The objective of this study is to evaluate the hypothesis that use of heliox would result in improvement of gas exchange when used with high flow nasal cannula in infants with RSV acute bronchiolitis.
MethodsAll patients that met the inclusion criteria were randomized to either heliox (70:30) or air–oxygen mixture 30% via high flow nasal cannula at 8L/min for a continuous 24h. Measurements were taken at baseline, after 2h, and at the end of the 24h.
ResultsThis prospective study included 48 patients. After 2h of treatment with heliox, the oxygen saturation and PaO2 significantly improved when compared with the air–oxygen group, 98.3% vs. 92.9%, 62.0mmHg vs. 43.6mmHg (p=0.04 and 0.01), respectively. Furthermore, PaO2/FiO2 ratio was significantly higher in the heliox group when compared with the air–oxygen group, 206.7 vs. 145.3. Nevertheless, CO2 showed better elimination when heliox was used, without significance. MWCA score dropped significantly in the heliox group, 2.2 points vs. 4.0 points in air–oxygen (p=0.04), 2h after starting the therapy.
ConclusionTransient improvement of oxygenation in infants with RSV acute bronchiolitis during the initial phase of the therapy is associated with heliox when provided with HFNC, may provide a precious time for other therapeutic agents to work or for the disease to resolve naturally, avoiding other aggressive interventions.
O objetivo deste estudo é avaliar a hipótese de que o uso da mistura heliox resultaria em melhora da troca gasosa quando usado com cânula nasal de alto fluxo em crianças com bronquiolite aguda por VSR.
MétodosTodos os pacientes que atenderam aos critérios de inclusão foram randomizados para receber a mistura heliox (70:30) ou a mistura ar/oxigênio a 30% por meio da cânula nasal de alto fluxo a 8 L/min por 24 horas contínuas. As medições foram feitas no início, depois de 2 horas e ao fim de 24 horas.
ResultadosRealizamos um estudo prospectivo em que foram incluídos 48 pacientes. Após 2 horas de tratamento com a mistura heliox, a saturação de oxigênio e a PaO2 apresentaram melhora significativa em comparação ao grupo da mistura ar/oxigênio: 98,3% em comparação a 92,9%, 62,0mmHg em comparação a 43,6mmHg (p=0,04 e 0,01), respectivamente. Além disso, a relação PaO2/FiO2 era significativamente mais alta no grupo da mistura heliox que no grupo da mistura ar/oxigênio, 2067 em comparação a 1453. Contudo, o CO2 apresentou melhor eliminação quando a mistura heliox foi utilizada, sem relevância. O Escore MWCA caiu significativamente no grupo da mistura heliox, 2,2 pontos em comparação a 4,0 pontos da mistura ar/oxigênio (p=0,04) 2 horas após o início da terapia.
ConclusãoA breve melhora da oxigenação em crianças com bronquiolite aguda por VSR na fase inicial da terapia está associada à mistura heliox quando administrada pela CNAF e poderá fornecer um tempo precioso para outros agentes terapêuticos funcionarem ou para a própria doença se curar naturalmente e evitar outras intervenções agressivas.
Helium is an inert gas, and its density is almost 15% that of air. Carbon dioxide (CO2) has the capability to diffuse through helium more easily than through air.1 Helium inhalation results in maintenance of more laminar flow and decreases turbulence through narrowed airways, which subsequently lowers the resistance to gas flow.2 Therefore, breathing an oxygen–helium mixture (heliox) will lead to a decline in resistance to the flow of that mixture within the airways, and in turn decrease the effort of breathing, especially in disorders that course with an increase in airway resistance.3 Therefore, the use of heliox in patients suffering from obstructive upper airways,4 bronchiolitis, and obstructive lower lung diseases is beneficial.5,6 Heliox can be delivered noninvasively in patients breathing spontaneously, or invasively, through mechanical ventilation.4
Respiratory syncytial virus (RSV) is a non-segmented enveloped RNA virus that belongs to the Paramyxoviridae family. Its incubation period reaches eight days. The virus initially replicates in nasopharyngeal lining, then spreads to the epithelial lining of bronchioles, triggering a lower respiratory tract infection. The main target of RSV is respiratory ciliated epithelial cells; however, other cells such as macrophages and dendritic cells can also be infected. RSV genome replication creates RNA intermediates that elicit an antiviral-mediated response, mediated by interferon and other cytokines.7 As infection occurs in the lower respiratory tract, a sequence of events occurs in epithelial cells including edema and abundant mucus production, followed by cell necrosis then regeneration. Subsequently, a small airway obstruction leads to air trapping, increased resistance of lower airways, and hypoxemia.8
Based on its properties, heliox was considered as an adjuvant therapy to improve oxygenation in patients with RSV lower respiratory tract infection while they were mechanically ventilated; however, its use in these patients has been inconclusive.3
This study aimed to evaluate whether the use of heliox would result in an improvement of gas exchange when continuously delivered through a high-flow nasal cannula (HFNC) in infants diagnosed with acute bronchiolitis caused by RSV.
