To compare the effectiveness of a single intramuscular dose of bromopride, metoclopramide, or ondansetron for treating vomiting.
MethodsRandomized controlled trial including children 1–12 years of age presenting with acute vomiting at the pediatric emergency department. Outcomes: Number of children that stopped vomiting at one, six, and 24h following treatment; episodes of diarrhea; acceptance of oral liquids; intravenous rehydration; return to hospital and side effects.
ResultsThere were 175 children who completed the study. Within the first hour after treatment, all drugs were equally effective, with ondansetron preventing vomiting in 100%, bromopride in 96.6%, and metoclopramide in 94.8% of children (p=0.288). Within six hours, ondansetron was successful in preventing vomiting in 98.3% of children, compared to bromopride and metoclopramide, which were successful in 91.5% and 84.4% of patients, respectively (p=0.023). Within 24h, ondansetron was superior to both other agents, as it remained efficacious in reducing vomiting in 96.6% of children, as opposed to 67.8% and 67.2% with bromopride and metoclopramide, respectively (p=0.001). The ondansetron group showed better acceptance of oral liquids (p=0.05) when compared to the bromopride and metoclopramide. The ondansetron group did not show any side effects in 75.9% of cases, compared to 54.2% and 53.5% in the bromopride and metoclopramide groups, respectively. Somnolence was the most common side effect.
ConclusionsA single dose of ondansetron is superior to bromopride and metoclopramide in preventing vomiting six hours and 24h following treatment. Oral fluid intake after receiving medication was statistically better with Ondansetronwhile also having less side effects compared to the other two agents.
Para comparar a eficácia de uma única dose intramuscular de bromoprida, metoclopramida ou ondansetrona no tratamento de vômito.
MétodosEnsaio controlado randomizado incluindo crianças de 1 a 12 anos de idade que apresentam vômito agudo no departamento de emergência pediátrica. Desfechos: Número de crianças que pararam de vomitar 1, 6 e 24 horas após o tratamento; episódios de diarreia; aceitação de líquidos orais; reidratação intravenosa, retorno ao hospital e efeitos colaterais.
Resultados175 crianças concluíram o estudo. Na primeira hora após o tratamento, todos os medicamentos foram igualmente eficazes, sendo que a ondansetrona preveniu vômito em 100%, a bromoprida em 96,6% e metoclopramida em 94,8% das crianças (p=0,288). Em 6 horas, a ondansetrona mostrou sucesso na prevenção do vômito em 98,3% das crianças, em comparação à bromoprida e à metoclopramida, que mostraram sucesso em 91,5% e 84,4% dos pacientes, respectivamente (p=0,023). Em 24 horas, a ondansetrona foi superior aos dois outros agentes, pois ela continuou eficaz na redução do vômito em 96,6% das crianças, diferente de 67,8% e 67,2% com bromoprida e metoclopramida, respectivamente (p=0,001). O grupo de ondansetrona mostrou melhor aceitação de líquidos orais (p=0,05) em comparação a bromoprida e metoclopramida. O grupo de ondansetrona não mostrou efeitos colaterais em 75,9% dos casos, em comparação a 54,2% e 53,5% dos grupos de bromoprida e metoclopramida. O efeito colateral mais comum foi sonolência.
ConclusõesUma única dose de ondansetrona é superior a bromoprida e metoclopramida no tratamento de vômito 6 horas e 24 horas após o tratamento. A ingestão de fluídos orais após receber medicação foi estatisticamente melhor com ondansetrona, ao mesmo tempo em que também apresentando menos efeitos colaterais em comparação aos outros dois agentes.
