To evaluate treatment adherence among perinatally-infected pediatric human immunodeficiency virus (HIV) patients followed in pediatric centers in Brazil.
MethodsThis was a cross-sectional multicenter study. Medical records were reviewed and adherence scale, assessment of caregivers’ quality of life (WHOQOL-BREF), anxiety, depression, and alcohol/substances use/abuse were assessed. Outcomes included self-reported 100% adherence in the last three days and HIV viral load (VL)<50 copies/mL. Statistical analyses included contingency tables and respective statistics, and multivariable logistic regression.
Results260 subjects were enrolled: 78% children and 22% adolescents; 93% of caregivers for the children and 77% of adolescents reported 100% adherence; 57% of children and 49% of adolescents had VL<50 copies/mL. In the univariate analyses, HIV diagnosis for screening due to maternal infection, lower caregiver scores for anxiety, and higher scores in physical and psychological domains of WHOQOL-BREF were associated with 100% adherence. Shorter intervals between pharmacy visits were associated with VL<50 copies/mL (p ≤ 0.01). Multivariable regression demonstrated that caregivers who did not abuse alcohol/other drugs (OR=0.49; 95% CI: 0.27-0.89) and median interval between pharmacy visits<33 days (OR=0.97; 95% CI: 0.95-0.98) were independently associated with VL<50 copies/mL; whereas lower caregiver scores for anxiety (OR=2.57; 95% CI: 1.27-5.19) and children's HIV diagnosis for screening due to maternal infection (OR=2.25; 95% CI: 1.12-4.50) were found to be independently associated with 100% adherence.
ConclusionsPediatric HIV programs should perform routine assessment of caregivers’ quality of life, and anxiety and depression symptoms. In this setting, pharmacy records are essential to help identify less-than-optimal adherence.
Avaliar a adesão ao tratamento antirretroviral entre portadores de HIV acompanhados em centros pediátricos.
MétodosTrata-se de estudo transversal multicêntrico. Os prontuários ambulatoriais foram revistos e aplicadas escala de adesão, avaliação de qualidade de vida (WHOQOL-BREF), ansiedade, depressão e uso indevido de álcool/substâncias entre cuidadores. Os desfechos incluíram autorrelato 100% de adesão nos últimos três dias e carga viral do HIV (CV)<50 cópias/mL.
Resultados260 indivíduos foram incluídos, 79% crianças e 21% adolescentes; 93% das crianças e 77% dos adolescentes relataram 100% de adesão; 57% das crianças e 49% dos adolescentes tinham CV<50 cópias/mL. Na análise univariada, diagnóstico do HIV por triagem devido à infecção materna, cuidador com pontuação menor para ansiedade e maior nos domínios físico e psicológico do WHOQOL-BREF se mostraram independentemente associados a 100% de adesão. Intervalos mais curtos entre visitas de farmácia foram associados com CV<50 cópias/mL (p ≤ 0,01). Regressão multivariada mostrou que os cuidadores sem abuso de álcool/outras drogas (OR=0,49; IC95% 0,27-0,89) e o intervalo médio entre visitas de farmácia<33 dias (OR=0,97; IC95% 0,95-0,98) foram associados com CV<50 cópias/mL; cuidador com menores escores para ansiedade (OR=2,57; IC95% 1,27-5,19) e diagnóstico de crianças por triagem devido à infecção materna (OR=2,25; IC95% 1,12-4,50) foram associados com 100% de adesão.
ConclusõesProgramas de HIV pediátrico devem avaliar qualidade de vida e sintomas de ansiedade e depressão dos cuidadores. Registros de farmácia são essenciais na identificação de adesão insatisfatória.
In Brazil, 13,758 cases of perinatally-acquired human immunodeficiency virus (HIV) infection were reported as of July 2013.1 Access to combined antiretroviral therapy (cART) has changed the course of HIV disease among Brazilian perinatally-infected children.2 Sustained adherence to therapy is the most important determinant of successful treatment and is especially challenging among HIV-infected children and adolescents due to reasons such as dependency on caregivers, attitudes of defiance/denial, and delay in diagnosis disclosure to children.3–5 This study's aim was to evaluate treatment adherence among perinatally HIV-infected children and adolescents based on a biomedical (viral suppression) and a behavioral (cART missed doses) outcome, and to explore possible barriers to satisfactory adherence among the Brazilian population.
