calculate the differences in the demographic, clinical, and hematological parameters between the obese and control groups, depending on the normality of distribution. For categorical response variables, differences between the two groups were assessed using the chi-squared test. Logistic regression was used when clinical variables were controlled between the two groups. To establish the correlation between the parameters in the obese group, Spearman's and Pearsons's correlations were used. The results were considered statistically significant at p<0.05.
ResultsComparative analysis between the obese and control groupsOne hundred and twenty obese children and adolescents, 61 boys and 59 girls, with ages between 6 and 17 years (mean age 11.65 years±2.96) were included in the study. The control group was made up of 41 healthy, non-obese children and adolescents, 29 boys and 12 girls, within the same age group (mean age 12.73 years±2.77).
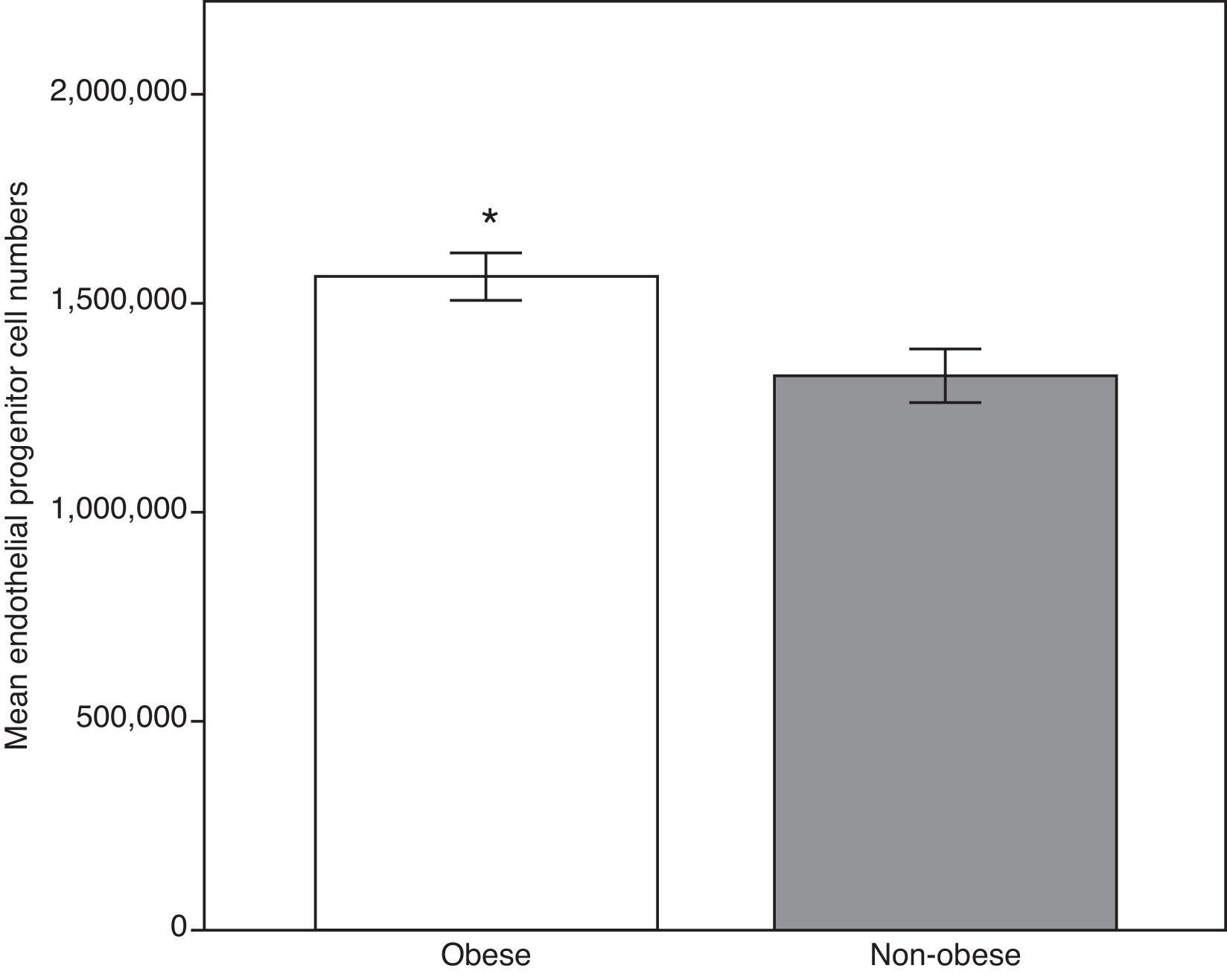
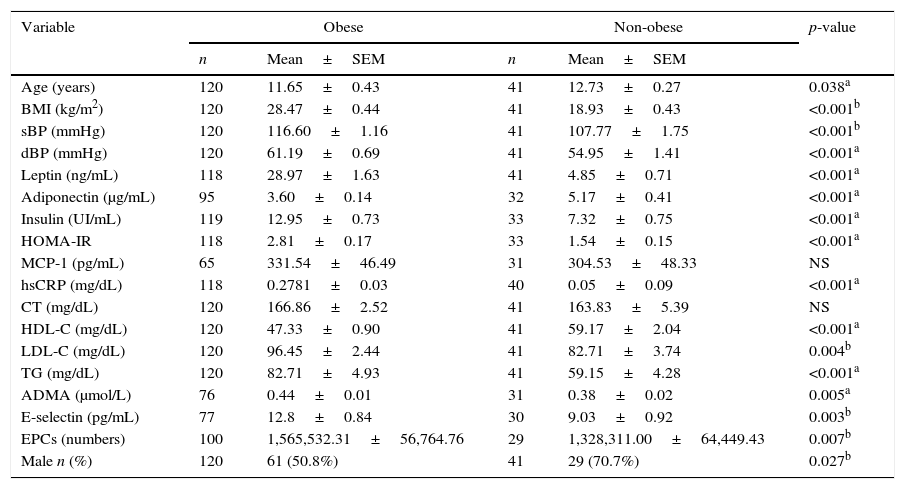
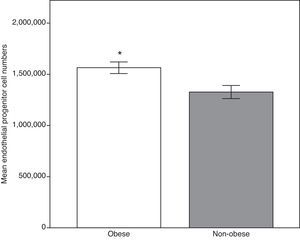
We firstly compared the two group's anthropometric, clinical and analytical parameters. As demonstrated in Table 1, all the means of the analyzed parameters were significantly higher in the obese group, including E-selectin, ADMA and circulating EPCS, except for adiponectin and HDL-C (Fig. 1). Total cholesterol and monocyte chemoattractant protein-1 showed no significant statistical differences.
Anthropometric, clinical, and analytical parameters of the obese and non-obese groups.
| Variable | Obese | Non-obese | p-value | ||
|---|---|---|---|---|---|
