The incidence of abnormal breathing and its consequences on craniofacial development is increasing, and is not limited to children with adenoid faces. The objective of this study was to evaluate the cephalometric differences in craniofacial structures and head posture between nasal breathing and oral breathing children and teenagers with a normal facial growth pattern.
MethodNinety-eight 7–16 year-old patients with a normal facial growth pattern were clinically and radiographically evaluated. They were classified as either nasal breathing or oral breathing patients according to the predominant mode of breathing through clinical and historical evaluation, and breathing respiratory rate predomination as quantified by an airflow sensor. They were divided in two age groups (G1: 7–9) (G2: 10–16) to account for normal age-related facial growth.
ResultsOral breathing children (8.0±0.7 years) showed less nasopharyngeal cross-sectional dimension (MPP) (p=0.030), whereas other structures were similar to their nasal breathing counterparts (7.6±0.9 years). However, oral breathing teenagers (12.3±2.0 years) exhibited a greater palate length (ANS-PNS) (p=0.049), a higher vertical dimension in the lower anterior face (Xi-ANS-Pm) (p=0.015), and a lower position of the hyoid bone with respect to the mandibular plane (H-MP) (p=0.017) than their nasal breathing counterparts (12.5±1.9 years). No statistically significant differences were found in head posture.
ConclusionEven in individuals with a normal facial growth pattern, when compared with nasal breathing individuals, oral breathing children present differences in airway dimensions. Among adolescents, these dissimilarities include structures in the facial development and hyoid bone position.
A incidência da respiração anormal e de suas consequências no desenvolvimento craniofacial aumenta e não é limitada a crianças com fácies adenoideanas. O objetivo deste estudo foi avaliar as diferenças cefalométricas nas estruturas craniofaciais e na postura da cabeça entre crianças e adolescentes com respiração nasal e respiração bucal com padrão de crescimento facial normal.
Método98 pacientes com idades entre 7-16 anos com padrão de crescimento facial normal foram avaliados de forma clínica e radiológica. Eles foram classificados como pacientes com respiração nasal ou respiração bucal de acordo com a predominância do modo de respiração por meio da avaliação clínica e histórica e da predominância da frequência respiratória conforme qualificado por um sensor de fluxo de ar. Os pacientes foram divididos em duas faixas etárias (G1: 7 a 9) (G2: 10 a 16) para contabilizar o crescimento normal facial relacionado à idade.
ResultadosAs crianças com respiração bucal (8,0±0,7 anos de idade) mostraram menor dimensão transversal nasofaríngea (MPP) (p=0,030), ao passo que outras estruturas foram semelhantes a seus pares com respiração nasal (7,6±0,9 anos de idade). Contudo, os adolescentes com respiração bucal (12,3±2,0 anos de idade) mostraram maior comprimento do palato (espinha nasal anterior-espinha nasal posterior (ENA-ENP)) (p=0,049), maior dimensão vertical na menor face anterior (Xi-ENA-Pm) (p=0,015) e menor posição do osso hioide a respeito do plano mandibular (H-PM) (p=0,017) que seus pares com respiração nasal (12,5±1,9 anos de idade). Não foram constatadas diferenças estatisticamente significativas na postura da cabeça.
ConclusãoMesmo em indivíduos com padrão de crescimento facial normal, em comparação a indivíduos com respiração nasal, as crianças com respiração bucal apresentam diferenças nas dimensões das vias aéreas. Entre os adolescentes, essas dissimilaridades incluem estruturas no desenvolvimento facial e na posição do osso hioide.
Physiological breathing is often affected by anatomic or functional problems, causing the respiratory cycle to be initiated not only through the nose but also through the mouth.1,2 Compared to nasal breathing (NB) children, oral breathing (OB) children are at higher risk for restless sleep, diaphoresis and enuresis at night, and, in some cases, even sleep apnea syndrome. The low-quality sleep materializes as daytime sleepiness, irritability, and headaches3 likely to negatively impact academic performance. Further, the presence of hyponasal speech or speech alterations4 increases the likelihood of being classified with a learning disability. In fact, many of these children are misdiagnosed with attention deficit hyperactivity disorder (ADHD) and sometimes erroneously medicated.5
Several studies postulate that OB children exhibit characteristics of the typical adenoid facies: a decrease in the facial prognathism, a small nose and nostrils, a short upper lip, and an open mouth posture which may be the source for a backward and downward rotation of the mandible that causes an increase in the vertical development of the lower anterior face and a narrower anteroposterior upper airway dimension.1,6–8 These patients’ muscle imbalance, owing to an anatomic recondition, may lead to cranio-cervical hyperextension and kyphotic posture.9,10 There are also reports of different types of malocclusion, such as open bites, anterior and/or posterior crossbites, class II malocclusion,11 constricted palates, and gummy smiles resulting in unattractive facial features.5 In addition, OB children often suffer from chronic gingivitis, periodontitis, candida infections,12 dental erosion, and cavities.13 Due to the difficulty of breathing and chewing simultaneously for extended periods, masticatory efficiency decreases.14 This, in turn, leads to OB children's preference for soft and oftentimes non-nutritious foods that increase the possibility of malocclusions and cavities.