MethodsThis was a cross-sectional study conducted in five Brazilian centers, each located in one of the five Brazilian macro-regions.
Perinatally HIV-infected children and adolescents (0 to 18 years) on cART for at least eight weeks were eligible. Retrievable laboratory evaluations within six months of the study's inception were recorded. Study participation was offered as patients attended regular clinic visits with their legal guardians. Study centers have routine adherence support initiatives that include individual and familiar counseling and group activities; these strategies were not modified during study enrollment.
After signing the informed consent, caregivers and adolescents answered structured questionnaires that included sociodemographic information, number of missed doses of cART in the last three days, and the short version of the World Health Organization (WHO) questionnaire for quality of life (WHOQOL-BREF).6 Adherence assessment was peformed by trained healthcare workers with caregivers whenever the patient was a child (< 13 years), and directly with adolescents.
All caregivers answered the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).7 Anxiety and depression were assessed among caregivers and adolescents using the Hospital Anxiety and Depression Scale (HADS).8 Medical records were reviewed aiming to collect data, including: acquired immunodeficiency syndrome (AIDS) defining diagnosis, previous hospitalizations, CD4+T lymphocyte cell counts, HIV viral load, and information about the reason for initial HIV test (symptomatic children vs. HIV-exposed children screened in the context of family screening). Pharmacy records were electronically available at every clinical center and routine dispensing was scheduled to occur on a monthly basis.
Sample size was calculated based on the clientele registered for follow-up and treatment at participating sites, considering results of a previous study in Brazil on adherence among children and adolescents,9 a statistical power of 80%, and an alpha error of 5%. Outcomes for optimal adherence were defined as no missed doses of cART in the last three days (100% adherence) and viral load below the limit of detection (< 50 copies/mL) in the exam closest to enrollment.
Data were stored and analyzed using SPSS 18.0 software (SPSS Statistics for Windows, Version 18.0. Chicago, USA). The level of significance adopted in uni- and multivariable logistic regression analyses was 0.05. Contingency tables and respective statistics were used to assess the putative association between predictor variables (e.g., sociodemographic, biological [extracted from patients’ case report charts], QOL scores, anxiety and depression scores, alcohol and substance use and abuse) and the two outcomes: cART adherence and viral suppression. Multiple logistic regression was used to assess factors that showed association with the outcomes with p-values<0.25 in the univariate analyses and to investigate independent predictors by controlling for possible confounders. Models fitness was verified by Hosmer-Lemeshow statistics and analysis of residuals (data not shown).
The research protocol was submitted and approved by the institutional review board of each site: Hospital dos Servidores do Estado do Rio de Janeiro (Rio de Janeiro, RJ, Brazil), Fundação de Medicina Tropical do Amazonas (Manaus, AM, Brazil), Instituto de Medicina Integral Prof. Fernando Figueira (Recife, PE, Brazil), Grupo Hospitalar Conceição (Porto Alegre, RS, Brazil), and Universidade Federal de Mato Grosso do Sul (Campo Grande, MS, Brazil).
ResultsFrom December of 2009 to April of 2011, 572 HIV-infected children (< 13 years) and adolescents (> 13 and<18 years) attended HIV clinics at the five participating sites. From this total, 60 were not using cART or had been using it for less than eight weeks; 24 were infected by means other than vertical transmission; 19 did not have a legal guardian; 74 consented but did not complete the study procedures; 48 did not agree to participate; and 87 were not invited due to conflicting schedules. Therefore, 260 pairs of perinatally HIV-infected children or adolescents and their respective caregivers were enrolled into the study and completed the study procedures. Sociodemographic characteristics of enrolled patients were similar to those of the whole group. However, viral suppression among patients on cART was significantly worse among the whole group (51%), when compared to those fully enrolled (58%) (p<0.01). The study population comprised 203 children (78%) and 57 adolescents (22%).