MethodsParticipantsEligible participants were infants aged 1 month up to 2 years. They were admitted to the pediatric intensive care unit, from September 2013 to May 2015, with RSV acute bronchiolitis clinically diagnosed and laboratory confirmed. The bronchiolitis diagnosis was based on criteria that included cough, increased respiratory rate, chest retraction, prolongation of expiratory time, sibilant rhonchi, and hyperinflated lungs on chest X-ray. RSV infection was confirmed by direct antigen detection test of nasopharyngeal discharges. The patients were eligible for inclusion into the study when they could not maintain their oxygen saturation ≥93% in room air and required supplemental oxygen on admission. The eligible patients, after obtaining a written consent from at least one of the parents, were randomized to receive either heliox therapy (70:30) or air–oxygen mixture at 8L/min through a HFNC, continuously for 24h.
Subjects were excluded from the study if they required mechanical ventilation or had a hemodynamically significant congenital heart defect (significant left-to-right intracardiac shunt with or without the presence of pulmonary arterial hypertension or right-to-left shunting) or if the parents/guardians refused to sign the informed consent. Patients with chronic lung disease, including bronchopulmonary dysplasia, and those previously diagnosed with hyper-reactive airway diseases were also excluded.
Study designThis was an interventional, prospective, single-center study. All participants that met the inclusion criteria were randomized by block randomization, after the informed consent was signed, and subjected to either heliox (70:30) or air–oxygen mixture with starting 30% oxygen fraction via HFNC at 8L/min for a continuous 24h. If the oxygen saturation was kept ≤93% despite fraction of inspired oxygen of 30%, an increment of 5% was done to maintain saturation >93%.
The modified Wood's Clinical Asthma Score9 is a scoring system used to assess respiratory distress alterations over a period of time, with a maximum score is 11. Measurements of vital signs were taken at baseline then hourly through the whole study, while PaO2, PaCO2, and Modified Wood's Clinical Asthma Score were measured and reported at baseline, 2h after starting the treatment, 12h after starting the treatment, and at the end of the 24h.
RSV detectionDetection of RSV in nasopharyngeal samples was done using the TRU RSV test (Meridian Bioscience, Inc., USA). The TRU RSV test is a rapid, qualitative capture immunoassay for the detection of RSV antigen in human samples. The test utilizes gold-linked monoclonal antibodies to RSV fusion protein and nucleoproteins (detector antibodies). Plastic-shafted cotton swabs were used to collect nasopharyngeal samples from study participants. Swabs were transported in a transport medium (0.85% saline) to the microbiology laboratory for further processing. Test procedure was performed according to the method provided by the manufacturer.
Logistics gas supplyHeliox (Advanced Technology Company, Kuwait) was administered via HFNC using the RT329 system, which was connected to the humidifier chamber's gas inlet port (MR850 Humidification system, Fisher & Paykel Healthcare, Madrid, Spain). The gas source for HFNC was heliox (30% oxygen and 70% helium) and, through the dry side of the humidifier chamber, a secondary source oxygen gas was added. The air–oxygen mixture was kept at 30% fraction of inspired oxygen (FIO2) supplied via HFNC using the RT329 system.
MeasurementsA chest radiograph was obtained for each participant before implementation of the study protocol to assess the presence of hyperinflation, which was demarcated on the chest X-ray as a diaphragmatic level below the sixth thoracic rib anteriorly. Arterial blood was collected to measure both partial pressure of oxygen in arterial blood (PaO2) and partial pressure of carbon dioxide in arterial blood (PaCO2) before starting the treatment; it was also collected at 2h and 24h after start of treatment. All patients were managed according to a unified management protocol.10
Oxygen saturation was continuously monitored with Masimo SET pulse oximeter (Masimo Corporation, Irvine, USA). Other vital signs were also monitored at hourly intervals for 24h until discontinuation of the heliox therapy. The collected data was then analyzed.
Outcome measuresThe measured outcomes were the improvement of arterial PaO2 and the extent of respiratory distress changes measured by the Modified Wood's Clinical Asthma Score 2h and 24h after start treatment.