Published evidence is inconclusive, in part, because growth patterns have not been taken into account, as certain physical characteristics are shared by subjects with a predominant vertical growth pattern, who, in turn, are more likely to be OB children.15 In addition, decreased adenoids and occlusal maturation have not been used as classification parameters when comparing across subjects.8 Moreover, different diagnostic tools have been used to classify breathing modes.
The main objective of this research was to evaluate the cephalometric differences in craniofacial structures (i.e., the form and position of the maxilla, mandible, upper airway, and hyoid bone) and head posture between NB and OB children and teenagers with a normal facial growth pattern, using a measurable diagnostic tool for breathing mode and a rigorous selection criteria of patients. It is hypothesized that there are anatomic differences in craniofacial structures in OB compared to NB children and teenagers, even in patients with a normal facial growth pattern.
MethodParticipantsParticipants were recruited at random during a routine clinic visit at the College of Integrated Child Dentistry at Seville University. Inclusion criteria were as follows: white boys and girls between 7 and 16 years of age; normal growth pattern appearance; free of any neurologic or congenital alterations, genetic syndromes, craniofacial malformations, severe systemic disease, respiratory allergies, obstructive sleep apnea syndrome (OSAS), or asthma. Exclusion criteria: any upper airway surgery, orthodontic or orthopedic procedures, prolonged use of a pacifier (more than six months) and/or baby bottle (more than two years), any habits like lip or finger sucking, or an evident anterior tongue position. Of the 187 children (11.3±0.2 years, 58.3% girls and 41.7% boys) evaluated for eligibility, 98 met the inclusion criteria. For all patients, one parent and/or legal guardian signed the informed consent form. The study and its protocol were approved by the Research Ethics Committee of the Virgen Macarena-Virgen del Rocio University Hospitals (Seville, Spain).
MeasuresNormal facial growth pattern was confirmed by cranial and facial index and cephalometric parameters (FP-MP) (n=68°±3.5°) to exclude children with a growth pattern predisposition. The cranial index measures transverse and anteroposterior diameters of the skull based on the following formula: maximum transverse diameter×100/maximum anteroposterior diameter. The scores are categorized as follows: dolichocephalic (<76), mesocephalic (76–81), or brachycephalic (>81). The facial index measures vertical and transverse parameters of the facies. The height of the face is determined starting on the superciliar plane (the line uniting the eyebrows) and measuring vertically to the gnathion point (i.e., the lowest point of the soft chin). The width of the face is measured based on the bizygomatic width as follows: maximum vertical diameter×100/maximum transverse diameter. The scores classify facies as: brachyfacial (<97), mesofacial (97–104), or dolichofacial (>104).16
Breathing mode (oral vs. nasal) was assessed by an Airflow Sensor for e-Health Platform, designed by Cooking Hacks (Libelium®, Libelium Comunicaciones Distribuidas S.L, Zaragoza, Spain). The sensor measured the nasal respiratory frequency accurately by detecting temperature changes in the airflow. This device consists of a set of two prongs placed in the nostrils and secured by a flexible thread that fits behind the ears. Breathing is measured by the sensors located inside the prongs. Two measurements were taken at different times to avoid punctual substantial fluctuations that could affect results. Patients underwent a complete clinical examination, and their clinical history and data were collected through a parent questionnaire. Based on this information, participants were classified as either OB or NB patients. OB children were defined by a lower nasal respiratory frequency (under 17 breaths per minute) as measured by the staff and based on parental reports that report predominant breathing through the mouth, showing an open mouth posture during the day and/or while sleeping (change from an upright to a supine position may cause a change in respiratory mode).1 Moreover, if the children frequently exhibited three or more of these symptoms, they were included: snoring, wheezing, drooling on the pillow, waking up during the night gasping for air, or getting up tired in the morning. Children were classified as nasal breathers if they had a high nasal respiratory frequency (above 18 breaths per minute), a closed mouth during the day and night, and the previously described symptoms were absent. The classification was supported by an otolaryngologist by means of rhinomanometry.