HIV testing and diagnosis108/260 (42%) of the patients had HIV testing and diagnosis elicited by a symptomatic/clinical condition (not necessarily an AIDS-defining condition); 76/260 (29%) were tested because someone else in the family had HIV (i.e., family screening), and 73/260 (28%) were found to be HIV-infected in the first months of life, during follow-up as an infant born to an HIV-infected mother. Information could not be obtained for three asymptomatic children. Due to these distinct situations that influenced the timing of HIV diagnosis, children began to attend the clinic at mean ages of 4.5 years (SD=4.1), 4.3 years (SD=3.5), and 0.8 years (SD=1.8), respectively. Children assessed for AIDS-related symptoms were compared vis-à-vis the other two groups (i.e. those enrolled after family screening or routine follow-up of infants born to an HIV-infected mother). Mean age at study entry was 9.2 years (SD=4.3).
Centers for Disease Control and Prevention (CDC) 1994 Classification10At the time of referral to HIV specialized care centers, 90/260 subjects were classified as CDC clinical category N (34.6%); 27/260, as clinical category A (10.4%); 73/260, as clinical category B (28.1%); and 68/260, as clinical category C (26.2%). Information could not be obtained for two children. 83/260 (31.9%) already had advanced immunodeficiency (CDC 3 immune category). Thus, 116/260 (44.6%) of children/adolescents had already progressed to AIDS when first admitted to care (CDC categories C and/or 3), and 35 (13.4%) of them were classified as C3.
cART useAt the time of study enrollment, the mean duration of cART was seven years (SD=3.7). Forty-five (17%) children had used mono- or dual therapy before starting cART. At enrollment, 61% of children/adolescents were using cART with protease inhibitors (PI) and 39% were using cART with a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen; 98/260 (37.7%) were on their first regimen, 62/260 (23.8%) on their second regimen, and 100/260 (38.5%) on their third regimen or beyond.
Adherence assessment188/203 (92.6%) of the children (caregivers’ information) and 44/57 (77.2%) of the adolescents reported no missed doses of cART in the last three days (100% adherence).
Viral suppression120/203 (57%) of children and 28/57 (49%) of adolescents had plasma viral loads bellow 50 copies/mL at the clinical visit closest to enrollment. The chance of viral suppression did not differ among those who had received mono- or dual therapy prior to cART initiation (OR=1.0; 95% CI: 0.70 – 1.5).
Association between optimal adherence and viral suppressionThe two proposed outcomes (viral load<50 copies/mL and no ART missed dose in the last three days) had no statistical association (p=0.34) for both children and adolescents.
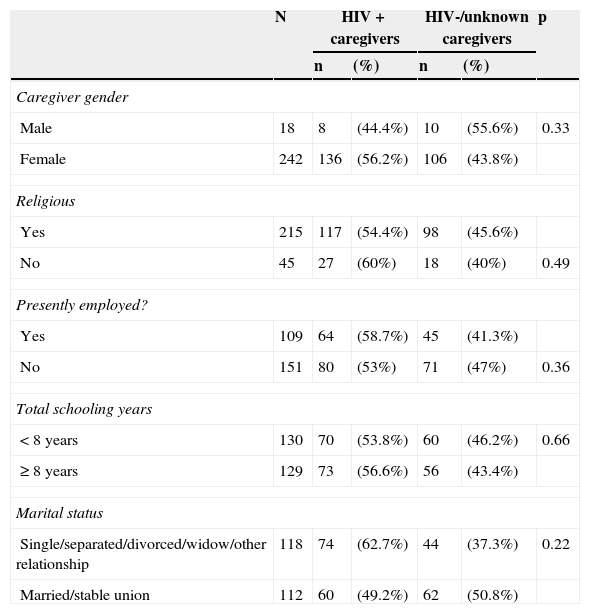
Caregiver parametersOver half of the caregivers (142/260; 54.6%) were HIV-infected (mostly a biological parent), 102/260 (39.2%) were not infected by HIV (“uninfected”), and 16/260 (6.2%) did not know their HIV status. Caregivers living or not living with HIV had similar sociodemographic characteristics, except that HIV-infected caregivers were significantly younger, compared to those who were not living with HIV (p<0.01), as shown in Table 1. HIV-infected caregivers were more likely to abuse alcohol or other substances than uninfected caregivers (p=0.02).