The study protocol has been approved by the Ethical Committee of the Faculty of Medicine of Mansoura University, and registered under Clinical trial No. ISRCTN99072045.
Statistical analysisStudent's t-test for independent sample was used for data analysis. Data was expressed as mean±standard deviation. Results were considered significant when p<0.05. All the statistical analysis was made using SPSS version 15.0 (Chicago, IL, USA).
ResultsThroughout the study, 86 infants were diagnosed with acute bronchiolitis and admitted to the pediatric intensive care unit. Of these, 48 (22 boys and 26 girls) met the inclusion criteria and were randomized into two groups of 24 patients each: the received heliox (70:30) group or air–oxygen mixture 30% group, via HFNC. The mean age of patients was 6.4 months (±1.2 months). Two patients with an underlying heart disease (known to have large ventricular septal defect), eight patients who were premature babies with chronic lung disease, two patients who had suffered from recurrent bronchiolitis, and 26 patients in whom RSV infection was discarded after nasopharyngeal swabs by direct antigen detection assay were excluded.
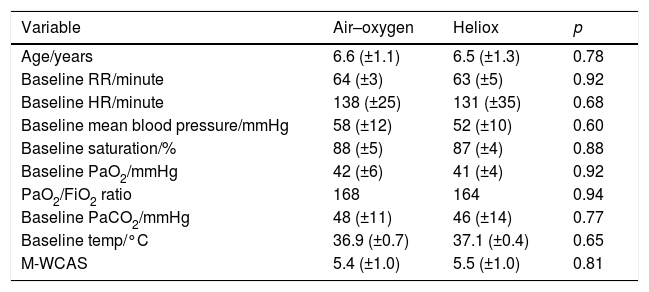
All baseline measurement of study subjects in both groups were similar (Table 1). In two patients in the air–oxygen group, FIO2 had to be increased to 35% for only 3h to maintain saturation >93% then back to the 30%. No patient in heliox group required FIO2 more than 30%.
Baseline study characteristics in both groups.
| Variable | Air–oxygen | Heliox | p |
|---|---|---|---|
| Age/years | 6.6 (±1.1) | 6.5 (±1.3) | 0.78 |
| Baseline RR/minute | 64 (±3) | 63 (±5) | 0.92 |
| Baseline HR/minute | 138 (±25) | 131 (±35) | 0.68 |
| Baseline mean blood pressure/mmHg | 58 (±12) | 52 (±10) | 0.60 |
| Baseline saturation/% | 88 (±5) | 87 (±4) | 0.88 |
| Baseline PaO2/mmHg | 42 (±6) | 41 (±4) | 0.92 |
| PaO2/FiO2 ratio | 168 | 164 | 0.94 |
| Baseline PaCO2/mmHg | 48 (±11) | 46 (±14) | 0.77 |
| Baseline temp/°C | 36.9 (±0.7) | 37.1 (±0.4) | 0.65 |
| M-WCAS | 5.4 (±1.0) | 5.5 (±1.0) | 0.81 |
RR, respiratory rate; HR, heart rate; PaO2, partial arterial oxygen; FIO2, fraction of inspired oxygen; PaCO2, partial arterial carbon dioxide; M-WCAS, modified Wood's Clinical Asthma score.
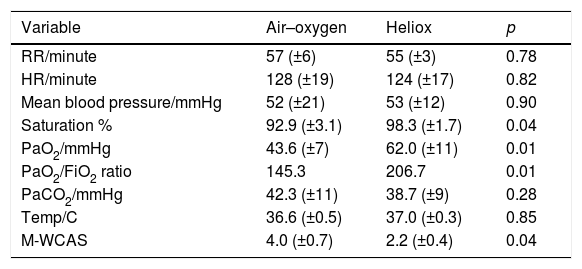
After the initial 2h of treatment, an improvement of all measured values was observed in both study groups. Oxygen saturation and PaO2 improved significantly more in the heliox group than in air–oxygen group, 98.3% (±1.7%) vs. 92.9% (±3.1%), 62.0mmHg (±11mmHg) vs. 43.6mmHg (±7mmHg), respectively (p=0.04 and 0.01, respectively). Furthermore, PaO2/FiO2 ratio was significantly higher in the heliox group than in the air–oxygen group, 206.7 vs. 145.3. Nevertheless, PaCO2 improvement failed to show the same significance between the studied groups 38.7(±9) mmHg vs. 42.3 (±11) mmHg (p=0.28; Table 2).