Acute gastroenteritis (AGE) is one of the most common causes of morbidity and mortality in children, contributing to numerous emergency department visits and pediatric hospitalizations. AGE is considered an important public health issue; according to the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF), there are about two billion cases of diarrheal disease worldwide every year, and 1.9 million children younger than 5 years of age perish from diarrhea each year, mostly in developing countries.1 Generally, AGE is an acute and self-limiting disease, which usually lasts 3–7 days.2
Vomiting is a common manifestation of AGE that causes discomfort; left untreated, it may lead to dehydration.1,2 Oral rehydration therapy (ORT) is the most suitable treatment for children with AGE, but it is challenging in the presence of persistent/refractory emesis.2 Guidelines state that ORT in children has a high chance of failure in the setting of persistent vomiting, and support the use of intravenous (IV) rehydration in this context.3,4 However a recent publication showed that ORT is efficacious even in children with vomiting in a high percentage of cases.3,4
When pharmacological intervention is used for persistent nausea and vomiting, it can prevent severe complications due to dehydration.2,5 Bromopride, metoclopramide, and ondansetron have been used in clinical practice in these situations.6,7
Bromopride has been used since the 1970s; its use has been fully incorporated by pediatricians since then. The recommendation of bromopride remains a reference as a therapeutic resource for various diseases in gastroenterology textbooks and published reviews on prokinetics.8
Metoclopramide is an anti-emetic widely used in the treatment of vomiting in children, with good results. A study comparing the therapeutic effectiveness of intravenous metoclopramide (72%) and ondansetron (81%) showed that both are effective in stopping vomiting.9 Some studies found that metoclopramide was associated with adverse effects, such as sedation, agitation, or extrapyramidal reaction.2,5,8,10 However, such adverse effects could be secondary to the dosing used; this remains to be clarified.
Ondansetron has been widely used as an anti-emetic in cases of vomiting associated with chemotherapy, radiotherapy, and surgical interventions. Recent studies have shown that the prescription of ondansetron is rising in pediatric emergency departments for treating AGE-associated vomiting.3,7,11,12 It has been shown in a recent meta-analysis that ondansetron compared with placebo increased the chance for vomiting cessation up to one hour after drug administration, but there was no difference between the groups after four, 24, and 48h. Treatment with ondansetron compared with placebo reduced the risk of failure of oral rehydration therapy and reduced the risk of hospitalization.4 A study conducted in the United States and Canada with ondansetron found that 86% of physicians recommended its use, considering its effectiveness effective in improving ORT success and its reasonable cost.13
Still, more evidence is needed concerning the safety and efficacy of antiemetic use for vomiting in children. The aim of this study was to assess the effectiveness of a single intramuscular dose of bromopride, metoclopramide, and ondansetron to treat acute onset vomiting in a pediatric emergency department.
MethodsTrial designControlled clinical trial phase IV, randomized in three parallel groups, to assess the therapeutic effectiveness of ondansetron, bromopride, and metoclopramide in treating vomiting. Patients were randomized in sequential blocks as they became eligible to participate in the study, and similar distribution in each treatment group was ensured. The assignment was made as 1:1:1 for each medication in blocks of fifteen children, including five from each group. The sequential block randomization was not predictable by treating clinicians.
Changes in trial designThere were no changes in the study design throughout its duration.
Study settingThe study was conducted in the Pediatric Emergency Department (PedER) of Hospital Universitário de Santa Maria (HUSM), in southern Brazil, between August 2013 and June 2014.
Eligibility criteria for participantsInclusion criteria were as follows: children 1–12 years of age, admitted to the PedER with two or more episodes of vomiting in the previous 24h, and considered to need intramuscular anti-emetic medication by the attending physician. The study did not consider the severity or details of the vomiting, but only the attending physician's decision to use anti-emetic medication.
Exclusion criteria were as follows: (a) use of anti-emetic medications in the previous 24h; (b) children with known allergy to bromopride, metoclopramide, and/or ondansetron; (c) severe dehydration, vomiting due to sedation, anesthesia, or chemotherapy; (d) pregnant and/or breast-feeding teenagers; (e) children with heart disease, advanced renal disease, urinary tract infection, epilepsy, intestinal obstruction, appendicitis, meningitis, diabetes mellitus, malnutrition, pneumonia, or brain tumor.
InterventionsOnce subjects were identified as meeting eligibility criteria, they were approached for recruitment. Immediately following recruitment, patients were randomized to a treatment group. The three treatment groups were: group A, bromopride (0.15mg/kg, maximum 10mg); group B, metoclopramide (0.15mg/kg, maximum 10mg); group C, ondansetron, (0.15mg/kg, maximum 8mg). They were all administered as a single intramuscular dose.
Children remained under observation in the PedER until discharged by the attending physician. At the time of discharge, a survey was given to parents or legal guardians to ascertain details on the evolution of symptoms in the 24h following the treatment. Children whose vomiting did not improve 60min following the intervention were prescribed other medications by the attending physician, as they deemed appropriate (typically either intravenous fluid or other anti-emetics).
