Fructooligosacharides and galactooligosacharides soften fecal bolus and increase frequency of depositions when added to infant formula. This study aimed to determine the effects of galactooligosaccharide in pediatric patients with chronic constipation.
MethodsFrom 2010 to 2012, 20 constipated patients (4–16 years of age) attended to at a primary healthcare unit were enrolled in a double-blinded, placebo-controlled crossover trial. Eleven children ingested galactooligosaccharide (1.7g) for 30 days, followed by a 15-day washout period, and a 30-day period of placebo (maltodextrin). Nine patients ingested maltodextrin for 30 days, followed by 15-day washout period, and galactooligosaccharide (1.7g) for 30 days. Constipation symptoms were considered as primary outcomes: bowel movements/week, straining during defecation, and stool consistency. Outcome symptoms were ranked according to a numerical scale elaborated for this study. Data were recorded at baseline, and on days 15 and 30 of each 30-day crossover period. Repeated-measures analysis of variance (ANOVA) was used to analyze symptoms along time.
ResultsAt baseline, there was no significant difference in symptoms severity between groups (p=0.45). Galactooligosaccharide ingestion was related to increase of the bowel movement frequency, p<0.0001; relief of defecation straining, p<0.0001; and decrease in stool consistency, p=0.0014, compared to placebo ingestion. Patients reported no side effects from galactooligosaccharide.
ConclusionGalactooligosaccharide was effective at improving clinical symptoms in this group of constipated children.
A adição de frutooligosacarídeos e galactooligosacarídeos a fórmulas infantis pode diminuir a consistência fecal e aumentar a frequência das evacuações. O objetivo do presente estudo foi determinar o efeito do galactooligosacarídeo em crianças com constipação crônica.
MétodosEntre 2010 e 2012, 20 pacientes constipados (4-16 anos), atendidos numa unidade básica de saúde, completaram ensaio clínico duplo cego, placebo-controlado e de delineamento crossover. Onze pacientes receberam galactooligosacarídeo (1,7g) por 30 dias, seguidos por 15 dias de washout, e, após, placebo (maltodextrina) por 30 dias; outros nove pacientes receberam placebo 30 dias, seguidos de 15 dias de washout e 30 dias de galactooligosacarídeo (1,7g). Os desfechos primários foram frequência semanal de evacuações, esforço evacuatório e consistência fecal, classificada por escala numérica elaborada para esse estudo e compilada no primeiro, 150 e 300 dias de cada período de crossover. Análise estatística foi feita por método de análise de variância (ANOVA) para medidas repetidas.
ResultadosIntensidade dos sintomas nos grupos foi semelhante no início do estudo (p=0,45). Durante a ingestão de galactooligosacarídeo constatou-se maior frequência de evacuações, p<0,0001, menor dificuldade evacuatória, p<0,0001 e diminuição da consistência fecal, p=0,0014. Efeitos colaterais não foram referidos durante a ingestão do prebiótico.
ConclusãoDurante a ingestão de galactooligosacarídeo os sintomas clínicos da constipação em crianças e adolescentes foram significantemente aliviados.