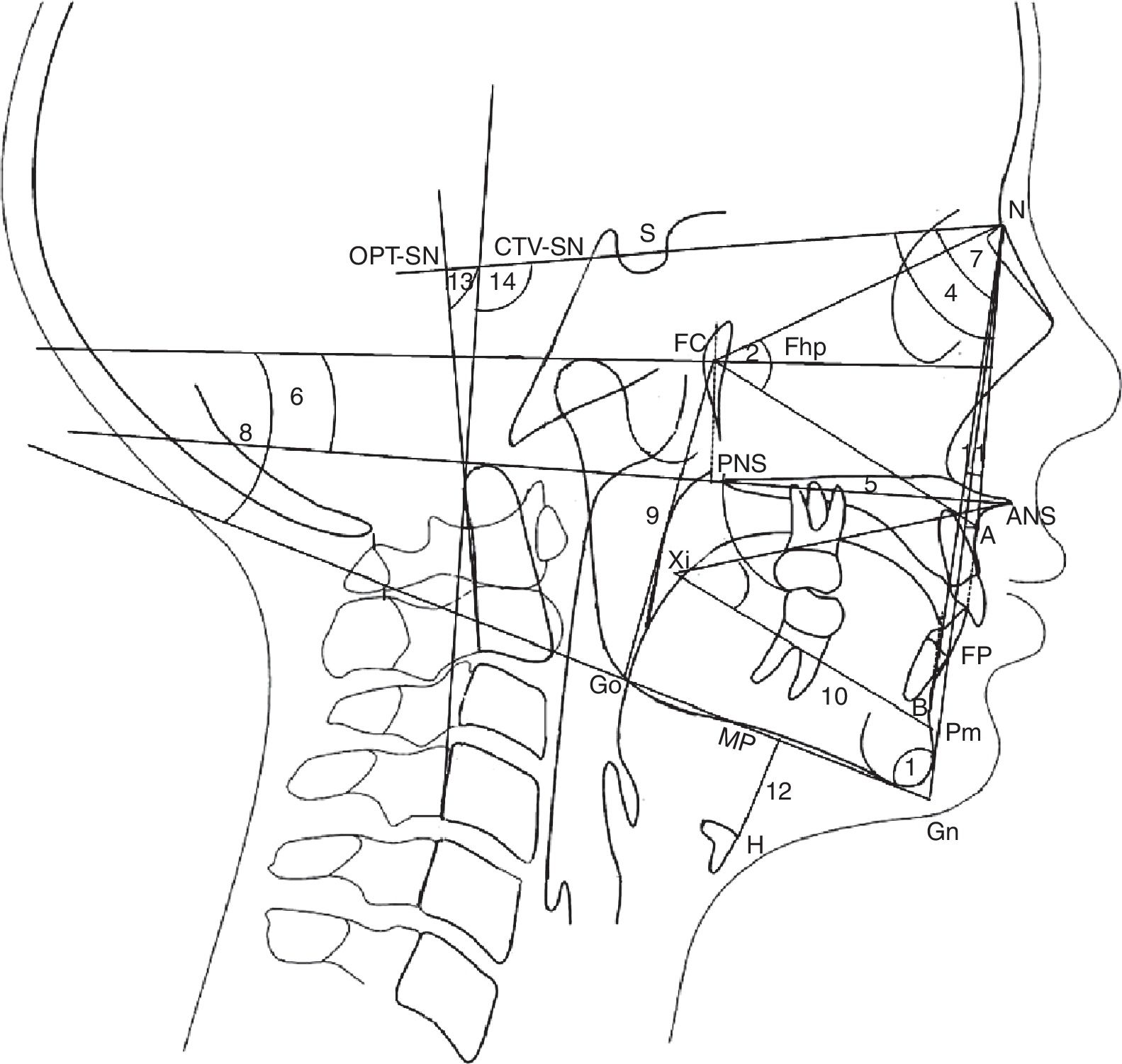
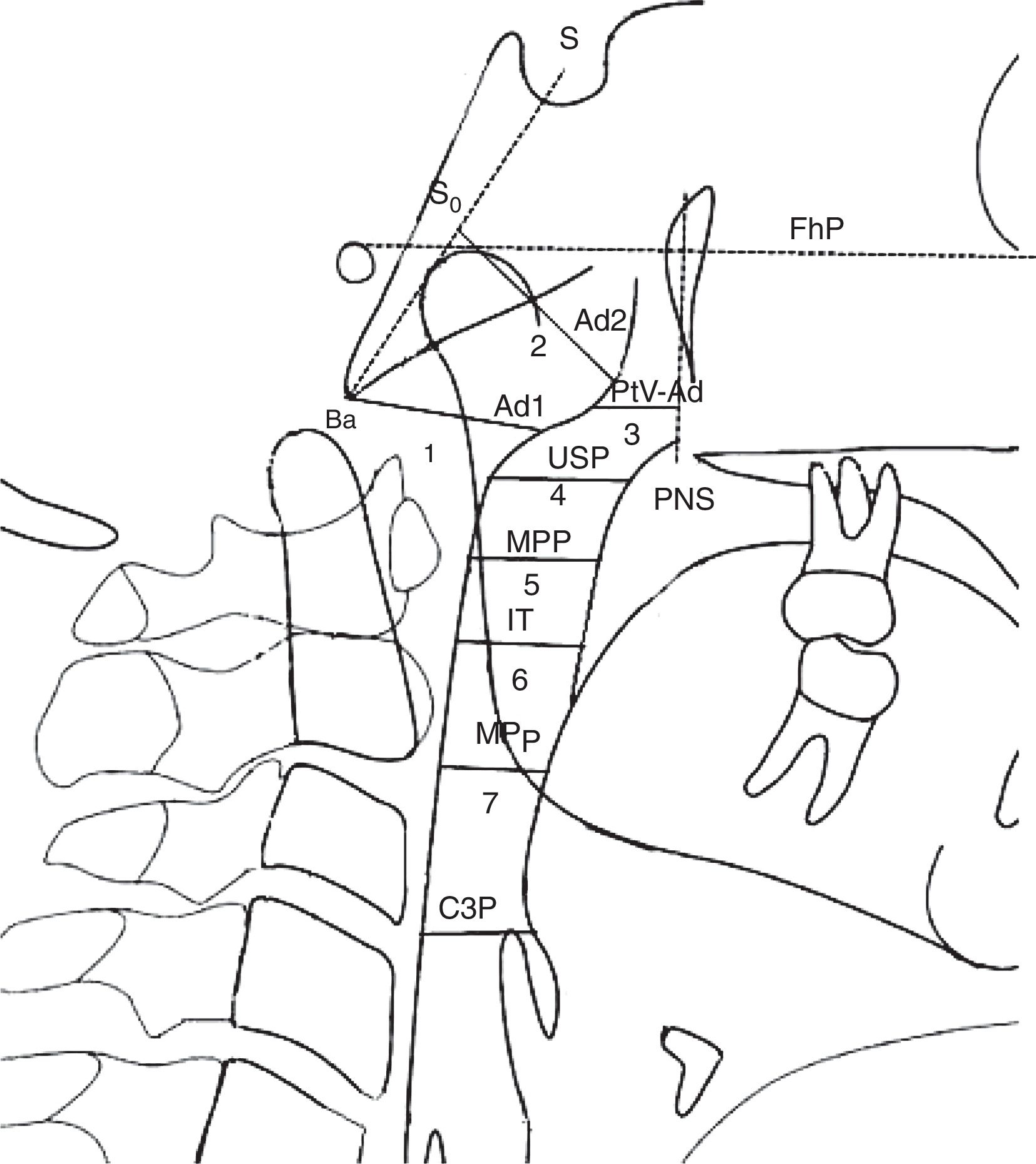
Lateral radiographs were taken standing with the body relaxed and with a natural head position (self-balance position)17 by X-ray equipment Planmeca Promax (Planmeca Oy), at the Faculty of Dentistry of Seville University. The cephalostat was placed without adding any pressure, so as to not affect the child's posture. Traditional cephalometric landmarks were hand-traced and digital radiographs were imported into a commercially available software system (Ortho TP®, Vimercate MicroLab, Vimercate, Italy) and analyzed again. The cephalometric parameters were chosen based on previous publications6,17–19 (Figs. 1 and 2). However, new measurements were added for airway dimensions:
- •
USP: Distance of a point of soft palate (5mm under to the upper point of the soft palate) (USP) to the horizontal counterpoint on the posterior pharyngeal wall parallel to the Frankfurt horizontal plane (FHP).
- •
IT: Distance of the posterior and inferior point of tonsil (T) (5mm upper to the down point of the tonsil) to horizontal counterpoint on posterior pharyngeal wall parallel to the FHP.
- •
MPP: Distance of the intersection points on anterior and posterior pharyngeal wall of the middle of the USP and IT parallel to the FHP.
- •
MPp: Distance of the intersection points on anterior and posterior pharyngeal wall of the mandibular plane (MP) parallel to the FHP.
- •
C3P: Distance between posterior pharyngeal from the most anterior and inferior point on the corpus of the third cervical vertebra (C3) and anterior pharyngeal (P) parallel to the FHP.
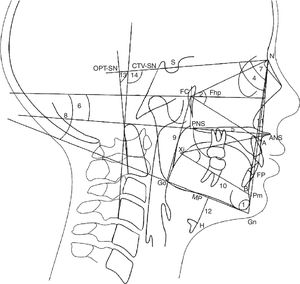