Sociodemographic characteristics of caregivers according to HIV status.
| N | HIV+caregivers | HIV-/unknown caregivers | p | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Caregiver gender | ||||||
| Male | 18 | 8 | (44.4%) | 10 | (55.6%) | 0.33 |
| Female | 242 | 136 | (56.2%) | 106 | (43.8%) | |
| Religious | ||||||
| Yes | 215 | 117 | (54.4%) | 98 | (45.6%) | |
| No | 45 | 27 | (60%) | 18 | (40%) | 0.49 |
| Presently employed? | ||||||
| Yes | 109 | 64 | (58.7%) | 45 | (41.3%) | |
| No | 151 | 80 | (53%) | 71 | (47%) | 0.36 |
| Total schooling years | ||||||
| <8 years | 130 | 70 | (53.8%) | 60 | (46.2%) | 0.66 |
| ≥ 8 years | 129 | 73 | (56.6%) | 56 | (43.4%) | |
| Marital status | ||||||
| Single/separated/divorced/widow/other relationship | 118 | 74 | (62.7%) | 44 | (37.3%) | 0.22 |
| Married/stable union | 112 | 60 | (49.2%) | 62 | (50.8%) | |
HIV, human immunodeficiency virus; HIV+, caregiver living with HIV; HIV-/unknown, caregiver not living with HIV or with unknown status for HIV.
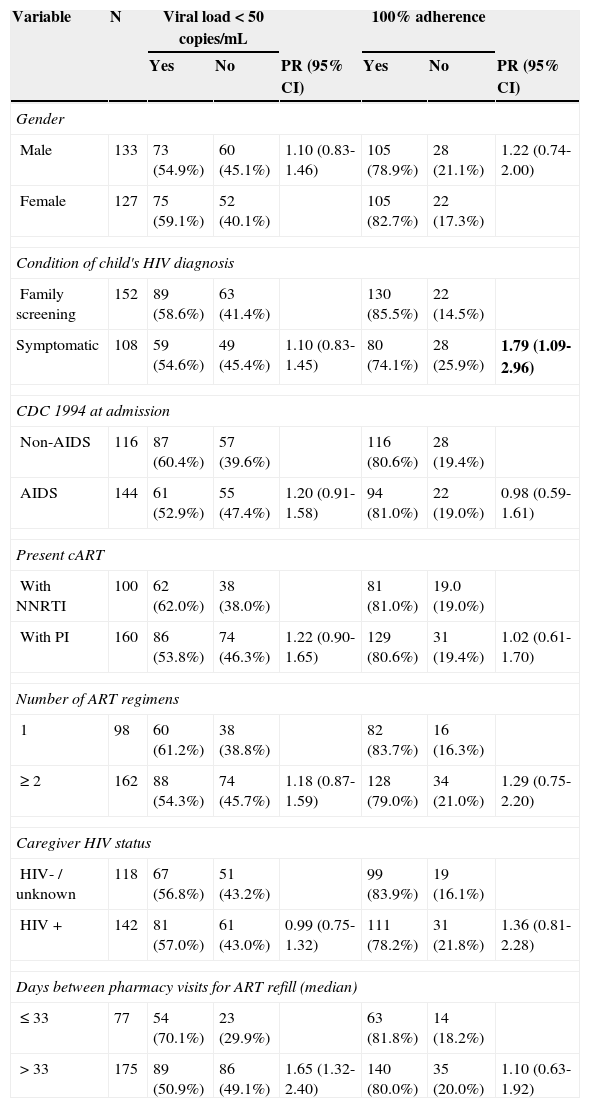
252/260 patients (97%) had available pharmacy records. Median interval between pharmacy visits for cART refill in the last twelve months was 32 days for children who showed viral suppression and 40 days for those with detectable viral load (prevalence ratio [PR]=1.65 [95% CI: 1.32-2.40]).
Univariate analysisTable 2 shows the distribution of key covariates from children/adolescents and their putative associations with the two outcomes under analysis. Table 3 summarizes findings on caregivers’ anxiety and depression assessment, screening for alcohol and other substance abuse, and scores related to quality of life and their putative association with the two outcomes among children/adolescents.