Data from the last decades have indicated childhood constipation as a common problem worldwide. Its reported prevalence has varied from 0.7% to 30%; in addition, in recent years, the number of patients has grown significantly in western world.1,2 Different therapeutics have been recommended for constipation management, including stool lubricants, dietary fiber supplementation, laxatives, prokinetics, and functional foods. Osmotic laxatives and dietary fibers are the most widely used therapeutic tools; however, there are very few evidence-based studies to support any treatment recommendations for constipation in pediatric patients.3,4
Functional foods containing probiotics or prebiotics have been identified as useful for regulating bowel habits in children.5 A multicenter controlled trial showed that consumption of fermented dairy products containing Bifidobacterium lactis was associated to increase in stool frequency in children with constipation and stool frequency <3 times/week.6
Concerning the effects of prebiotics on laxation, studies conducted in pediatric patients have included predominantly infants fed exclusively on milk formulas. Ingestion of prebiotics was proposed to be effective for treating constipation, since consumption of fructooligosaccharides and galactooligosaccharides added to infant formula has been shown to increase fecal bolus and the frequency of depositions.7–10
The rationale of prebiotics’ therapeutic effects on constipation is based on the fact that 4′galactooligosaccharide (GOS) affects the host health by stimulating the growth and/or activity of colonic bifidobacteria.11 Bifidobacteria, mainly Lactobacillus acidophillus or Bifidobacterium bifidum, act by fermenting carbohydrates, producing short chain fatty acids (SCFAs), the major anion on the large intestine; SCFAs are able to increase colonic blood flow and muscular activity, enhancing fecal wet weight and thus promoting laxation.12,13
The current study was aimed at evaluating the effectiveness of the prebiotic GOS in the treatment of constipation in children and adolescents.
MethodsDesign: An interventional, non-randomized, double-blinded, placebo-controlled, crossover assignment study was conducted from June 8, 2010 to March 25, 2012.
Setting: A primary healthcare unit managed by medical school staff.
Patients: Subjects aged 4–16 years old who spontaneously sought medical care and, when eligible after initial examination, were invited to participate in the study.
Eligibility criteriaInclusion criterion: Diagnosis of constipation based on the Rome III criteria for functional disorders.14 Furthermore, in order to exclude lactose intolerance, to be enrolled patients were required to report a daily ingestion of at least 250mL of cow's milk without referring symptoms, and they were asked specifically about pain, cramping, diarrhea, or flatulence after milk ingestion.
Non-inclusion criterion: Patients with other comorbid conditions, those already under treatment for constipation, and those who used antibiotics or probiotics for the last 6 months.
Study productsTest product: The prebiotic GOS was produced from lactose through the action of β-galactosidase enzymes, produced by the microorganism Scopulariopsis sp.15 A daily 6mL volume of prescribed maltodextrin solution (placebo) and GOS was ingested in the morning. In the GOS mixture, a volume of 6mL containing 1.7g GOS was used. Parents/guardians and participants were informed of possible GOS ingestion side effects, such as abdominal distention, flatulence, abdominal cramping, and diarrhea.
Control: The placebo consisted of a maltodextrin solution.
BlindingThe two treatments, prebiotic GOS and placebo, were identical in viscidity, color, smell, taste, and packaging. All research staff and patients involved were unaware of the treatment administered to the patient.
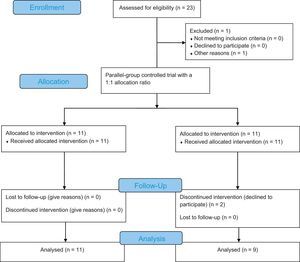
Order of patients inclusion in study and flow diagramThe trial consisted of a two-sequence, two-period, two-treatment crossover design. Each crossover period lasted 30 days, separated by 15 days as the washout period. The sequence of GOS or placebo ingestion defined by the first patient enrolled in the study was chosen by lot. The order of ingestion in the followed patients was systematically alternate. Fig. 1 shows a study flowchart.16
Study flowchart according to the Consolidated Standards of Reporting Trials statement.30
Clinical data collection related to constipation symptoms, substance delivery (GOS or placebo), and compliance control were achieved during home visits every two weeks: at baseline, day 15, and day 30, in both 30-day study periods. Compliance with substances was measured by the return of previously used flasks.