Cephalometric landmarks, angles, and reference planes. Growth pattern: 1. FP-MP Angle formed by facial plane (N-Pg) and mandibular plane (Gn-Go). Facial plane is formed by nasion (N) and pgonion (Pg). Mandibular plane is formed by gnation (Gn) and gonion (Go); facial height: 2. FCNA Angle formed by facial center point (FC) and line FC-nasion (N) and line FC-subspinale (A); 3. Xi-ANS-Pm Angle formed by center of the ramus point (Xi) and line Xi-anterior nasal spine (ANS) and Xi-suprapogonion (Pm); Maxilla: 4. SNA Angle formed by skull base line (SN) and line N-subspinale (A). Skull base is a plane from sella (S) to nasion (N); 5. ANS-PNS Distance from anterior nasal spine (ANS) to posterior nasal spine (PNS); 6. ANS-PNS-FhP Angle formed by palatal plane (ANS-PNS) and Frankfurt plane (FhP). Frankfurt plane is formed by orbitale (Or) and ponion (Po); Mandible: 7. SNB Angle formed by skull base line (SN) and line N-supramentale (B); 8. MP-FhP Angle formed by mandibular plane (MP) and Frankfurt plane (FhP); 9. Go-FC Distance from gonion (Go) to the facial center (FC); 10. Xi-Pm Distance from Xi to suprapogonion (Pm); Maxilla-Mandible: 11. ANB Angle formed by subspinale (A) and nasion (N) line and line N-supramentale (B); Hyoid bone: 12. H-MP Distance from the most anterior and superior point of hyoid bone (H) perpendicular to mandibular plane (MP); Craniocervical Posture: 13. OPT-SN Angle formed by (SN) and odontoides (OPT). OPT is formed by a line through the postero-superior point and postero-inferior point of odontoides; 14. CVT-SN Angle formed by (SN) and cervical (CVT). CVT is formed by a line through the postero-superior point and postero-inferior point of the four cervical.
