This study aimed to analyze the neural encoding of verbal and nonverbal stimuli in individuals with autism spectrum disorder using brainstem auditory evoked potentials.
MethodologyThirty individuals between 7 and 12 years of age and of both genders participated in this study. Fifteen were diagnosed with autism spectrum disorder, and 15 had typical development. All subjects had normal hearing and no other impairments. An electrophysiological hearing assessment was performed using brainstem auditory evoked potentials with click and speech stimuli.
ResultsIn the brainstem auditory evoked potentials with click stimuli, the mean wave I latency was longer for the right ear in both groups, and interpeak intervals III–V were greater for the individuals with autism spectrum disorder. For brainstem auditory evoked potentials with speech stimuli, wave V latency was shorter in individuals with autism spectrum disorder.
ConclusionThese data suggest that individuals with autism spectrum disorder may have a dysfunction of the central auditory nervous system for nonverbal stimuli and faster neural encoding of the initial part of the verbal stimulus, suggesting hypersensitivity to complex sounds such as speech.
Este estudo visou analisar a codificação neural de estímulos verbais e não verbais em indivíduos com transtorno do espectro do autismo com o uso de potenciais evocados auditivos de tronco encefálico.
MetodologiaParticiparam 30 indivíduos entre sete e 12 anos e de ambos os sexos; 15 foram diagnosticados com transtorno do espectro do autismo e 15 apresentaram desenvolvimento típico. Todos os indivíduos apresentaram audição normal e nenhuma outra alteração. Foi feita uma avaliação eletrofisiológica da audição com o uso dos potenciais evocados auditivos de tronco encefálico com estímulos de clique e fala.
ResultadosNos potenciais evocados auditivos de tronco encefálico com estímulos de clique, a média do valor de latência da onda I foi maior para o ouvido direito nos dois grupos e os intervalos interpicos III-V foram maiores para os indivíduos com transtorno do espectro do autismo. Para os potenciais evocados auditivos de tronco encefálico com estímulos de fala, a latência da onda V foi menor nos indivíduos com transtorno do espectro do autismo.
ConclusãoNossos dados sugerem que os indivíduos com transtorno do espectro do autismo podem ter uma disfunção do sistema nervoso auditivo central para estímulos não verbais e codificação neural mais rápida da parte inicial dos estímulos verbais, o que sugere hipersensibilidade a sons complexos, como a fala.
Autism spectrum disorder (ASD) is a central disorder characterized by the inability to establish interpersonal relationships and to react normally to situations early in life, with an emphasis on social withdrawal.1
According to the Diagnostic and Statistical Manual of Mental Disorders,2 individuals with ASD may exhibit behavioral patterns that include restricted interests and repetitive and stereotyped behaviors. Furthermore, changes in reciprocal social interactions and modes of communication are observed. Additionally, it is known that individuals with ASD may have perceptual, cognitive, and memory disorders of attention that can be mistaken for auditory deficiencies.2
Moreover, individuals with ASD may be hyper- or hyposensitive to sensory stimuli and can be very sensitive to sounds; they may be troubled by loud sounds (hyperacusis) or be unable to hear and respond to loud sounds or noises (hypoacusis). Such sensitivities are also observed in relation to light and tactile sensation.3 Consequently, it is important to perform a complete audiological assessment that investigates both the peripheral and the central auditory pathways.4
Although they are very useful in routine hearing assessments, conventional or conditioned tonal audiometry, which are used to assess hearing acuity, and the battery of tests used to assess central auditory processing have limited clinical applicability for individuals with ASD because they require active responses from the patient. Individuals with ASD are not always able to respond accurately to the stimuli presented and/or perform the necessary tasks.
Consequently, it is important to include electrophysiological tests in the assessment of individuals with ASD. Such tests may be able to both predict the hearing threshold of these individuals and to identify possible changes in the central auditory pathways, thus aiding in diagnosis and early intervention.