Sample size calculationData from 10 subjects who first completed the study were analyzed by ANOVA to calculate sample size.17 SAS software for sample size calculation was used, v. 9.2 of the SAS System for Windows (SAS Institute Inc. Cary, NC, USA). Statistical analysis using analysis of variance (ANOVA) for repeated measures was applied. ANOVA was used to analyze comparative treatments, considering the difference in outcomes. Power analysis was applied in the context of ANOVA by assuming a two-sequence, two-period, two-treatment crossover design, effect size in the population, sample size, and significance level. A statistically significant effect in ANOVA was monitored with follow-up tests, in order to assess which variable was different between groups. Follow-up tests were performed post hoc. SAS software “fpower” was then used to calculate sample size after specifying the power of study at 90% and alpha=0.05. Such analysis showed that using a power analysis of 90%, nine subjects would be required in each group.
Further dietary fiber supplementation was not allowed until completion of the trial. For those patients who refused to participate in the study or those who completed the trial, dietary fiber was prescribed.
OutcomesThree symptoms were considered as primary outcomes: bowel movements per week, fecal consistency and straining/pain during fecal passage. These symptoms were ranked according to numerical values. Bowel movements frequency was ranked from 1 to 3, as follows: 1=greater than three times per week; 2=one to two times per week; and 3=less than once per week. Intensity of straining/pain during stool passage ranged from 1 to 3 (1=no discomfort or pain, 2=episodic pain and/or discomfort, and 3=pain and/or discomfort during every stool passage). Fecal consistency was defined according to a photographic scale18 and varied from 3 to 0 (3=hard, separate lumps, 2=banana with deep cracks, 1=banana with superficial cracks, and 0=soft banana/separate soft pieces/fluffy pieces). The sum of values ranged from 2 to 9 and was considered a clinical score characterizing symptom intensity. The physical evaluation and patient scores were compiled during the baseline interview, and on day 15 and 30 in each period.
Statistical analysisDescriptive analyses were performed, including measures of central tendency and dispersion for numerical variables. At baseline, clinical and demographic data were compared (Mann–Whitney test).
GOS effect was determined according to a two-period, two-treatment crossover assignment: GOS and placebo. The Kolmogorov–Smirnov test was performed to test normality of data, rejecting the null hypothesis of normality. For statistical analysis, ANOVA was used for repeated measurements with rank-transformation (ANOVA on ranks). Ranking is a procedure used to transform data that violate the assumptions of normality. ANOVA on ranks signifies that a standard analysis of variance has been calculated on rank-transformed data. The significance level adopted for statistical tests was 5%.
The study was approved by the Ethics Committee of the institution, No. 366/2009, CAAE: 0280.0.146.000-09. All participants and/or parents/guardians signed an informed consent. The study was reviewed and published on the site ClinicalTrials.gov, identifier number: NCT02183766.
ResultsTwenty-three patients were initially included, but three patients were lost to follow-up due to a change of address without any previous communication. Twenty children and adolescents aged 4–16 years (mean 8.8±4.1 SD) were included. Twelve children were female. After unblinding, it was observed that 11 patients received the sequence of GOS to placebo and nine patients received the sequence of placebo to GOS. None of the patients refused treatment or had a low acceptance of medication. At the baseline interview, all patients reported fewer than three bowel movements per week or hard stool consistency.
Since patients were systematically allocated to groups according to consecutive first health care visit, the effect of allocation (order of product ingestion) was calculated by ANOVA for repeated measures; there was no difference between the groups’ scores determined by group allocation, p=0.9427.
Clinical scores over timeTable 1 shows demographic clinical data from 20 patients who completed the trial. Tables 2 and 3 show the distribution of clinical scores, considering GOS effect and time effect, respectively, i.e., clinical scores over time. Mean clinical scores decreased significantly during GOS ingestion in both groups (Table 2). Mean clinical scores over time showed that the effect of GOS lasted throughout its ingestion, since the washout period stopped the effect of GOS. There was no group/time interaction (Table 3).