Differences between study groups 2h after the start of intervention.
| Variable | Air–oxygen | Heliox | p |
|---|---|---|---|
| RR/minute | 57 (±6) | 55 (±3) | 0.78 |
| HR/minute | 128 (±19) | 124 (±17) | 0.82 |
| Mean blood pressure/mmHg | 52 (±21) | 53 (±12) | 0.90 |
| Saturation % | 92.9 (±3.1) | 98.3 (±1.7) | 0.04 |
| PaO2/mmHg | 43.6 (±7) | 62.0 (±11) | 0.01 |
| PaO2/FiO2 ratio | 145.3 | 206.7 | 0.01 |
| PaCO2/mmHg | 42.3 (±11) | 38.7 (±9) | 0.28 |
| Temp/C | 36.6 (±0.5) | 37.0 (±0.3) | 0.85 |
| M-WCAS | 4.0 (±0.7) | 2.2 (±0.4) | 0.04 |
RR, respiratory rate; HR, heart rate; PaO2, partial arterial oxygen; FIO2, fraction of inspired oxygen; PaCO2, partial arterial carbon dioxide; M-WCAS, modified Wood's Clinical Asthma score.
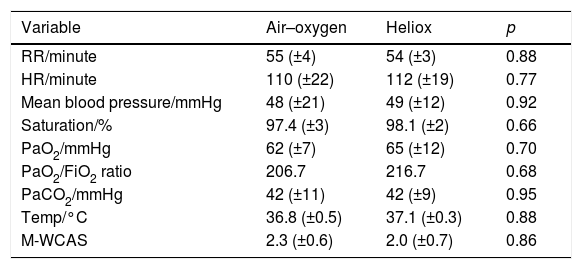
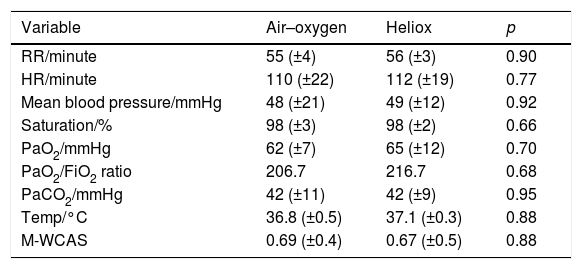
The improvement in oxygen saturation, PaO2, PaO2/FiO2 ratio, and PaCO2 also was better in the heliox group after 12h of treatment, but the difference was not statistically significant (98.1% [±2.0%] vs. 97.4% [±3.0%], 65.0mmHg [±12mmHg] vs. 62.0mmHg [±7mmHg], 216.7 vs. 206.7, and 42.0mmHg [±9mmHg] vs. 42.0mmHg [±11mmHg]; p=0.66, 0.70, and 0.95, respectively; Table 3). At the end of the 24h after treatment onset, all the measured parameters in both groups, including PaO2, oxygen saturation, and PaCO2, improved from baseline but the difference was not statistically significant (p=0.95, 0.7, and 0.88 respectively; Table 4).
Differences between study groups 12h after the start of intervention.
| Variable | Air–oxygen | Heliox | p |
|---|---|---|---|
| RR/minute | 55 (±4) | 54 (±3) | 0.88 |
| HR/minute | 110 (±22) | 112 (±19) | 0.77 |
| Mean blood pressure/mmHg | 48 (±21) | 49 (±12) | 0.92 |
| Saturation/% | 97.4 (±3) | 98.1 (±2) | 0.66 |
| PaO2/mmHg | 62 (±7) | 65 (±12) | 0.70 |
| PaO2/FiO2 ratio | 206.7 | 216.7 | 0.68 |
| PaCO2/mmHg | 42 (±11) | 42 (±9) | 0.95 |
| Temp/°C | 36.8 (±0.5) | 37.1 (±0.3) | 0.88 |
| M-WCAS | 2.3 (±0.6) | 2.0 (±0.7) | 0.86 |
RR, respiratory rate; HR, heart rate, PaO2, partial arterial oxygen; FIO2, fraction of inspired oxygen; PaCO2, partial arterial carbon dioxide; M-WCAS, modified Wood's Clinical Asthma score.