Of the tests used for the electrophysiological assessment of hearing, brainstem auditory evoked potentials (BAEPs) are able to reflect synchronized electrical activity of the brainstem's neural elements in response to acoustic stimulation, and this neuronal activity can be observed in the first milliseconds after the presentation of the sound stimulus.5,6
Two types of acoustic stimuli can be used to elicit electrophysiological responses: verbal and nonverbal. These stimuli have different characteristics and elicit different responses; verbal stimuli are composed of a more complex temporal structure, and the response to these stimuli requires synchronic neuronal activation.7 The perception of these acoustic characteristics is related to neural encoding, an essential function of the central auditory nervous system, which must simultaneously process multiple cues to decode a linguistic message.8
Several studies have evaluated the role of the auditory pathway at the level of the brainstem in individuals with ASD using BAEPs.4,9–17 In general, the literature demonstrates that this population may present alterations in BAEPs; the most common findings are increased latency of waves III and V4,9,10 and an increase in the interpeak intervals for I–III and I–V, suggesting damage to the brainstems of these patients.4,10,12,13 However, these studies have evaluated this population using only one type of acoustic stimuli: clicks. The results have differed, however, and more studies are needed in this area to strengthen the hypotheses.
As the assessment of sound information processing is of interest to scientific research in this population, the present study is innovative in its assessment of BAEPs with both verbal and nonverbal stimuli in individuals with ASD. This research is of great importance because it can reflect the changes in sound conduction via the central auditory pathways, allowing a better understanding of acoustic information processing. The present study used two different types of stimuli; it makes an important contribution to the literature because only one previous study compared both stimulus types. Although it is not a pioneering study on the subject, most previous studies of BAEPs in ASD used only nonverbal stimuli.
Considering that individuals with ASD are hypo- or hypersensitive to sounds, the hypothesis of this study is this population will present abnormalities in BAEPs with both speech and click stimuli. Thus, this study aimed to analyze the neural encoding of verbal and nonverbal stimuli in individuals with ASD using BAEPs.
Materials and methodsThis was a prospective, cross-sectional clinical study approved by the Research Ethics Committee under number 227/15. All subjects who participated in the survey and their parents received explanations and signed informed consent forms.
SampleThe study included 30 individuals aged between 7 and 12 years and of both genders. The sample was divided into two groups:
- •
ASD group: A total of 26 families of individuals diagnosed with ASD were initially contacted. Of these, only 15 individuals participated and were able to complete all assessments (two females and 13 males, with a mean age of 9.07±1.75 years). All the individuals with ASD were diagnosed by psychiatrists and neurologists according to the DSM-IV criteria. The Autism Behavior Checklist was used to guarantee the homogeneity of the group, and the participating individuals scored between 75 and 85. The Wisconsin Card Sorting Test was used to assess cognitive development; the results were analyzed by an experienced psychologist and indicated performance around the low limits of normal development. All the individuals with ASD communicated verbally with major deficits in the functional use of language. The absence of any neurological disorder (such as seizures and/or syndromes) was an inclusion criterion.
- •
Typical development group (TD): This group comprised 15 normal, healthy individuals who were matched by age and gender to the ASD group. They did not present any psychiatric disorders, neurological antecedents, or auditory and/or speech or language complaints, and were spontaneously and voluntarily recruited.
In addition to the abovementioned inclusion criteria, only children with normal hearing were considered. To rule out possible hearing loss, an initial anamnesis was conducted with the parent or guardian to investigate the individual's medical history, and meatoscopy was performed to rule out possible obstruction of the external ear canal.
Subsequently, the following procedures were performed, and the following normality criteria were applied:
- •
Acoustic immittance measurement: Tympanometry was performed using an AT235 (Interacoustics®, AT235, Denmark) with ipsilateral and contralateral acoustic reflex analysis at frequencies of 0.5, 1, 2, and 4kHz. The inclusion criterion was the presence of a type A curve with acoustic reflexes.
- •
Pure tone audiometry: A audiometer (Grason-Stadler®, GSI 6, USA) was used. This was performed at frequencies of 0.5, 1, 2, and 4kHz, and in some cases, play conditioning was used. The accepted normality criterion was an audibility threshold of up to 20dB HL for all measured frequencies.
- •
Speech audiometry: A audiometer (Grason-Stadler®, GSI 6, USA) was used. The speech recognition threshold (SRT) and the speech recognition percentage index (SRPI) were assessed using the word lists suggested by Santos and Russo.18 The normality criteria were an SRT of up to 10dB HL above the mean pure tone threshold for the frequencies of 0.5, 1, and 2kHz; for the SRPI, more than 88% correct responses at an intensity of 30dB above the SRT was considered acceptable.
All tests were conducted in a soundproof booth, and the sound stimuli were presented through supra-aural headphones.