| n | Mean±SEM | n | Mean±SEM | ||
| Age (years) | 120 | 11.65±0.43 | 41 | 12.73±0.27 | 0.038a |
| BMI (kg/m2) | 120 | 28.47±0.44 | 41 | 18.93±0.43 | <0.001b |
| sBP (mmHg) | 120 | 116.60±1.16 | 41 | 107.77±1.75 | <0.001b |
| dBP (mmHg) | 120 | 61.19±0.69 | 41 | 54.95±1.41 | <0.001a |
| Leptin (ng/mL) | 118 | 28.97±1.63 | 41 | 4.85±0.71 | <0.001a |
| Adiponectin (μg/mL) | 95 | 3.60±0.14 | 32 | 5.17±0.41 | <0.001a |
| Insulin (UI/mL) | 119 | 12.95±0.73 | 33 | 7.32±0.75 | <0.001a |
| HOMA-IR | 118 | 2.81±0.17 | 33 | 1.54±0.15 | <0.001a |
| MCP-1 (pg/mL) | 65 | 331.54±46.49 | 31 | 304.53±48.33 | NS |
| hsCRP (mg/dL) | 118 | 0.2781±0.03 | 40 | 0.05±0.09 | <0.001a |
| CT (mg/dL) | 120 | 166.86±2.52 | 41 | 163.83±5.39 | NS |
| HDL-C (mg/dL) | 120 | 47.33±0.90 | 41 | 59.17±2.04 | <0.001a |
| LDL-C (mg/dL) | 120 | 96.45±2.44 | 41 | 82.71±3.74 | 0.004b |
| TG (mg/dL) | 120 | 82.71±4.93 | 41 | 59.15±4.28 | <0.001a |
| ADMA (μmol/L) | 76 | 0.44±0.01 | 31 | 0.38±0.02 | 0.005a |
| E-selectin (pg/mL) | 77 | 12.8±0.84 | 30 | 9.03±0.92 | 0.003b |
| EPCs (numbers) | 100 | 1,565,532.31±56,764.76 | 29 | 1,328,311.00±64,449.43 | 0.007b |
| Male n (%) | 120 | 61 (50.8%) | 41 | 29 (70.7%) | 0.027b |
BMI, body mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment-insulin resistance; MCP-1, monocyte chemoattractant protein-1; hsCRP, high-sensitivity C-reactive protein; CT, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglycerides; ADMA, asymmetric dimethylarginine; EPCs, circulating endothelial progenitor cell count; NS, non-significant p-value; n, sample number; SEM, standard error of the mean.