All assessments within the 24h period of data collection (i.e., response to treatment at one, six, and 24h post treatment) were made by members of the research team, who were blinded to the intervention. Following discharge, information was collected by phone calls to the caregivers by members of the research team.
OutcomesVariables were assessed for the three drugs, at 60min, six hours, and 24h after intervention with IM medication. At 60min, data was obtained regarding the number of children in whom vomiting ceased, acceptance of oral fluids and amount (mL), need for intravenous rehydration, and side effects. After receiving the medication, children were restrained from receiving any oral fluid for 30min; then, children were allowed to drink 300mL of either oral rehydration solution or tap water, given in small amounts using a 10mL syringe by their accompanying parents, to avoid stimulating the emetic reflex as much as possible. The amount of fluids taken orally was measured one hour after initiating its administration. After full oral fluid intake was tolerated, light solid food intake was advised at discharge from the PedER.
Six hours post anti-emetic medication, the number of children that ceased vomiting was recorded, as well as whether they had been discharged home or remained in the PedER. Side effects were also recorded, such as diarrhea, somnolence, or others. Information collected 24h post anti-emetic medication included the number or children that ceased vomiting, returns to PedER due to vomiting or side effects, and side effects at any point in time – like diarrhea, somnolence, or others.
The sample size was calculated based on previous studies, considering the vomit cessation rate at 24h,14 using the expected values for each medication under study: 60% (ondansetron), 45% (bromopride), and 30% (metoclopramide). The estimated sample was 48 subjects per group (144 children). Estimating attrition of 20%, the final sample size was set at 180 patients.