ProceduresElectrophysiological hearing assessments were performed using the Smart EP USB Junior (Intelligent Hearing System 5020, USA) in an acoustically treated room with the subject sitting comfortably in a reclining chair.
The participant's skin was cleansed with abrasive paste, and the electrodes were placed using electrolytic paste and microporous tape according to the IES 10-20 international standard.19 The impedance values of the electrodes were maintained below 5kΩ. BAEPs were measured using click and speech stimuli transmitted via insert earphones.
BAEPs with a click stimulus were performed with rarefied polarity at 80dB HL and were presented at a rate of 27.7 stimuli per second for a total of 2048 stimuli. The recording window was 24ms. Two tests were performed on each ear so that reproducibility of the trace and, consequently, the presence of the response could be verified. After the traces were collected, the absolute latencies of waves I, III, and V, and interpeak intervals I–III, III–V, and I–V were identified and analyzed.
BAEPs with speech stimuli were performed using the syllable /da/, which was delivered to the right ear only at an intensity of 80dB HL, a rate of 11.1 stimuli per second and a recording window of 60ms. Two sweeps of 3000 stimuli were performed for a total of 6000 stimuli. After collection, the traces were summed; in the trace resulting from this sum, the absolute latencies of waves V, A, C, D, E, F, and O and the V-A amplitude were identified and analyzed.