Endothelial progenitor cell count in the obese and the non-obese groups. The higher levels in the obese group may reflect the response by repairing mechanisms secondary to early endothelial activation. Data expressed as means±standard error of the mean. Obese group, n=100; non-obese group, n=29. *Student's t-test, p<0.01 vs. non obese group.
As age (p=0.038) and gender (p=0.027) were significantly different between the two groups, the analysis was repeated using logistic regression, and the results did not differ significantly, namely, MCP-1 (p=0.334), ADMA (p=0.005), E-selectin (p=0.008), and EPCs (p=0.043). Total cholesterol and MCP-1 continued to show no significant differences.
Circulating EPC count were directly, but weakly correlated, to BMI (r=0.21; p=0.016), leptin (ρ=0.25; p=0.006), triglyceride levels (r=0.24; p=0.031), and E-selectin (ρ=0.30; p=0.004).
Analysis of the obese groupBody mass index was directly and moderately correlated to MCP-1 (ρ=0.51; p<0.001), systolic blood pressure (ρ=0.47; p<0.001), leptin (ρ=0.40; p<0.001), and HOMA-IR (ρ=0.35; p<0.001); weakly correlated to hsCRP (ρ=0.22; p=0.018); and inversely but weakly correlated to adiponectin (ρ=−0.28; p=0.007) and HDL-C (ρ=−0.26; p=0.004).
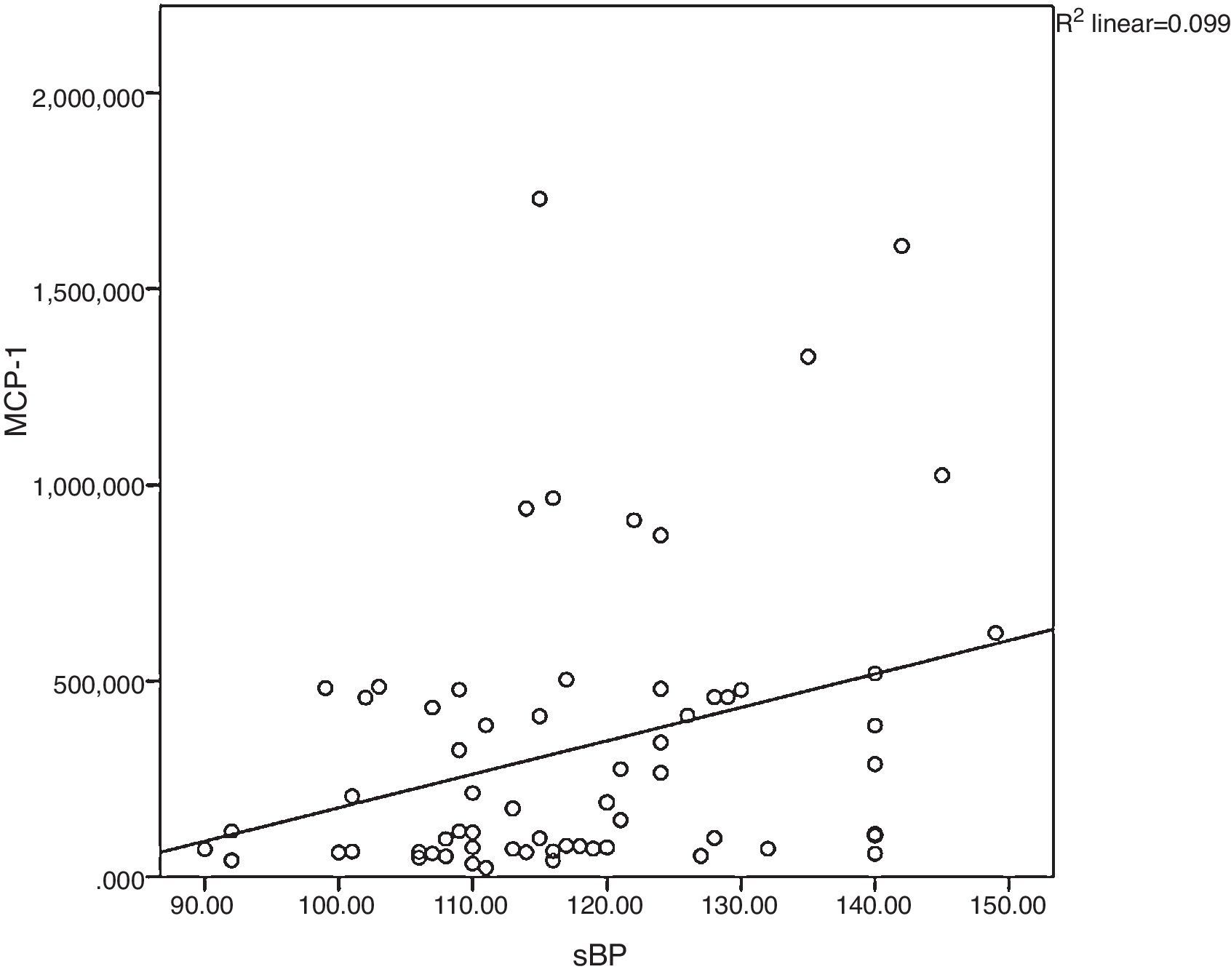
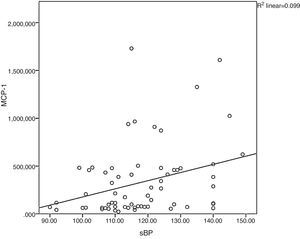
Monocyte chemoattractant protein-1 was directly correlated to systolic blood pressure (ρ=0.34; p=0.005), as shown in Fig. 2. High sensitivity C-reactive protein was weakly correlated to leptin (ρ=0.32; p<0.001) and LDL-C (ρ=0.23; p=0.011).