Baseline demographics and clinical characteristics.
| GOS→Placebo Group 1 (n=11) | Placebo→GOS Group 2 (n=9) | p-Valuea | |
|---|---|---|---|
| Variable | |||
| Sex, M:F | 4:7 | 4:5 | NS |
| Age, (years) (mean±SD) | 8.7±4.8 | 8.8±3.5 | NS |
| Age at early symptoms, (years, mean±SD) | 5±4.7 | 3.8±2.4 | NS |
| Constipation started on 1st year of life | 3/11 | 1/9 | NS |
| Duration of constipation (years) | 3.8±0.7 | 5.4±3.45 | NS |
| Bowel movements/week (Baseline) | 1.5±0.5 | 1.7±0.7 | NS |
| Stool consistencyb | |||
| Mean±SD | 2.8±0.2 | 2.6±0.2 | 0.07 |
| Median (min-max) | 3 (1–2) | 2.5 (2.5–3) | |
| Baseline scorec | 6.5±1 | 6.1±1.4 | 0.45 |
| Score during GOS | 4.6±1.2 | 4.3±1 | 0.70 |
| Score during placebo | 5.8±1.1 | 6±1.3 | 0.65 |
Clinical score is presented as mean±SD.
GOS, galactooligosaccharide.
Clinical scores based on bowel frequency, fecal consistency, and defecation discomfort.
| Period 1 Mean±SD | Period 2 Mean±SD | |||||
|---|---|---|---|---|---|---|
| Baseline | Day 15 | Day 30 | Baseline | Day 15 | Day 30 | |
| Group 1 (initial sequence: GOS→placebo) (n=11) | 6.82±1.47 | 3.73±1.68 | 3.09±1.64 | 5.55±1.57 | 6±1.67 | 6.18±1.72 |
| Group 2 (initial sequence: placebo→GOS) (n=9) | 6.11±1.45 | 6.11±1.36 | 5.78±1.39 | 6.11±1.36 | 3.67±1.22 | 3.11±1.36 |
| p-Valuea | 0.1739 | 0.0004 | <0.0001 | 0.1237 | <0.0001 | <0.0001 |
GOS, galactooligosaccharide.
Clinical data and time effect (clinical data over time) using analysis of variance (ANOVA).
| Score/group | Period 1 Mean±SD | p-Value Time effect | Period 2 Mean±SD | p-Value Time effect | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 15 | Day 30 | Baseline | Day 15 | Day 30 | |||
| Group 1 GOS→Placebo (n=11) | 6.82±1.47 | 3.73±1.68 | 3.09±1.64 | <0.0001a | 5.55±1.57 | 6±1.67 | 6±1.72 | <0.0001b |
| Group 2 Placebo→GOS (n=9) | 6.11±1.45 | 6.11±1.36 | 5.78±1.39 | 0.4493 | 6.11±1.36 | 3.67±1.22 | 3.11±1.36 | 0.0105b |
Clinical score ranged from 9 (greatest severity) to 2 (no symptoms).
GOS, galactooligosaccharide.
The prebiotic GOS ingested by constipated children was effective at increasing bowel movement frequency, relieving pain or discomfort during stool passage, and softening stool consistency. No adverse effects were reported.
GOS has been shown to be effective for improving constipation in elderly subjects, pregnant women, and infants.19,20 Nevertheless, to the best of the authors’ knowledge, no clinical trials have studied GOS effects in constipated children or adolescents. Studies including schoolchildren or adolescents while managed in outpatient clinics are highly burdensome and require strict control on drug adherence. In the present study, it took considerable effort to measure adherence and symptoms by frequent home visits.
The main symptoms of constipated children seeking Brazilian primary health care settings refer to hard stools and large intervals between bowel movements. Less than 15% of patients reported fecal retentive incontinence.21 Primary care treatment prescribed for early constipation symptoms in children varies considerably, and usually less than 50% of these patients are successfully treated.22 GOS ingestion promoted relief of complaints during its ingestion, regardless other adjuvant recommendation.