After the data were collected, they were submitted to descriptive and inferential statistical analyses. For the BAEPs with a click stimulus, the descriptive analysis was performed using summary measures for the absolute latencies of waves I, III, and V in milliseconds (ms) and for interpeak intervals I–III, III–V, and I–V in microvolts by ear (right and left) and by group (TD and ASD). In the inferential analysis, each latency and interpeak interval was analyzed using an adjusted normal mixed model in which the patient effect was considered random and group and ear effects were considered fixed.20 For each model, the diagnostic analysis showed that the adjusted model performed well.21
A significance level of 0.05 was adopted for each hypothesis test. First, the hypothesis that there was no interaction effect between group and ear was tested. When this hypothesis was not rejected (p-value >0.05), the hypotheses that there were no group or ear effects were tested.
For the BAEPs with speech stimulus, the equality of the two groups’ means for all variables was tested using Student's t-test for two independent samples with the same variance.22 Homoscedasticity and normality assumptions were verified and were shown to be satisfied.
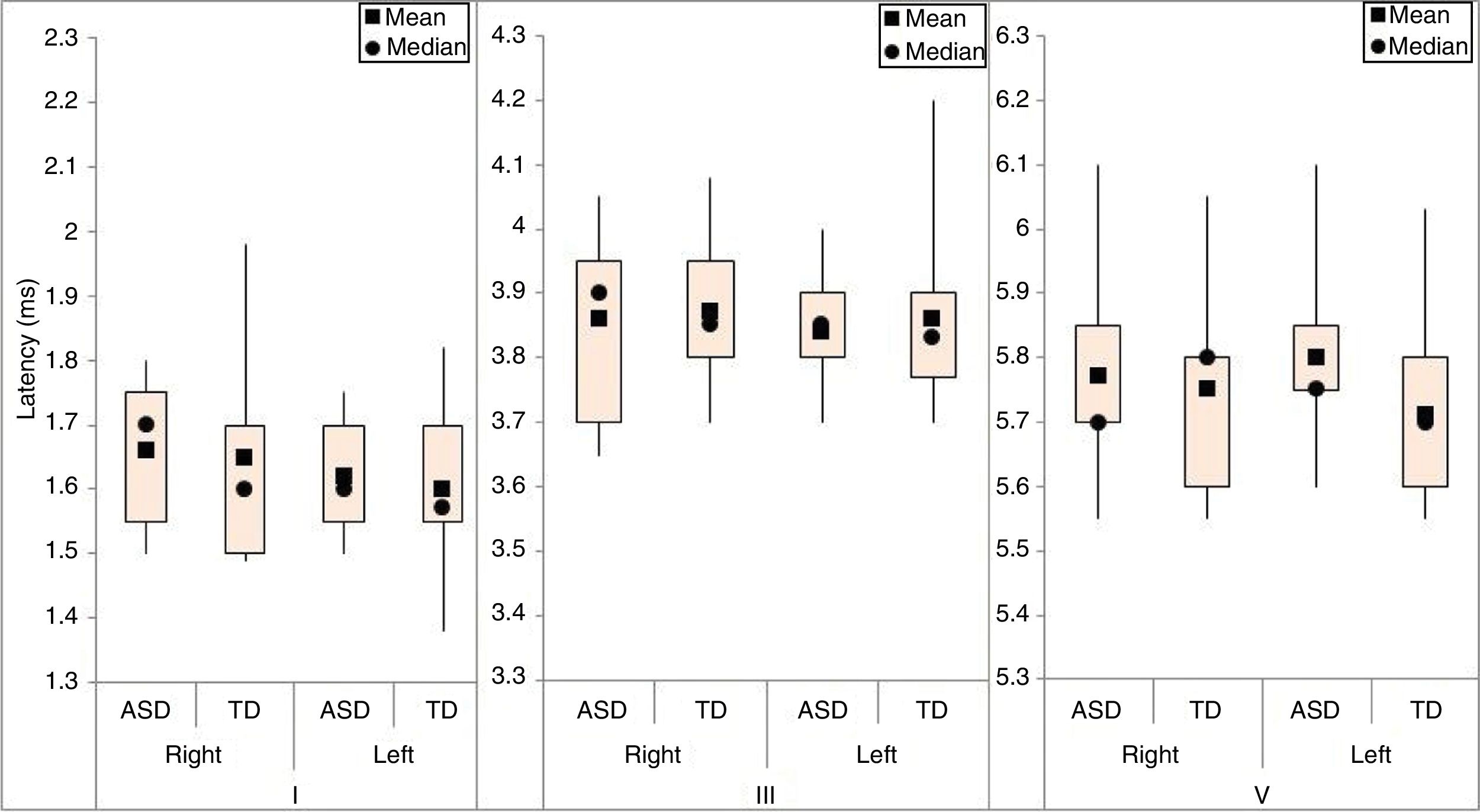
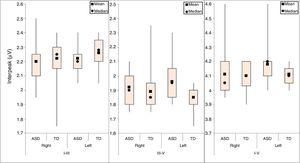
ResultsThe BAEP results were analyzed using descriptive measures for the absolute latency of waves I, III, and V in the right and left ears of both groups (Fig. 1).
For each of the absolute latencies of waves I, III, and V, an adjusted model that took into account the group and ear variables was applied. The interactions between these variables for each wave (p-values of 0.783, 0.606, and 0.179 for waves I, III and V, respectively) were compared for each group irrespective of ear and for both ears irrespective of group.