Continuous variables were expressed as means and standard deviation (SD) or medians with interquartile ranges, depending on their distribution. Similarly, Student's t-test or the Kruskal–Wallis test was used when appropriate. The categorical variables were expressed by percentage and compared by Pearson's chi-squared test or Fisher's exact test. Data was analyzed using SPSS software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. NY, USA).
The study was approved by the institutional Review Board (CAAE: 12188513.9.0000.5336), and all parents or legal guardians agreed to participate by signing an informed consent. Clinical Trial registration number: RBR-5ky3ks.
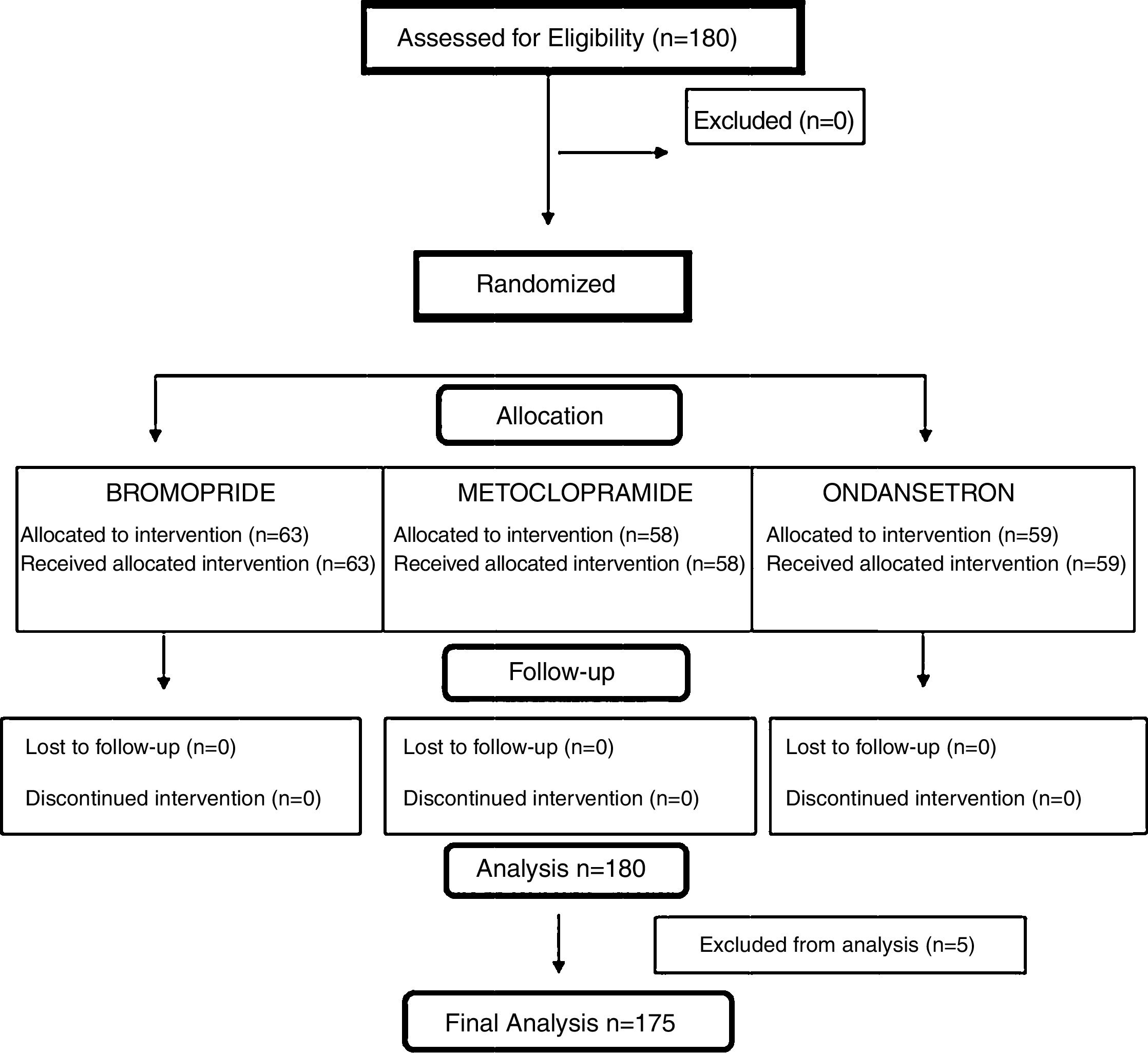
ResultsParticipant flowOne hundred and eighty children were selected and randomized to receive treatment for vomiting in the PedER (Fig. 1).
Losses and exclusionsFive children were excluded (four from the bromopride group and one from the ondansetron group) due to the prescription of a different antiemetic drug by the attending physician.
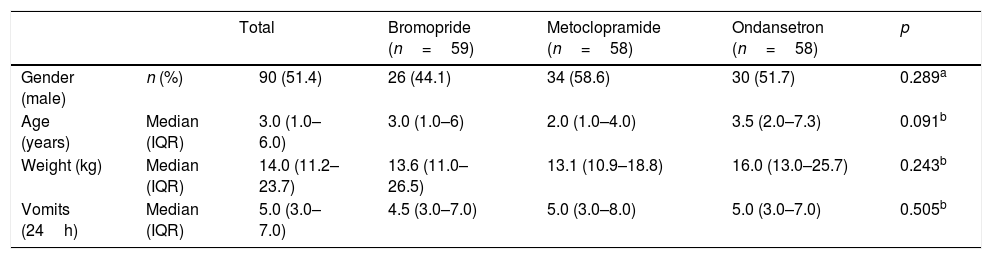
Baseline dataNo significant differences were observed between study groups in terms of sex, age, weight, and number of episodes of vomiting before administering the study drug (Table 1).
Baseline characteristics of children treated for vomiting with bromopride, metoclopramide, and ondansetron intramuscular.
| Total | Bromopride (n=59) | Metoclopramide (n=58) | Ondansetron (n=58) | p | ||
|---|---|---|---|---|---|---|
| Gender (male) | n (%) | 90 (51.4) | 26 (44.1) | 34 (58.6) | 30 (51.7) | 0.289a |
| Age (years) | Median (IQR) | 3.0 (1.0–6.0) | 3.0 (1.0–6) | 2.0 (1.0–4.0) | 3.5 (2.0–7.3) | 0.091b |
| Weight (kg) | Median (IQR) | 14.0 (11.2–23.7) | 13.6 (11.0–26.5) | 13.1 (10.9–18.8) | 16.0 (13.0–25.7) | 0.243b |
| Vomits (24h) | Median (IQR) | 5.0 (3.0–7.0) | 4.5 (3.0–7.0) | 5.0 (3.0–8.0) | 5.0 (3.0–7.0) | 0.505b |
IQR, interquartile range; n, number of patients.
175 children completed the study, distributed as follows: group A (bromopride), 59; group B (metoclopramide), 58, and group C (ondansetron), 58. There were no losses in the follow up.
OutcomesOf all study participants, only five children (2.9%) vomited in the first hour after receiving medication. Two (3.4%) belonged to group A, and three (5.2%) to group B, with no significant difference between groups (Table 2).