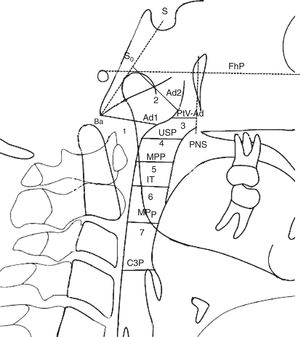
Airway dimensions. Nasopharynx: 1. Ad1-Ba Distance of (ad1) to basion (Ba); Ad1 is the intersection point of posterior pharyngeal wall and the line from posterior nasal spine (PNS) to basion (Ba); 2. ad2-S0 Distance of (ad2) to (S0). Ad2 is the intersection point of posterior pharyngeal wall and the line from the midpoint (S0) of the line from sella (S) to basion (Ba) to posterior nasal spine (PNS); 3. PtV-Ad Distance of (PtV) point to adenoid (Ad). PtV is a vertical line perpendicular to FhP passing through the most posterior point of the fossa pterigomaxilar. PtV point is located 5mm upper to PNS. Oropharynx: 4. USP Distance of a point of soft palate (5mm under to the upper point of Soft Palate) (USP) to the horizontal counterpoint on the posterior pharyngeal wall parallel to Frankfurt Plane (FhP). 5. MPP Distance of the intersection points on anterior and posterior pharyngeal wall of the middle of (USP) and (IT) parallel to FhP. 6. IT Distance of the posterior and inferior point of tonsil (T) (5mm upper to the down point of the tonsil) to horizontal counterpoint on posterior pharyngeal wall parallel to FhP. 7. MPP Distance of the intersection points on anterior and posterior pharyngeal wall of the mandibular plane (MP) parallel to FhP; Hypopharynx: 8. C3P Distance between posterior pharyngeal since the most anterior and inferior point on the corpus of the third cervical vertebra (C3) and anterior pharyngeal.
To detect errors in landmark identification and measurements, twenty randomly selected lateral cephalometric radiographs were measured and compared by the same investigator two weeks later.
Finally, patients were divided into two age groups (G1=7–9 years) (7.8±0.5 years) and (G2=10–16 years) (12.3±1.0 years) for three main reasons: (1) to avoid confusing breathing mode influence on craniofacial development with normal changes in growth; (2) to account for the process of occlusal maturation—associated with changes in the vertical dimension of the face—based on the variation in the eruption of permanent teeth to replace mixed dentition; and (3) to account for the decrease of adenoids that starts between the ages of 7 and 10, which widens the differences in nasopharyngeal dimensions. In children younger than 7 years old, adenoids are still physiologically present in a considerable volume in NB and OB children; therefore, it may difficult to find differences in the adenoids zone.20
Statistical analysesData were analyzed using descriptive statistical methods. Quantitative variables were described with means and standard deviations, and differences were tested for significance with Student's t-test for independent samples. Differences in non-parametric variables were tested for significance with the Mann–Whitney U test for independent samples. Statistical significance was set at two-sided p<0.05. Bonferroni correction was used as the adjustment method to maintain the probability of type I error below 5% (0.05). Accordingly, the p-value to consider statistically significant differences was 0.05/22=0.002. Statistical tests were performed using SPSS (SPSS for Windows, Version 16.0. Chicago, USA).
ResultsThe average respiratory rate was 18 breaths per minute; the lowest respiratory rate detected was 12 breaths per minute and the highest rate was 25 breaths per minute. The average cranial and facial index scores were 79.3±2.4 and 101.5±1.7, respectively.