There was evidence of an ear effect; the mean absolute latency of wave I in the right ear was greater than that of the left ear (p-value=0.037) irrespective of group; the mean absolute latencies of waves III and V did not differ between ears (p-values of 0.326 and 0.613, respectively).
There was no evidence of a group effect; the mean absolute latencies of waves I, III, and V did not differ between groups (p-values of 0.666, 0.682, and 0.211, respectively) for either the right or left ear.
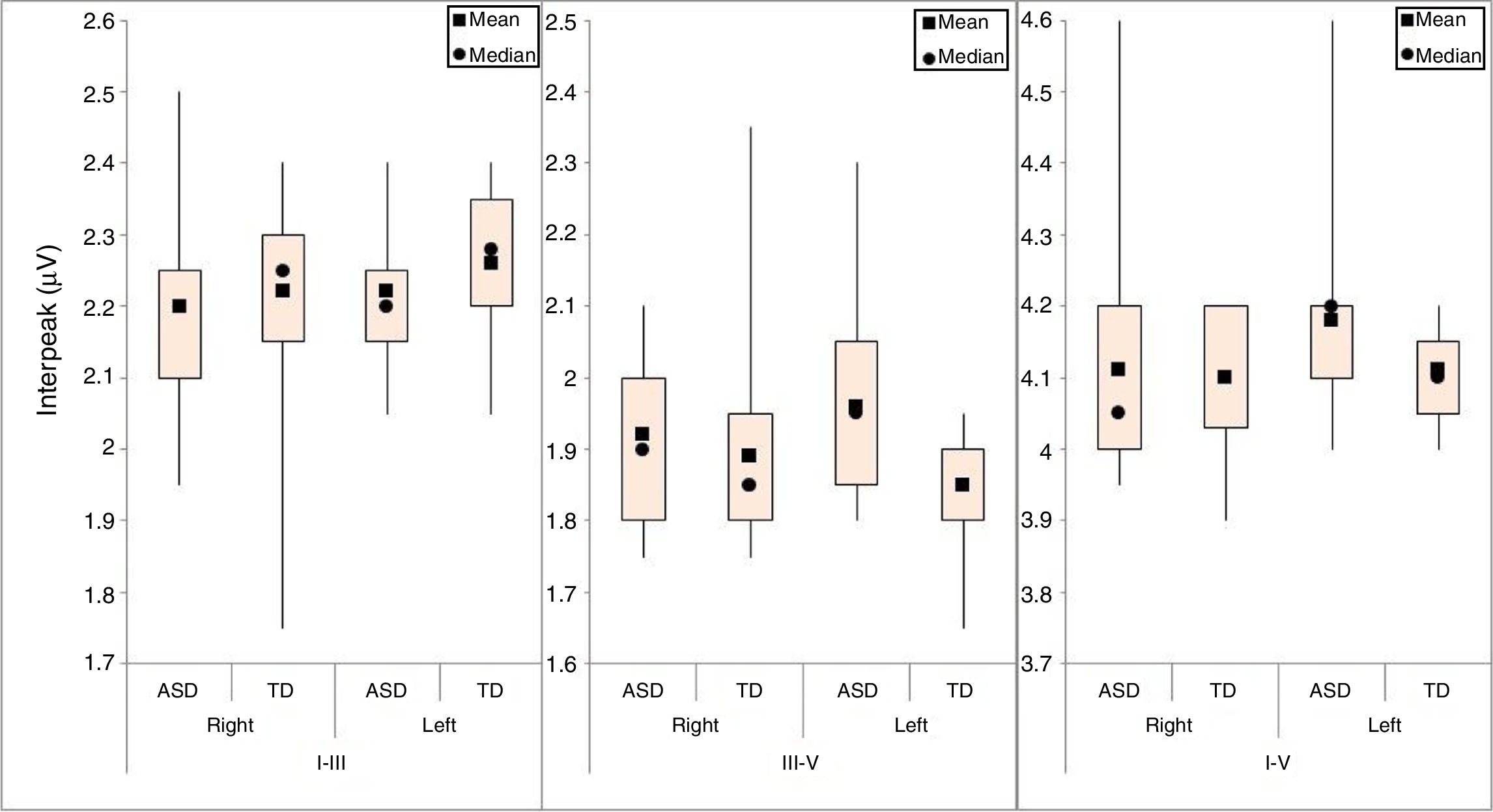
For interpeak intervals I–III, III–V, and IV, a descriptive analysis was performed on the right and left ears for both groups (Fig. 2).
Similar to the method used for the latencies, the inferential analysis of interpeak intervals I–III, III–V, and I–V used an adjusted model that took into account the group and ear variables. As there was no interaction among these variables (p-values of 0.634, 0.107, and 0.099 for interpeak intervals I–III, III–V, and I–V, respectively), the ears were compared irrespective of group, and the groups were compared irrespective of ear.
The results showed evidence of a group effect. The mean of interpeak interval III–V in the ASD group was greater than that of the TD group (p-value=0.046). The means of interpeak intervals I–III and I–V did not differ between the two groups (p-values of 0.368 and 0.332, respectively). Interpeak intervals I–III, III–V, and I–V did not differ between the ears (p-values of 0.261, 0.918, and 0.069, respectively).