Comparison between bromopride, metoclopramide and ondansetron groups at 60min, six hours, and 24h after medicating.
At 24h, the anti-emetic effect, in terms of number of children having stopped vomiting, was statistically superior in group C compared to groups A and B (96.6% vs. 67.8% and 67.2%, respectively [p=0.001]) (Table 2).
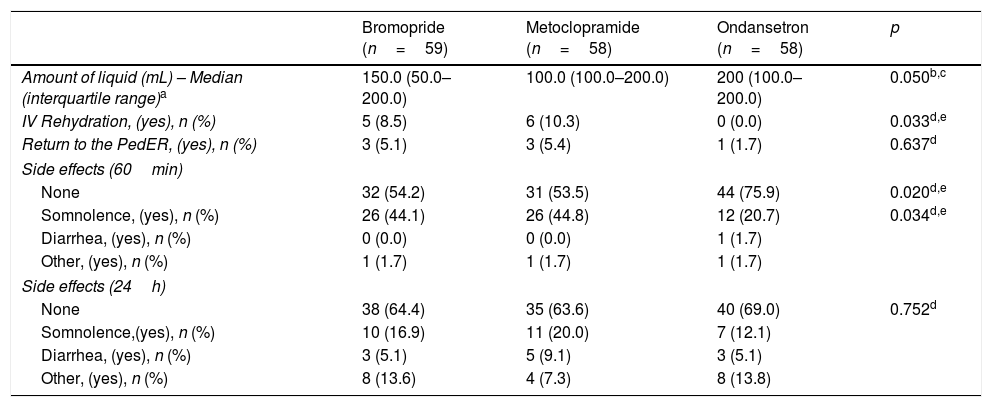
Oral fluid intake (Table 3), evaluated 30min after receiving medication, was statistically better (p=0.050) for group C (200mL), compared to groups A (150mL) and B (100mL).
Secondary outcome measures comparing different medications.
| Bromopride (n=59) | Metoclopramide (n=58) | Ondansetron (n=58) | p | |
|---|---|---|---|---|
| Amount of liquid (mL) – Median (interquartile range)a | 150.0 (50.0–200.0) | 100.0 (100.0–200.0) | 200 (100.0–200.0) | 0.050b,c |
| IV Rehydration, (yes), n (%) | 5 (8.5) | 6 (10.3) | 0 (0.0) | 0.033d,e |
| Return to the PedER, (yes), n (%) | 3 (5.1) | 3 (5.4) | 1 (1.7) | 0.637d |
| Side effects (60min) | ||||
| None | 32 (54.2) | 31 (53.5) | 44 (75.9) | 0.020d,e |
| Somnolence, (yes), n (%) | 26 (44.1) | 26 (44.8) | 12 (20.7) | 0.034d,e |
| Diarrhea, (yes), n (%) | 0 (0.0) | 0 (0.0) | 1 (1.7) | |
| Other, (yes), n (%) | 1 (1.7) | 1 (1.7) | 1 (1.7) | |
| Side effects (24h) | ||||
| None | 38 (64.4) | 35 (63.6) | 40 (69.0) | 0.752d |
| Somnolence,(yes), n (%) | 10 (16.9) | 11 (20.0) | 7 (12.1) | |
| Diarrhea, (yes), n (%) | 3 (5.1) | 5 (9.1) | 3 (5.1) | |
| Other, (yes), n (%) | 8 (13.6) | 4 (7.3) | 8 (13.8) | |
IV, intravenous.
Intravenous (IV) rehydration was given to 11 children, five from the bromopride group and six from the metoclopramide group (p=0.658). No patients needed IV rehydration in the ondansetron group (p=0.033). Seven children returned to the PedER due to vomiting (Table 3).
Side effectsAt 60min post-treatment, the group that showed fewest adverse effects was Group C (24.1%), while groups A and B reported twice as many adverse events; 44.8% and 46.5%, respectively (p=0.020). The most common side effect was somnolence in all three groups; however, this was less prevalent in Group C than Groups A and B (Table 3). At 24h, Group C showed less side effects than Groups A and B, but this was not statistically significant (p=0.752).
There were no statistical differences in diarrhea episodes after using the anti-emetics in any of the medication groups.