Differences between study groups 24h after the start of intervention.
| Variable | Air–oxygen | Heliox | p |
|---|---|---|---|
| RR/minute | 55 (±4) | 56 (±3) | 0.90 |
| HR/minute | 110 (±22) | 112 (±19) | 0.77 |
| Mean blood pressure/mmHg | 48 (±21) | 49 (±12) | 0.92 |
| Saturation/% | 98 (±3) | 98 (±2) | 0.66 |
| PaO2/mmHg | 62 (±7) | 65 (±12) | 0.70 |
| PaO2/FiO2 ratio | 206.7 | 216.7 | 0.68 |
| PaCO2/mmHg | 42 (±11) | 42 (±9) | 0.95 |
| Temp/°C | 36.8 (±0.5) | 37.1 (±0.3) | 0.88 |
| M-WCAS | 0.69 (±0.4) | 0.67 (±0.5) | 0.88 |
RR, respiratory rate; HR, heart rate; PaO2, partial arterial oxygen; FIO2, fraction of inspired oxygen; PaCO2, partial arterial carbon dioxide; M-WCAS, modified Wood's Clinical Asthma score.
The modified Wood's Clinical Asthma Score did not show any significant difference between both groups before treatment initiation; however, it dropped significantly after 2h of treatment, from 5.5 points (±1.0 points) in the heliox group vs. 5.4 points (±0.9 points) in the air–oxygen group, to 2.2 points (±0.4 points) vs. 4.0 points (±0.7 points), respectively (p=0.04). At the end of the 24h of treatment, the modified Wood's Clinical Asthma Score decreased in both groups, but the difference was not statistically significant (p=0.88).
No treatment-related adverse events were observed throughout the study period. All enrolled participants have completed the study. None of the enrolled participants required mechanical ventilation. Furthermore, all patients have fully recovered without any complications.
DiscussionHelium is a low molecular weight gas that, when mixed with oxygen, yields a mixture less dense than air.11 Therefore, such mixture can be used in cases of airway obstruction, through reduction of flow resistance and consequently a reduction in respiratory effort.12–14 Furthermore, heliox has been proved to enhance alveolar ventilation through its high diffusion coefficient and CO2 elimination.11,14,15
In the literature, there are recent references for the non-invasive use of heliox in the management of acute bronchiolitis in infants.16 The present study has shown that heliox, when combined with HFNC, will increase its efficacy regarding gas exchange and significantly reduce clinical scores evaluating respiratory distress in the initial phase of treatment in patients with RSV acute bronchiolitis. The response seen with heliox regarding increase of oxygenation boomed rapidly within the first 2h of the study intervention and was maintained throughout therapy, coherent with its way of action. The degree of clinical and gas exchange improvement observed in the present study when heliox was used with HFNC is similar to previous findings where heliox was used with nasal continuous positive airway pressure (n-CPAP) ventilation.17,18 However, other authors referred the improvement in oxygenation to be most likely due to n-CPAP ventilation rather than the heliox effect itself.17 Moraa et al., in a recent Cochrane review, showed evidence of short-term benefit of heliox inhalation as an adjuvant therapy with oral or intramuscular dexamethasone in children suffering from moderate to severe croup.4 Chowdhury et al., in a multicenter trial, found that heliox therapy did not reduce length of treatment when given via nasal cannula. Nevertheless, they have stated that the heliox effect was greater in tight-fitting facemasks or CPAP.19
The lack of CO2 clearance improvement in the present work was consistent with the earlier report by Gross et al.3 They failed to demonstrate any beneficial effect of various heliox mixtures on arterial CO2 in infants with mechanical ventilation due to moderate to severe RSV lower respiratory tract disease. Kneyber et al. reported the same results; however, their participants probably had a more severe illness than those in the present study, with higher PaCO2.20 Previously, they observed a favorable effect of heliox in obstructive airway among infants.
None of the present patients showed any adverse effect, regardless of the administered therapy. The techniques of gas delivery with HFNC and the inert nature of helium were well tolerated by all patients. However, the present study has some limitation. Randomization was not blind because of the discrepancy between both gas delivery systems. Discrimination between clinical phenotypes based on chest radiology was hindered by the small size of the study sample, but further research of this subject is encouraged.
The present data has shown the transient beneficial effect of heliox when provided with HFNC in terms of clinical condition and blood gas exchange in infants with RSV acute bronchiolitis during the initial phase of the non-invasive therapy. Moreover, such transient improvement may provide a precious time interval for other therapeutics to be effective or for the natural resolution of disease during the initial phase of therapy. Moreover, other interventions, such as intubation and mechanical ventilation may be avoided. Clearly, further work is needed to evaluate other aspects, such as ideal time for treatment onset, proper heliox mixture parentage to use, duration of therapy, and identification of non-responders. On the meantime, the mixture of helium and oxygen delivered through HFNC may be beneficial for RSV acute bronchiolitis management among infants.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Seliem W, Sultan AM. Heliox delivered by high flow nasal cannula improves oxygenation in infants with respiratory syncytial virus acute bronchiolitis. J Pediatr (Rio J). 2018;94:56–61.