In present study, mean duration was from 3 to 5 years. It is likely that most patients had already been treated but did not continue with therapy. No patient referred symptoms of retentive fecal incontinence or had fecal retention palpable in abdomen or identified by rectal examination. It appears that the patients have mild constipation, responsive to oral laxatives and with no complications. Such a clinical picture is found commonly in constipated children treated in primary healthcare centers. These data show that as a group, children and adolescents improve while using GOS, and no changes in symptom were recorded with placebo. No other sort of therapy was recommended besides strict adherence to the products.
In spite of a paucity of data providing conclusive evidence to support the current recommendations of fiber intake for constipated pediatric patients, pediatric clinical trials have shown that dietary fiber increases the frequency of defecation and stool softness.23 Diet modification to increase fiber consumption is considered an important component in the management of constipation. Dietary fiber supplementation was not prescribed during this trial, in order to better evaluate the isolated GOS effect on fecal characteristics. Dietary fiber was prescribed to subjects who declined to participate in the study or to those who had completed the trial.
Broad age range and sample size should be discussed as limitations of the present study. The pathogenesis of non-organic constipation is closely related to stool toileting refusal caused by pain during stool passage, generally occurring during the toilet-training period. Such patients usually have a previous history of hard feces, and infrequent and painful evacuation often accompanied by screaming and stool-holding maneuvers. When symptoms are not addressed, the condition progresses until a diagnosis is made. In around 50% of children, a long-term outcome can be observed, and 25% of children with functional constipation continue to experience symptoms into adulthood.24 Long-term constipation is associated with fecal impaction, retentive incontinence, and megarectum. Irrespective of complications and age, good clinical outcomes were defined as at least three bowel movements per week for at least 4 weeks, with no more than two episodes of fecal incontinence per month, irrespective of laxative use.24 Considering pathogenesis context, it must be pointed out that despite age variability, the included subjects were very similar in their constipation characteristics, with no subjects presenting signs of complications such as fecal impaction and requirement of aggressive laxatives. In respect to the small sample issue, the crossover design was chosen because this design enabled researchers to make comparisons between small samples; the sample size was primarily defined as sufficient by statistical analysis. Accepting these considerations, it is possible to conclude that the present results are true for patients with mild symptoms.
In studies conducted to evaluate prebiotic effects on infant constipation, treatment duration ranged from 21 days to 8 weeks for symptom evaluation. A 4-week period was proposed based on such studies.25–27
The fixed GOS dosage was chosen to provide an adequate balance between efficacy and tolerance, according to results from adult trials. It was not possible to examine bifidogenic effect on microbiota or the increased production of fecal SCFAs. According to the literature, the addition of 12g/day of GOS was shown to be effective and well-tolerated, resulting in increased fecal bifidobacteria in healthy individuals.28,29 It was shown that the estimated safe adult dose ranging from 0.3 to 0.4g/kg/day and 2.5g GOS is sufficient to induce a bifidogenic effect.30 An excessive amount of GOS could promote adverse osmotic effects leading to osmotic diarrhea, symptoms not referred by the present patients during GOS ingestion.
This pilot study showed that GOS improves clinical constipation symptoms and may represent a supportive measure in the treatment of constipation. To confirm these findings, a larger, randomized, placebo-controlled trial is required.
FundingTo carry out the project, funding was obtained from FAEPEX (Support Education Fund, the Research and Extension), School of Medical Sciences, Universidade Estadual de Campinas (UNICAMP), Sao Paulo, Brazil released on 12/11/2009 under the Agreement N°: 519,294.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Beleli CA, Antonio MA, dos Santos R, Pastore GM, Lomazi EA. Effect of 4′galactooligosaccharide on constipation symptoms. J Pediatr (Rio J). 2015;91;567–73.
Study linked to School of Medical Sciences and Faculty of Food Engineering, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brasil.
Study conducted at Basic Health Unit in the outskirts of Campinas, coordinated by a team of pediatricians of the Department of Pediatrics, School of Medical Sciences, Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brasil.