DiscussionInterpretationThe results showed that bromopride, metoclopramide, and ondansetron are effective anti-emetic medications for treating vomiting in children, with relatively benign safety profiles. Of the three drugs we tested, ondansetron demonstrated to have the best therapeutic profile in terms of effectiveness and amount of oral fluids accepted after the administration of the drug; it was also associated with a lower frequency of side effects.15
The scientific literature supports the use of anti-emetics for treating persistent vomiting.7 A study conducted in the USA revealed that most emergency pediatricians prescribe anti-emetics to treat vomiting, considering that it is distressing and unpleasant to children and caregivers.13 A study conducted in Italy had similar results.16
Apart from their efficacy, clinicians will often use medications based on their safety profile. Ondansetron has been shown to be effective in preventing vomiting, allowing oral rehydration and limiting IV rehydration use for patients with AGE, while having relatively few side effects.4,7 Several studies have compared the effectiveness of ondansetron to placebo, and have shown that ondansetron is more effective in treating vomiting, reducing admission rates; however, some studies report diarrhea with the use of ondansetron, probably due to its prokinetic effect.11,12,17
According to different authors, six hours after the administration of anti-emetic medication may be the most suitable time period to assess its effectiveness. There appears to be benefits from using ondansetron in comparison to bromopride and metoclopramide. In the present study, ondansetron was more effective six (p=0.023) and 24h (p≤0.001) after administration in reducing the incidence of vomiting, compared to the other drugs. It showed a low rate of side effects, was more successful in oral rehydration, prevented IV rehydration, and prevented hospital admission (p≤0.05). Bromopride and metoclopramide were considered effective but caused adverse effects, mainly in the first hour after medication, such as somnolence, which may increase the observation time and hospital costs.
Cubeddu et al. compared the therapeutic effectiveness of ondansetron, metoclopramide, and a placebo in control of vomiting, 24h after receiving anti-emetics.14 Ondansetron was more effective in controlling vomiting compared to metoclopramide and the placebo. The limitation of this study was the small sample size. A study conducted by Al-Ansari et al. evaluated the time to vomiting cessation during episodes of AGE and revealed that both ondansetron and metoclopramide were equally effective in this context.9 Some authors concerned about side effects from anti-emetics, like diarrhea or dystonic reaction, have described that metoclopramide can cause extrapyramidal symptoms and sedation in children.2,7,10
The present study investigated whether the anti-emetic medications studied were associated with the development of diarrhea. Neither drug was shown to cause significant diarrhea in the cohort studied. Al-Ansari et al., also failed to show an association between anti emetic use and diarrhea.9 Cubeddu et al. had previously suggested that diarrhea may be related to the use of metoclopramide.14 Although the prokinetic effect of metoclopramide could contribute to this side effect, one would expect ondansetron to cause similar symptoms given its mechanism of action. A study mentions that the increased incidence of diarrhea can be considered as resulting from toxin retention, which could have been eliminated by vomiting.6
To the authors’ knowledge, this is the only study that has compared the therapeutic effectiveness of bromopride, metoclopramide, and ondansetron in the treatment of acute onset vomiting in a pediatric cohort treated at the emergency department.
LimitationsThe main limitation of the present study is that the clinicians caring for the children in the emergency department, as well as parents and caregivers, were not blinded to the intervention. However, randomization of the medication minimized this potential bias. In addition, there was no placebo arm; however, these children were treated as per routine clinical practice that includes the use of anti-emetic medications. Specific diagnosis was not included in the eligibility criteria; however, vomiting without a definitive diagnosis is a common complaint in pediatric emergency departments, and randomization controlled this potential bias.
In conclusion, it was found that bromopride, metoclopramide, and ondansetron are effective anti-emetic medications for treating vomiting in children, hence preventing dehydration, use of IV fluids, and hospitalization. Ondansetron was superior to the other two agents studied in achieving emesis control and also had fewer side effects.
Registration number: RBR-5ky3ksName of trial registry: Randomized clinical trial, comparing the therapeutic effectiveness of intramuscular bromopride, metoclopramide, and ondansetron in the treatment of vomiting.
Protocol: The Brazilian Clinical Trials Registry/clinicaltrials.gov. Identifier: RBR-5ky3ks.
FundingThe study received funding from HUSM.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Epifanio M, Portela JL, Piva JP, Ferreira CH, Icaza EE, Mattiello R. Bromopride, metoclopramide, or ondansetron for the treatment of vomiting in the pediatric emergency department: a randomized controlled trial. J Pediatr (Rio J). 2018;94:62–8.