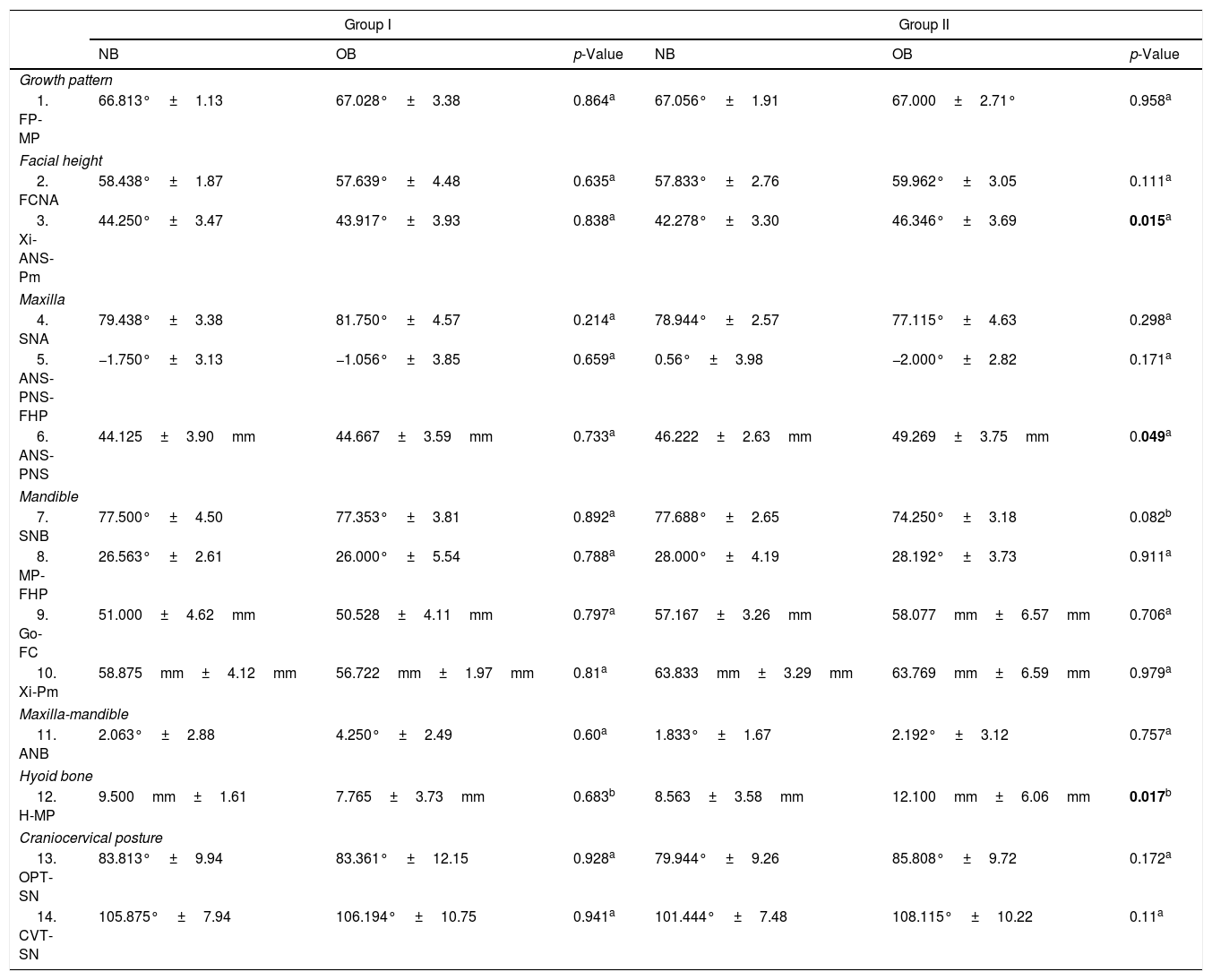
Means and standard deviations for cephalometric variables—craniofacial, hyoid position, head posture (Table 1), and airway parameters (Table 2)—from 56 OB patients (64.6%) and 42 NB patients (35.4%) were compared by age group. According to the lateral cephalometric analysis, in G1 the airway distance in the region of the tonsils (MPP) was lower in OB (8.0±0.7 years) than NB (7.6±0.9 years) (p=0.03). No statistically significant differences in the airway were found in G2. However, in G2, the lower anterior facial height (Xi-ANS-Pm) and the palate length (ANS-PNS) were higher in OB (12.3±2.0 years) than in NB (12.5±1.9 years) (p=0.015 and p=0.049, respectively). Also, the hyoid bone was located in a lower position relative to the mandibular plane (H-MP) in OB teenagers than NB ones (p=0.017). Finally, no statistically significant differences were found in the head posture between OB and NB patients in either age group (Table 2).