Univariate analysis of sociodemographic and clinical data of children/adolescents and their association with proposed outcomes (viral load<50 copies/mL and 100% adherence).
| Variable | N | Viral load<50 copies/mL | 100% adherence | ||||
|---|---|---|---|---|---|---|---|
| Yes | No | PR (95% CI) | Yes | No | PR (95% CI) | ||
| Gender | |||||||
| Male | 133 | 73 (54.9%) | 60 (45.1%) | 1.10 (0.83-1.46) | 105 (78.9%) | 28 (21.1%) | 1.22 (0.74-2.00) |
| Female | 127 | 75 (59.1%) | 52 (40.1%) | 105 (82.7%) | 22 (17.3%) | ||
| Condition of child's HIV diagnosis | |||||||
| Family screening | 152 | 89 (58.6%) | 63 (41.4%) | 130 (85.5%) | 22 (14.5%) | ||
| Symptomatic | 108 | 59 (54.6%) | 49 (45.4%) | 1.10 (0.83-1.45) | 80 (74.1%) | 28 (25.9%) | 1.79 (1.09-2.96) |
| CDC 1994 at admission | |||||||
| Non-AIDS | 116 | 87 (60.4%) | 57 (39.6%) | 116 (80.6%) | 28 (19.4%) | ||
| AIDS | 144 | 61 (52.9%) | 55 (47.4%) | 1.20 (0.91-1.58) | 94 (81.0%) | 22 (19.0%) | 0.98 (0.59-1.61) |
| Present cART | |||||||
| With NNRTI | 100 | 62 (62.0%) | 38 (38.0%) | 81 (81.0%) | 19.0 (19.0%) | ||
| With PI | 160 | 86 (53.8%) | 74 (46.3%) | 1.22 (0.90-1.65) | 129 (80.6%) | 31 (19.4%) | 1.02 (0.61-1.70) |
| Number of ART regimens | |||||||
| 1 | 98 | 60 (61.2%) | 38 (38.8%) | 82 (83.7%) | 16 (16.3%) | ||
| ≥ 2 | 162 | 88 (54.3%) | 74 (45.7%) | 1.18 (0.87-1.59) | 128 (79.0%) | 34 (21.0%) | 1.29 (0.75-2.20) |
| Caregiver HIV status | |||||||
| HIV- / unknown | 118 | 67 (56.8%) | 51 (43.2%) | 99 (83.9%) | 19 (16.1%) | ||
| HIV+ | 142 | 81 (57.0%) | 61 (43.0%) | 0.99 (0.75-1.32) | 111 (78.2%) | 31 (21.8%) | 1.36 (0.81-2.28) |
| Days between pharmacy visits for ART refill (median) | |||||||
| ≤ 33 | 77 | 54 (70.1%) | 23 (29.9%) | 63 (81.8%) | 14 (18.2%) | ||
| >33 | 175 | 89 (50.9%) | 86 (49.1%) | 1.65 (1.32-2.40) | 140 (80.0%) | 35 (20.0%) | 1.10 (0.63-1.92) |
PR, prevalence ratio; CI, confidence interval; HIV, human immunodeficiency virus; CDC, Centers for Disease Control and Prevention; HIV+, caregiver living with HIV; HIV-/unknown, caregiver not living with HIV or with status unknown for HIV; cART, combined antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; ART, antiretroviral therapy.
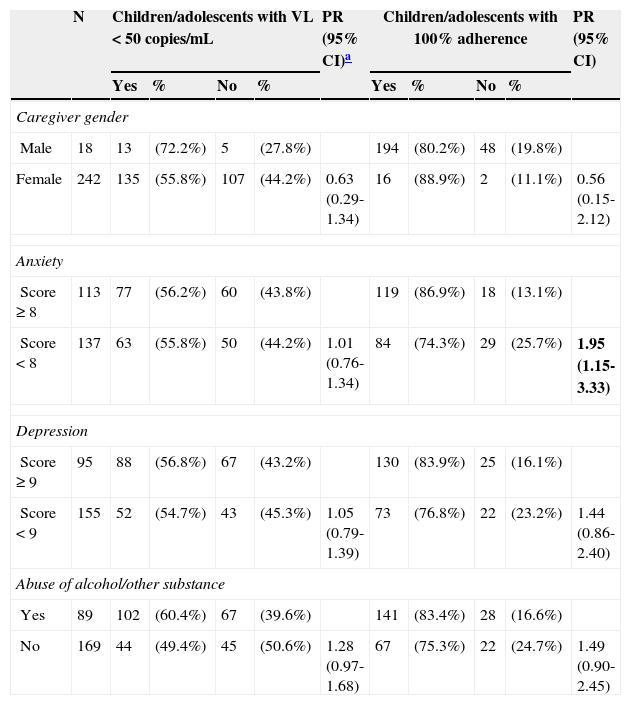
Results of caregivers’ evaluations of anxiety and depression, screening for misuse of alcohol and other substances (ASSIST scores ≥ cut-off) and quality of life (WHOQOL-BREF with its domains) and their association with studies outcomes children/adolescents.