Correlation between monocyte chemoattractant protein-1and systolic blood pressure. In obesity, cardiovascular risk factors cluster and contribute to the inflammatory process, as observed in the correlation between systolic blood pressure and MCP-1 in the present study. sBP, systolic blood pressure; MCP-1, monocyte chemoattractant protein-1.
Systolic blood pressure was inversely correlated to adiponectin (ρ=−0.33; p=0.001) and directly correlated to HOMA-IR (ρ=0.23; p=0.012) and, as expected, moderately correlated to age (ρ=0.50; p<0.001). Diastolic blood pressure had less pronounced correlations.
The HOMA-IR was correlated to ADMA (ρ=0.34; p=0.003) providing the most direct link to a marker of endothelial activation. This was evident particularly at HOMA-IR values above 3 (p=0.003), the cut-off value we used to define insulin resistance. It was also directly correlated to leptin (ρ=0.39; p<0.001), systolic blood pressure (ρ=0.23; p<0.012), and diastolic blood pressure (ρ=0.20; p<0.033), and inversely correlated to adiponectin (r=−0.21; p=0.040).
Metabolic syndrome was observed in 11.7% (n=14) of the obese patients. As expected, being part of the definition, systolic (p=0.005), but not diastolic blood pressure (p=0.728), was elevated, as were triglyceride levels (p<0.001), HDL-C was lower (p=0.007), and no significant differences were observed in glucose levels. Regarding the markers of endothelial activation and repair mechanisms, ADMA was directly, but weakly correlated to E-selectin (ρ=0.25; p=0.046), and the latter was weakly correlated to circulating EPC count (ρ=0.22; p=0.047).