Craniofacial, hyoid position, and head posture parameters in nasal and oral breathing children and teenagers.
| Group I | Group II | |||||
|---|---|---|---|---|---|---|
| NB | OB | p-Value | NB | OB | p-Value | |
| Growth pattern | ||||||
| 1. FP-MP | 66.813°±1.13 | 67.028°±3.38 | 0.864a | 67.056°±1.91 | 67.000±2.71° | 0.958a |
| Facial height | ||||||
| 2. FCNA | 58.438°±1.87 | 57.639°±4.48 | 0.635a | 57.833°±2.76 | 59.962°±3.05 | 0.111a |
| 3. Xi-ANS-Pm | 44.250°±3.47 | 43.917°±3.93 | 0.838a | 42.278°±3.30 | 46.346°±3.69 | 0.015a |
| Maxilla | ||||||
| 4. SNA | 79.438°±3.38 | 81.750°±4.57 | 0.214a | 78.944°±2.57 | 77.115°±4.63 | 0.298a |
| 5. ANS-PNS-FHP | −1.750°±3.13 | −1.056°±3.85 | 0.659a | 0.56°±3.98 | −2.000°±2.82 | 0.171a |
| 6. ANS-PNS | 44.125±3.90mm | 44.667±3.59mm | 0.733a | 46.222±2.63mm | 49.269±3.75mm | 0.049a |
| Mandible | ||||||
| 7. SNB | 77.500°±4.50 | 77.353°±3.81 | 0.892a | 77.688°±2.65 | 74.250°±3.18 | 0.082b |
| 8. MP-FHP | 26.563°±2.61 | 26.000°±5.54 | 0.788a | 28.000°±4.19 | 28.192°±3.73 | 0.911a |
| 9. Go-FC | 51.000±4.62mm | 50.528±4.11mm | 0.797a | 57.167±3.26mm | 58.077mm±6.57mm | 0.706a |
| 10. Xi-Pm | 58.875mm±4.12mm | 56.722mm±1.97mm | 0.81a | 63.833mm±3.29mm | 63.769mm±6.59mm | 0.979a |
| Maxilla-mandible | ||||||
| 11. ANB | 2.063°±2.88 | 4.250°±2.49 | 0.60a | 1.833°±1.67 | 2.192°±3.12 | 0.757a |
| Hyoid bone | ||||||
| 12. H-MP | 9.500mm±1.61 | 7.765±3.73mm | 0.683b | 8.563±3.58mm | 12.100mm±6.06mm | 0.017b |
| Craniocervical posture | ||||||
| 13. OPT-SN | 83.813°±9.94 | 83.361°±12.15 | 0.928a | 79.944°±9.26 | 85.808°±9.72 | 0.172a |
| 14. CVT-SN | 105.875°±7.94 | 106.194°±10.75 | 0.941a | 101.444°±7.48 | 108.115°±10.22 | 0.11a |
Bold values represent the statistically significant difference.
NB, nasal breathing; OB, oral breathing.
Airway parameters in nasal and oral breathing children and teenagers.
| Group I | Group II | |||||
|---|---|---|---|---|---|---|
| NB | OB | p-Value | NB | OB | p-Value | |
| Airway Nasopharynx | ||||||
| 1. ad1-Ba | 23.833±5.57mm | 21.529±7.07mm | 0.261b | 21.611±3.87mm | 22.192±5.54mm | 0.789a |
| 2. ad2-S0 | 22.750±3.17mm | 21.417±3.41mm | 0.358a | 21.625±4.07mm | 20.800±6.28mm | 0.647b |
| 3. PtV-Ad | 11.500±3.76mm | 10.000±3.69mm | 0.724b | 14.556±6.00mm | 13.692±4.99mm | 0.717a |
| Oropharynx | ||||||
| 4. USP | 12.688±2.01mm | 11.333±2.72mm | 0.221a | 14.222±3.98mm | 12.577±3.38mm | 0.309a |
| 5. MPP | 11.000±2.46mm | 8.765±2.15mm | 0.030b | 10.000±2.34mm | 9.346±2.60mm | 0.554a |
| 6. IT | 10.063±2.51mm | 10.472±1.89mm | 0.650a | 10.167±4.13mm | 11.077±2.72mm | 0.539a |
| 7. MPp | 10.500±2.34mm | 9.972±2.69mm | 0.637a | 9.056±2.81mm | 10.731±3.94mm | 0.287a |
| Hypopharynx | ||||||
| 8. C3P | 9.938±2.77mm | 9.86±3.63mm | 0.958a | 8.444±3.14mm | 10.692±3.82mm | 0.162a |
Bold value represents the statistically significant difference.
NB, nasal breathing; OB, oral breathing.