| N | Children/adolescents with VL<50 copies/mL | PR (95% CI)a | Children/adolescents with 100% adherence | PR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | % | No | % | Yes | % | No | % | ||||
| Caregiver gender | |||||||||||
| Male | 18 | 13 | (72.2%) | 5 | (27.8%) | 194 | (80.2%) | 48 | (19.8%) | ||
| Female | 242 | 135 | (55.8%) | 107 | (44.2%) | 0.63 (0.29-1.34) | 16 | (88.9%) | 2 | (11.1%) | 0.56 (0.15-2.12) |
| Anxiety | |||||||||||
| Score ≥ 8 | 113 | 77 | (56.2%) | 60 | (43.8%) | 119 | (86.9%) | 18 | (13.1%) | ||
| Score<8 | 137 | 63 | (55.8%) | 50 | (44.2%) | 1.01 (0.76-1.34) | 84 | (74.3%) | 29 | (25.7%) | 1.95 (1.15-3.33) |
| Depression | |||||||||||
| Score ≥ 9 | 95 | 88 | (56.8%) | 67 | (43.2%) | 130 | (83.9%) | 25 | (16.1%) | ||
| Score<9 | 155 | 52 | (54.7%) | 43 | (45.3%) | 1.05 (0.79-1.39) | 73 | (76.8%) | 22 | (23.2%) | 1.44 (0.86-2.40) |
| Abuse of alcohol/other substance | |||||||||||
| Yes | 89 | 102 | (60.4%) | 67 | (39.6%) | 141 | (83.4%) | 28 | (16.6%) | ||
| No | 169 | 44 | (49.4%) | 45 | (50.6%) | 1.28 (0.97-1.68) | 67 | (75.3%) | 22 | (24.7%) | 1.49 (0.90-2.45) |
| Quality of life (Domains)b | Mean rank | Mean rank | Mann-Whitney U | Mean rank | Mean rank | Mann-Whitney U | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical | 109 | 27.13 | 139 | 27.53 | 0.52 | 46 | 26.26 | 202 | 27.61 | 0.04 |
| Psychological | 109 | 21.8 | 141 | 22.26 | 0.38 | 47 | 20.69 | 203 | 22.37 | 0.00 |
| Social | 101 | 10.28 | 129 | 10.79 | 0.22 | 44 | 10.02 | 186 | 10.69 | 0.18 |
| Environmental/contextual | 108 | 23.91 | 141 | 25.14 | 0.09 | 46 | 23.76 | 203 | 24.8 | 0.17 |
ASSIST, Alcohol, Smoking and Substance Involvement Screening Test; WHOQOL-BREF, short version of the WHO questionnaire for quality of life; VL, viral load; PR, prevalence ratio; CI, confidence interval.
Clinically relevant covariates associated with the outcomes at the level of p<0.25 in the univariate analyses were included in the model. All intermediary steps and the final model were controlled for age. Multivariable logistic regression showed that caregivers who did not abuse alcohol and other drugs (OR=0.49; 95% CI: 0.27-0.89), as well as those who came to the pharmacy for cART refill with median interval less than 33 days (OR=0.97; 95% CI: 0.95-0.98) were independently associated with viral load<50 copies/mL among children and adolescents; whereas caregivers with anxiety score less than 8 at HAD (OR=2.57; 95% CI: 1.27-5.19) and children who had HIV diagnosis as a result of family screening (OR=2.25; 95% CI: 1.12-4.50) were found to be independently associated with no missed doses of ART in the last three days.