DiscussionThe present study clearly demonstrates that, unlike their lean counterparts, obese children and adolescents with evidence of ongoing low-grade inflammation and endothelial activation have raised EPC count. Correlations between adiposity, inflammatory markers, and obesity-related comorbidities such as arterial hypertension and insulin resistance were also observed.
Monocyte chemoattractant protein-1 regulates migration and infiltration of monocytes and macrophages16 into the vascular wall and plays an important role in inflammation-mediated diseases, such as obesity.17 As scant translational research has been done in this field, the authors were interested in evaluating the relationship of MCP-1 to obesity related comorbidities. Unlike the findings by Breslin et al.,18 no differences were observed in MCP-1 levels between the obese and control groups nor what an association with dyslipidemia retrieved (data not shown), possibly due to a different sample size (39 obese children and adolescents) and ethnicity (American-Mexican). However, in the obese group, MCP-1, unlike hsCRP, had a direct correlation with systolic blood pressure. This trend was not found in the control group. It is, thus tempting to postulate that in childhood obesity, MCP-1 contributes to arterial hypertension, and implicitly is implicated in the pathophysiological mechanisms leading to cardiovascular disease later in life. Based on the present evidence, MCP-1, compared to hsCRP, would appear to be a more robust cardiovascular risk marker. However, due to the sample size, further research needs to be done in this field. To the best of the authors’ knowledge, these associations have not been previously described.
The prevalence of arterial hypertension in childhood varies from 3% to 5%.19 The obese group had significantly higher systolic and diastolic blood pressure values (p<0.001) than the control group. The prevalence of arterial hypertension in the obese group was 9.2%, and almost a third had pre-hypertension (26.7%). Apart from MCP-1, and in agreement with the findings by Moser et al.,20 systolic blood pressure was directly correlated to BMI and inversely related to adiponectin and HDL-C. This cluster of mediators correlates visceral adiposity, inflammation, insulin resistance, and hypertension, some of the components of the metabolic syndrome, found in 11.7% of the obese group. A particular interesting finding was the inverse correlation between adiponectin and arterial hypertension, thus corroborating the findings of the study by Brambilla et al.,21 in which adiponectin was proposed as one of the mechanisms related to hypertension in childhood obesity. This implies that the loss of its antiatherogenic and anti-inflammatory properties contribute to the underlying mechanisms of obesity-related hypertension.
Furthermore, the loss of adiponectin's insulin sensitizing properties that occurs in obesity has also been implicated in insulin resistance.22 Unlike the findings by Lee et al.,23 where adiponectin was found to be a strong independent predictor of insulin sensitivity in obese children and adolescents of different racial backgrounds, in the present study adiponectin was shown to be correlated to hyperinsulinaemia (r=−0.23; p=0.033), but not to insulin resistance (n=38%; p=0.277), despite striking differences in adiponectin levels between the obese and control groups (p<0.001).
The present study included only white subjects from a different geographical area, which, together with the sample size (n=45 with HOMA-IR >3), might account for the observed differences, although adiponectin gene polymorphisms might also play a role. This hypothesis is merely speculative, as it was not tested in the present study. However, this rationale could not be applied to leptin, which had a stronger correlation to hyperinsulinaemia (r=0.35; p<0.001) and insulin resistance (p<0.001), findings in accordance with previously published works.24 A connection between MCP-1 (p=0.168), hsCRP (p=0.375), and insulin resistance was not observed.
The authors further attempted to relate ADMA and E-selectin, both markers of endothelial activation, to insulin resistance. In adults, raised levels of asymmetric dimethylarginine are considered a cardiovascular risk marker, and as a surrogate of nitric oxide bioavailability, reflects endothelial integrity. A direct correlation between HOMA-IR and ADMA (r=0.35; p=0.003) was observed, particularly evident in the insulin-resistant group (p=0.003), findings not previously reported. Some reports have suggested that ADMA contributes to insulin resistance as decreased nitric oxide bioavailability reduces blood flow to insulin sensitive tissues compromising glucose uptake in response to insulin.25 Conversely, insulin resistance itself has been found to contribute toward endothelial dysfunction. Under these circumstances, it is tempting to speculate on ADMA's permissive role on the interplay between insulin resistance and endothelial dysfunction, through its actions on nitric oxide bioavailability. A direct correlation between leptin and ADMA was also observed, but only in girls. This is not surprising, as leptin levels were significantly higher in obese girls (p<0.001). Nevertheless, this finding, not previously reported, potentially links leptin, and hence adiposity, to endothelial activation. Comparatively, linear regression by logarithmic transformation showed that insulin resistance had a greater influence on ADMA.