Previous studies report that OB children have a hyperdivergent facial pattern21–24 and a greater lower anterior facial height6,11 that it was observed in our research in OB teenagers (G2) with a normal facial pattern but not in G1. A greater inclination of the mandible plane22,23 was observed which, together with a posterior rotation of palatal plane,25 might indicate the vertical direction of mandibular growth11,26 and the development of a class II skeletal malocclusion.11 However, the OB patients (63.9%) presented class I skeletal occlusion. According to previous findings, OB patients’ maxilla and mandible were more retruded in relation to their skull base.23 Nevertheless, Ucar et al.25 observed that only the maxilla was more retrognathic, whereas others found that only the mandible was more retruded.26
The present results show a low position of the hyoid bone relative to the mandibular plane in OB in G2, which supports previous findings by Cuccia et al.27
Nasopharyngeal sectional dimensions increase with the rest of body tissues during the growth period, but the adenoid tissue starts to diminish between the ages of 7 and 10, only to disappear during adulthood. The measurements of the upper airway space were smaller in OB than in NB children (G1) in the region of the tonsils (MPP) but not in G2, supporting previous work.26 Therefore, tonsils are more hypertrophic in children than teenagers. This result could be affected by the possibility that G1 patients’ adenoids were still at the onset of their reduction, whereas G2 patients’ adenoids were already shrunken. In addition, the new airway measurements in this study could affect the ability to compare, because they were parallel to the Frankfurt plane (a constant plane) to avoid incorrect comparative results based on a variable plane.
Several studies report OB patients with cranio-cervical hyperextension,27 whereby postural problems are significantly more common among these children.9 The present research showed a cervical spine postural change in 90.3% of OB but, as both groups presented high percentages of craniofacial hyperextension, differences were not statistically significant. It is speculated that the intense use of new technological devices by the young, such as cell phones and tablets, might contribute this lack of substantial differences in craniofacial hyperextension.
A previous study found myofunctional and craniofacial alterations among OB children between the ages of 7–10,23 whereas the present study found these alterations in OB children with a mean age of 12.3±2.0 (G2). The discrepancy may result from these studies failing to take the growth patterns into account. In the present study, patients with an abnormal growth pattern were excluded based on the cranial and facial index and cephalometric parameters, because patients with a vertical growth pattern have common skeletal features, i.e., narrower anteroposterior dimension of the airway, retrusion of the maxilla and the mandible, vertical maxillary excess, and a higher class II skeletal discrepancy.19,28 These patients’ characteristics might be a compensatory mechanism that could trigger the transition from NB to OB. Conversely, horizontal growth pattern is usually characterized by a more anterior mandible. This results in a wider lower pharyngeal airway, which favors nasal breathing. Therefore, growth patterns could affect or benefit physiological respiratory function.29
It is important to be able to detect patients with an OB predomination. Early referral for the correction of this pathological function is key for the prevention of irregularities in craniofacial development and orthodontic problems. By eliminating the growth pattern confounding in this study and comparing patients according to their growth stage, it was possible to detect if real differences exist between NB and OB children (G1) and teenagers (G2).
This study has certain limitations. First, in a cross-sectional study, associations do not imply causal relationships. In fact, Shanker et al.28 found that several children switched between oral and nasal respiratory mode during the four years of their investigation. However, as with any treatable or preventable condition, the possibility of an observational longitudinal study without intervening once OB is detected is precluded for ethical reasons. Second, the small sample size resulting from the strict selection criteria may have limited the power of the analyses to detect further differences. Third, because this study did not recruit NB as OB participants, the power of the analyses may have suffered from this substantial difference in the sizes of the subgroups being compared. Despite these limitations, these findings may help medical professionals better manage patients with breathing disturbances, knowing that this might indicate a developmental imbalance.
The study also has its strengths. The highly precise selection criteria, by including only patients with normal growth pattern, reduced the potential bias of including children with a genetic predisposition for OB. In addition, occlusal maturation and the physiological decrease of the adenoids were taken into account when comparing the results. Finally, a sensor that supplied measurable data to better classify patients’ mode of breathing was utilized. To the best of the authors’ knowledge, this measurement device had never been used in this context. This combined with the fact that a constant plane was used as reference for the airway measurements, may prove these data to be more accurate than those of much of previous research.
After examining children and teenagers with a normal growth pattern, this study shows that there are cephalometric differences between individuals with oral breathing and nasal breathing modes. Compared to nasal breathers, a lower anteroposterior dimension of the airway in oral breathing children is found; whereas in teenage oral breathers, there is a greater lower anterior facial height, a longer palate, and a lower position of the hyoid bone relative to the mandibular plane.
These findings are of practical interest to clinicians when diagnosing, treating, and/or referring patients to specialists for breathing disturbances-related issues. As these issues might indicate a development imbalance, early diagnosis is important to correct or ameliorate any negative effects with timely treatment.
Future research examining larger samples of patients with same selection criteria as used here is needed in order to examine their craniofacial development according to mode of breathing, while taking into account growth pattern, age, and gender. Such a study may provide further evidence of the substantial influence of breathing in craniofacial development and head posture.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Hosanna Soler-Vilá, Ph.D. for her remunerated medical editing services. Moreover, they thank Angelo Vanella, radiologist, for the Ortho TP software configuration.
Please cite this article as: Chambi-Rocha A, Cabrera-Domínguez ME, Domínguez-Reyes A. Breathing mode influence on craniofacial development and head posture. J Pediatr (Rio J). 2018;94:123–130.