DiscussionAt study admission, over half (59%) of the children and half of the adolescents had viral suppression, whereas roughly 93% of children and 77% of adolescents reported no missed doses of ART in the last three days, which suggests both caregivers for the children and adolescents tended to overestimate their actual adherence. It is very unlikely that such pronounced discrepancies between self-reported adherence and viral load is, above all, due to secondary to errors in prescription and/or the emergence of drug resistance over short periods of time. The study was performed in referral centers, where patients were managed by experienced physicians and multidisciplinary health teams, with full access to a comprehensive portfolio of ARV medicines, at no cost at the point of delivery.
The present results were comparable to other recent Brazilian studies.11,12 Filho et al. interviewed 101 adolescents in Rio de Janeiro regarding missed doses of cART in the last three days and observed that 80% were adherent to treatment. Ernesto et al. studied 108 children and adolescents in Campinas, and interviewed them regarding the use of cART in the last 24h and the last seven days, as well as checked their pharmacy records. The authors found that 54.6% of the study population could be defined as non-adherent, considering at least one of these outcomes.
There is no gold standard with respect to the proper measurement of adherence, for either adults or children/adolescents. Questionnaires about missed doses of cART are frequently used to evaluate adherence in pediatric populations.9,13–18 Other commonly used methods include pill counts, records of pharmacy visits, medication diaries, electronic devices such as Medication Event Monitoring System (MEMS) (MWV Healthcare, Sion, Switzerland) caps, and therapeutic drug monitoring. A systematic literature review on adherence evaluation, which included 176 studies, demonstrated the use of a single method in most studies; in 71%, self-administered questionnaires were the method of choice.19
Many studies involving HIV-infected patients using of cART have found good association between viral suppression and adherence scale scores, including validated scales assessing self-reported missed doses.14,15,20,21 However, the present results corroborate those of Mellins et al.15 and Allison et al.,21 presenting findings from studies conducted in the United States. These two studies did not demonstrate significant associations between reported missed doses of ART in the last days and viral suppression. A meta-analysis on the association between adherence to cART and viral suppression among children, adolescents, and young adults found that this relationship was stronger for longitudinal studies when compared to cross-sectional studies, and that this association tended to be weaker (or absent) when the informant was the caregiver.22
In the present study, “no missed cART doses in the last three days”, as reported by patients/caregivers, was found to be associated with improved quality of life of caregivers, low anxiety scores, as well as the context and timing of HIV diagnosis, with better adherence found among those diagnosed by family screening. Most of these findings are in accordance with previous pediatric studies. In 2004, Mellins et al. interviewed 75 caregivers in the United States about their children's ART missed doses in the last month and observed that non-adherence was associated with worse parent-child communication, higher levels of stress, and lower quality of life among caregivers, as well as poor cognitive functioning and less open disclosure of caregivers’ HIV status to others.15 A systematic review on adherence to cART versus quality of life among adults living with HIV identified 12 studies, most of them aiming to evaluate how adherence has been associated with quality of life as a treatment outcome.23 Different results suggest that a virtuous cycle may exist, with optimal adherence and a high quality of life boosting each other through a feedback loop. Anxiety has been previously identified as a predictor of non-adherence among HIV-infected adults. A comprehensive survey implemented in the United States on behalf of the HIV Cost and Services Utilization Study found anxiety disorder, depression, and drug use as key risk factors for non-adherence.24 Findings from a Brazilian study conducted in adults using the same instrument as that of the present study (Hospital Anxiety and Depression Scale) were similar to these results. In this previous study, high scores for anxiety were found to be associated with an increased risk of non-adherence, whereas no association was found with depression scores.25
Caregivers of children and adolescents who were diagnosed in the context of routine screening of perinatally-exposed children were more likely to report no missed cART dose in the last three days when compared to those whose HIV testing had been motivated by clinical events/symptoms (not necessarily an AIDS-defining illness). The present findings differ from those shown by PENTA 5, where sicker children had better adherence to treatment.14 The present data suggest that families who accept screening procedures for children whenever a parent or sibling has a diagnosis of HIV infection may be more likely to adhere to treatment. The WHO estimates that only 15% of HIV-exposed infants are tested in the first months of life in low- and middle-income countries. In Brazil, many programs devoted to prevention of mother-to-child-transmission of HIV (PMTCT) seek to stimulate the full integration of maternal and pediatric care, and offer continued family follow-up, which is the case of the five participating sites in this study. This study's results reinforce the importance of this familiar approach, which enables the early diagnosis of infected children and may contribute to improved adherence.