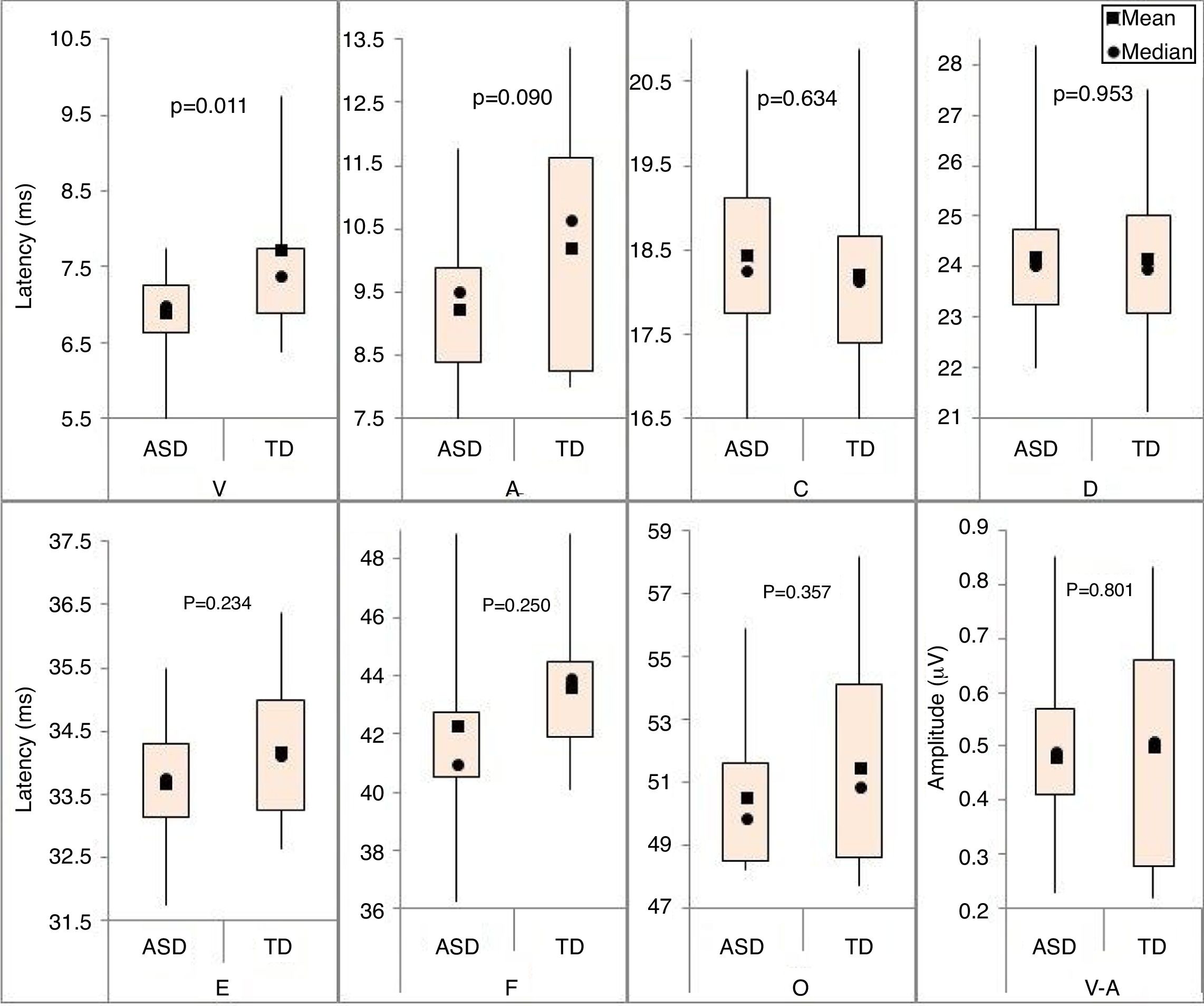
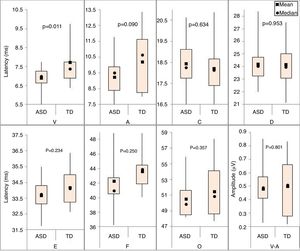
For the BAEPs with speech stimulus, descriptive measures were performed for the absolute latencies of waves V, A, C, D, E, F, and O, and the V-A amplitude in both groups (Fig. 3).
Significant differences were observed between the two groups only for the absolute latency of wave V (p-value=0.011), and the mean of the TD group was longer than that of the ASD group.
DiscussionBAEPs are objective measures that do not require the individual's active response. Consequently, one of the main clinical applications of BAEPs is the assessment of populations that are difficult to assess using behavioral methods, such as individuals with neurological and psychiatric disorders, including children with ASD.4,6
This study aimed to analyze the neural encoding of verbal and nonverbal stimuli of individuals with ASD using the BAEPs. To that end, BAEPs were elicited using click and speech stimuli in a group of children with ASD, and the results were compared with those of TD children.
Regarding the BAEPs elicited by click stimuli, no differences were observed between the absolute wave latency values of individuals with ASD and TD children. Regarding the interpeak intervals, there was a group effect for interpeak interval III–V, for which the ASD group showed greater values than the TD group.