E-selectin is a specific endothelial adhesion molecule whose levels are raised in endothelial dysfunction, promoting the migration of inflammatory cells to the intima. In this analysis, obese children and adolescents had significantly higher levels than healthy controls, implying the presence of endothelial activation, which indicates an early stage of atherosclerosis. Considering the entire cohort, a direct, but weak correlation to hsCRP (ρ=0.21; p=0.033) was found, reinforcing the role of inflammation in endothelial dysfunction and in agreement with published literature,26 but a similar pattern was not demonstrated within the obese group, possibly due to its homogeneity. We also observed, within the obese group, a direct correlation between ADMA and circulating EPC count. We found these correlations particularly interesting as they highlight various pathophysiological pathways related to endothelial activation, namely, inflammatory cell adhesion triggered by E-selectin, increased expression of ADMA, who competitively inhibits eNOS, reducing nitric oxide bioavailability and, ultimately, repair of injured tissue by EPCS. The authors believe that the relationship between E-selectin and ADMA, in childhood obesity, has not been previously published.
The term EPCs should be reserved for a progenitor cell restricted specifically to the endothelial lineage. In 1997, Asahara et al.27 reported for the first time on the existence of EPCs. To date, no specific marker has been found to clearly identify these cells, which makes their isolation controversial, as their identification is based on cell surface markers shared by other hematological cell lines. As such various methods have been used to identify EPCs. In order to minimize false positives various cell surface markers have been integrated to identify these cells, namely those linked to immature cell lines (e.g. CD34) with endothelial commitment (e.g. kinase insert domain receptor).
As reported by various authors, the count and function of circulating EPCS not only correlate inversely with cardiovascular risk factors, but also may predict the occurrence of cardiovascular events, particularly in the adult population.28 Data regarding EPCs and childhood obesity is scarce. A report by Jung et al. 29 observed a direct correlation to E-selectin. Contrary to the present study, this correlation only applied to obese adolescents. In the present study, EPCs and E-selectin were directly correlated in the pre-adolescent obese group, a finding not previously reported and that reinforces the fact that endothelial dysfunction is present in very young individuals.
In this analysis, the obese group had a significantly higher number of EPCs, implying that elevated EPCs in obese children and adolescents represent an attempt at repairing underlying vascular damage. Thus, at this stage, it would be possible to reverse the underlying endothelial damage if steps are taken to control weight.
The main strength of the present study is that it demonstrates that obese children and adolescents are potentially at risk for cardiovascular events during early adulthood due to the presence of an adverse proatherogenic profile. However, it appears that, at this stage, repairing mechanisms may be at play to reverse potentially ongoing vascular damage. The inference is that therapeutic interventions aimed at weight loss ought to be aggressively instituted in order to reverse endothelial damage and obesity-related comorbidities, such as arterial hypertension and insulin resistance.
This study has several limitations. Firstly its sample size, particularly its control group. It also only included a white-only population, limited to a particular geographical area, the former due to local race distribution. The study included both genders, as well as, different age groups. In the results, these differences were adjusted for, and therefore their interference was nullified.
Conflict of interestThe authors declare no conflicts of interest.
Please cite this article as: Pires A, Martins P, Paiva A, Pereira AM, Marques M, Castela E, et al. Circulating endothelial progenitor cells in obese children and adolescents. J Pediatr (Rio J). 2015;91:560–6.
Study linked to the Hospital Pediátrico, Centro Hospitalar e Universitário de Coimbra (CHUC); Laboratório de Fisiologia and Laboratório de Estatística, Instituto de Imagem Biomédica e Ciências da Vida, Faculdade de Medicina, Universidade de Coimbra; and to the Instituto Português do Sangue e Transplantação, Coimbra, Portugal.