These findings on viral suppression are similar to previous studies carried out in the present cART era. A previous Brazilian study, in Campinas, SP, found that 50% of children and adolescents had a viral load<50 copies/mL in their last exam.12 A recent study among 65 HIV-infected children in Gambia found that 58% had viral suppression after 36 months of cART initiation; in Kenya, 47% and 67% of 43 children achieved viral suppression to less than 100 copies/mL and 400 copies/mL, respectively, after six months of cART. In a United States-based study, among 126 children aged 3-13 years using cART for an average of 2.6 years, 36% achieved viral suppression. Among 437 children from 13 European countries, 53% achieved viral suppression at 12 months of treatment. In the present study, no significant differences were found with respect toviral suppression in children and adolescents previously exposed to mono- or dual therapy, compared with those whose first regimen was cART. This finding is in accordance with the observation that starting treatment with mono- or dual therapy did not have significant impact on survival among children transitioning to cART.26
The independent association of alcohol and drug abuse among caregivers as measured by ASSIST7 and viral suppression among children indicates the usefulness of this instrument in pediatric HIV clinics. This finding is not surprising, since alcohol use among HIV-infected adults has been associated with lower treatment adherence, incomplete viral suppression, and disease progression. In a systematic review of the literature, a negative association of moderate/heavy alcohol consumption and viral suppression was made evident.27 The incorporation of a screening instrument for drug use among caregivers may contribute to strategies aiming to improve adherence in the pediatric population, since among HIV-infected adults, the proper management of substance abuse has been associated with commitment to cART treatment.28 In fact, substance abuse has been identified as a relevant public health problem and the WHO recommends the use of ASSIST as a screening tool to be incorporated in primary health care settings and in all contexts where a significant proportion of patients may be exposed to it, such as in sexually transmitted disease clinics. According to WHO guidelines, the routine use of ASSIST increases the opportunities for early detection of drug abuse and for launching a discussion about it with both patients and their families.
This study confirmed that pharmacy reports are accurate predictors of viral suppression. Similar results have been found among HIV-infected adults in Africa,29 as well as in Brazilian adults, children, and adolescents.12,30 Pharmacy reports can provide useful information that can be easily incorporated into routine care as a monitoring tool. The Brazilian AIDS department defines pharmacy visits with intervals greater than 38 days as indicative of non-adherence, and advises health units to utilize mechanisms to alert professionals whenever this happens. The present results reinforce the need to trigger the alert mechanism as soon as delays occur.
The limitations of this study include putative biases related to patient selection at the sites (probably comprising intra- as well as inter-site unmeasured heterogeneities) and the cross-sectional design, which did not allow the evaluation of adherence over time and the proper definition of the directionality of some of the observed associations (i.e., those in which bidirectional associations are plausible). Unfortunately, data regarding HIV genotyping was not available for all patients, and thus this aspect of virological failure was not evaluated. The relatively small number of patients enrolled in some sites did not allow for a statistical analysis stratified by geographical location (i.e., the respective five Brazilian macro-regions).
We report the findings from a Brazilian multicenter study on cART adherence in a pediatric population. This study's findings indicate the usefulness of pharmacy reports along with the use of an integrated package of standard instruments, such as WHOQOL-BREF and ASSIST, which should be used in an attempt to provide a more comprehensive approach and optimal management and care for families with children and adolescents living with HIV/AIDS.
FundingThis study was realized with technical and financial support of the Ministério da Saúde / Secretaria de Vigilância em Saúde / DST, Aids e Hepatites (MS / SHS / STD-AIDS e hepatite), through the Projeto de Cooperação Técnica Internacional 914/BRA/1101 between the brazilian government and the Organização das Nações Unidas para a Educação, a Ciência e a Cultura (UNESCO).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge the families that participated in the study and colleagues from participating sites. They would also like to thank Dr. Karin Nielsen, from University of California, Los Angeles (UCLA), for the review of English language and suggestions.
Please cite this article as: Cruz ML, Cardoso CA, Darmont MQ, Souza E, Andrade SD, D’Al Fabbro MM, et al. Viral suppression and adherence among HIV-infected children and adolescents on antiretroviral therapy: results of a multicenter study. J Pediatr (Rio J). 2014;90:563–71.