The scientific literature is inconsistent regarding BAEP findings. Some studies confirm the lack of changes in the absolute wave latencies of BAEPs12,15–17; however, other authors have found an increase in the absolute latencies of waves III and IV,9 an increase in the latencies of waves I and V,10 and increased latencies of wave V in the right ear and wave I in the left ear in individuals with ASD.23
The dysfunctions observed along the auditory pathway in individuals with ASD have also been highlighted in other studies that observed changes in the interpeak intervals. One study that assessed children with ASD also found changes only in the III–V interpeak interval in these children compared with children with TD.12 Other studies have also observed increases in this interpeak interval, along with other changes.10,12,13,23
Additional studies have found changes interpeak intervals other than those observed in this study, such interpeak interval III–IV,9 interpeak intervals I–III and I–V,15 and interpeak interval I–III.24 In contrast, other studies have reported no statistically significant changes in BAEPs with click stimuli in individuals with ASD.16,17
Considering the findings described in the literature, it can be determined that individuals with ASD may have a distinct pattern of sound transmission throughout the nervous system; additionally, some studies describe alterations in the lower and/or upper brainstem; despite this, an extended onset of wave V has been demonstrated to be the most frequent alteration among individuals with ASD. Although the results of the present study show normal absolute latency for wave V, the increase in the III–V interpeak interval is also suggestive of a possible change in the upper brainstem region in these individuals.
The changes in the latency values, interpeak intervals, and wave amplitudes of BAEPs are known to reflect changes in the myelinationi, axon diameter, and synaptic efficacy of the auditory pathways at the brainstem level.25,26 Thus, the results of this study suggest that children with ASD exhibit abnormal cortical structures at the brainstem level, which impairs the conduction of neuroelectrical impulses of nonverbal stimuli between the cochlear nuclei and the lateral lemniscus.
Regarding the BAEPs elicited by speech stimuli, a statistically significant difference was found between the two groups in the absolute latency of wave V, which was shorter in the ASD group than in the TD group. However, the absolute latencies of waves A, C, D, E, F, and O, and the V-A amplitude showed no statistically significant differences between the groups.
Speech stimuli are complex and consist of numerous acoustic characteristics.8 To capture these signals, encoding must occur at the beginning of the stimulus in the brainstem.7
Some individuals with ASD may be hypersensitive to sounds and other sensory stimuli, which may make them more sensitive to the acoustic characteristics of a sound. This hypersensitivity could explain the decrease in the latency of wave V in the ASD group, which suggests faster neural encoding of the initial portion (onset) of the speech stimulus. This possible hyperresponsiveness to processing the immediate start of the acoustic stimulus does not seem to be sustained through the end of the stimulus (offset), as this difference was not observed for the other components (waves A, C, D, E, F, and O).3
The findings of this study differ from those of another study in which absolute latencies of waves V, A, C, and F of the BAEPs were longer in the ASD group than in the TD group.27
A study using click and speech stimuli in children with ASD found that the children with ASD had normal BAEPs in response to click stimuli; however, when speech stimuli were used, approximately 20% of the children with ASD exhibited aberrant encoding compared to controls. The authors suggested that speech stimuli were more sensitive than click stimuli for detecting subtle difficulties in language processing.28
This variation was not considered in all of the comparative studies above, which may have resulted in a very heterogeneous research sample. Currently, individuals with ASD can be subdivided into low- and high-functioning groups.25 This variation was not considered in all of the comparative studies above, which may have resulted in a very heterogeneous research sample. Furthermore, the discrepancies in the results could be due to differences in the mechanisms of auditory pathway dysfunction in along the spectrum of this disorder or to differences in the mechanisms used to process stimuli (click versus speech).29,30
The present study drew participants from a single center and was performed with a small sample; thus, the generalization of its findings is limited. Therefore, future studies should perform electrophysiological examinations of hearing in a greater number of individuals with ASD and should use these potentials to investigate neuronal plasticity in advance of therapeutic intervention.
FundingThis research was funded by São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP 2015/13239-7).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Kamita MK, Silva LA, Magliaro FC, Kawai RY, Fernandes FD, Matas CG. Brainstem auditory evoked potentials in children with autism spectrum disorder. J Pediatr (Rio J). 2020;96:386–92.
Study conducted at the Universidade de São Paulo, Faculdade de Medicina, Departamento de Fisioterapia, Fonoaudiologia e Terapia Ocupacional, São Paulo, SP, Brazil.