This study aims to investigate the role of metabolic syndrome (MetS) and the hypertriglyceridemic-waist (HW) phenotype in determining cardiometabolic risk factors and elevated liver enzymes in a national sample of Iranian pediatric population.
MethodThis nationwide study was conducted in the framework of the third survey of a surveillance program. Students, aged 10–18 years, were recruited from 27 provinces in Iran. The prevalence of cardiometabolic risk factors was compared in students with and without HW and MetS. The association of HW with different cardiometabolic risk factors was determined.
ResultsThe mean age of studied population was 14.73±2.41 years. Prevalence of HW and MetS was 3.3% and 4%, respectively. Sixty-nine (71.1%) participants with HW had MetS. The prevalence of obesity, elevated systolic blood pressure, hypercholesterolemia, and elevated alanine aminotransaminase (ALT) was significantly higher in subjects with HW phenotype and MetS than in their peers (p<0.05). A significant association was observed between HW and elevated levels of cholesterol and ALT, as well as between obesity and low HDL-C (p<0.05).
ConclusionsThe current findings serve as complementary evidence to previous studies, which have been mainly conducted among adults, suggesting that the HW phenotype is associated with cardiometabolic risk factors, especially with elevated cholesterol and ALT. The authors propose that, in primary care settings and in large epidemiological studies, the measurement of all MetS components can be replaced by studying HW as a screening tool for identifying children at high risk for cardiometabolic disorders.
Este estudo visa investigar o desempenho da síndrome metabólica e do fenótipo de cintura hipertrigliceridêmica (CH) na determinação de fatores de risco cardiometabólico e enzimas hepáticas elevadas em uma amostra nacional da população pediátrica iraniana.
MétodoEste estudo nacional foi realizado na estrutura da terceira pesquisa de um programa de vigilância. Foram recrutados alunos de 10-18 anos de 27 províncias do Irã. A prevalência de fatores de risco cardiometabólico foi comparada em alunos com e sem CH e SM. Foi determinada a associação da CH com diferentes fatores de risco cardiometabólico.
ResultadosA média de idade da população estudada foi de 14,73±2,41 anos. A prevalência de CH e SM foi de 3,3% e 4%, respectivamente. 69 (71,1%) dos participantes com CH apresentaram SM. A prevalência de obesidade, pressão arterial sistólica elevada, hipercolesterolemia e ALT elevada foi significativamente maior em meninos e meninas com fenótipo CH e SM que em seus outros pares (P<0,05). A associação de CH foi significativa com elevados níveis de colesterol e ALT, bem como obesidade e HDL-C baixo (P<0,05).
ConclusõesOs achados atuais servem de evidência complementar de estudos anteriores, conduzidos principalmente com adultos, e sugerem que o fenótipo CH está associado a fatores de risco cardiometabólico, principalmente com colesterol e ALT altos. Propomos que, em ambientes de cuidados básicos e em grandes estudos epidemiológicas, a medição de todos os componentes de SM possa ser substituída pelo estudo da CH como ferramenta de triagem para identificar crianças com alto risco de apresentarem distúrbios cardiometabólicos.
Non-communicable diseases, the leading cause of both mortality and morbidity in most populations, origin from early life.1 A clustering of risk factors increases the risk of chronic diseases. Different combinations of risk factors are suggested to identify children at risk for non-communicable diseases. Metabolic syndrome (MetS) is one of these combinations that has been well documented as a predisposing factor for most chronic diseases. However, examining all five components of MetS in large population-based studies is difficult and costly. Moreover, there is substantial controversy between the various definitions of MetS and the clinical screening parameters and cut-off points proposed by different organizations.2 Currently. there is no universally accepted definition for MetS in the pediatric age group. Therefore, simple screening indexes should be developed for population-based screening studies. Hypertriglyceridemic waist (HW), i.e. the coexistence of abdominal adiposity and hypertriglyceridemia, is a simple combination of risk factors.3–5 Both MetS and HW were found to be associated with increased cardiometabolic risk, including insulin resistance, atherogenic dyslipidemia, hypertension, endothelial dysfunction, low-grade inflammation, and impaired hemostasis.6,7
Lemieux et al.3 were the first authors to document the association of HW phenotype with increased cardiometabolic risk in adult men. In particular, the HW phenotype was associated with the atherogenic triad of hyperinsulinemia, elevated concentrations of apolipoprotein B. and small, dense low-density lipoprotein cholesterol (LDL-C) particles. Further studies confirmed the association of HW with cardiometabolic risk factors8–10; however, most of these studies have been conducted in adult populations.
A growing body of evidence suggests the association of liver function tests with MetS components. This correlation has been demonstrated even for children and adolescents.11,12 There is limited experience on the association of HW phenotype with cardiometabolic risk factors and elevated liver enzymes in the pediatric age group.
This study aimed to compare the frequency of cardiometabolic risk factors and elevated liver enzymes in children and adolescence with HW phenotype and MetS, to investigate the performance of the HW phenotype in determining the aforementioned risk factors in this population.
MethodsThis cross-sectional study was conducted in the framework of the third survey of a national school-based surveillance program entitled Childhood and Adolescence Surveillance and PreventIon of Adult Non-communicable disease (CASPIAN-III) study. Its detailed methodology has been previously published13; it will be briefly described herein.
This project was approved by the Research Ethics Committees and other relevant national regulatory organizations. A written informed consent and oral assent were obtained from parents and students, respectively. The project team members were trained, and a comprehensive operation manual was given to them. The Data and Safety Monitoring Board of the project has taken into account for different levels of quality control. A group of external evaluators and supervisors assessed the performance of the personnel, and monitored and calibrated the equipment.
The present study included 5625 students aged 10–18 years, recruited by multistage random cluster sampling from urban and rural areas of 27 provinces in Iran. For the present study, eligible schools were randomly selected from the list of schools, which were stratified according to database from the Iranian Ministry of Education. Students were also selected randomly from each selected school. Those students who had any chronic disease or received medications were not included in the survey.
Physical examinationA team of trained physicians, nurses, and healthcare professionals conducted the physical examination under standard protocols and using calibrated instruments. Weight and height of students were measured with light clothes and without shoes. Body mass index (BMI) was calculated as weight (kg) divided by the height squared (m2). Waist circumference (WC) was measured using a non-elastic tape to the nearest 0.2cm at the end of expiration at the midpoint between the top of iliac crest and the lowest rib in standing position. The maximum level of hip without any pressure to the body surface was considered for measuring hip circumference. Systolic and diastolic blood pressures (SBP and DBP) were measured under standard protocol by using appropriate cuff size. BP was measured twice after at least five minutes of rest. SBP was considered as the clear hearing of the first sound (first Korotkoff phase) and DBP as disappearance of sound (fifth Korotkoff phase).14
Laboratory testsFor blood sampling, students, accompanied by one of their parents, attended the nearest health center to their school after 12h of fasting. Venous blood sample was obtained between 8:00 and 9:30 am from the ante-cubital vein. Blood samples were centrifuged for 10min at 3000rpm, within 30min of venipuncture. Fresh samples were analyzed by standard kits (Pars Azmoun, Tehran, Iran) in the Central Provincial Laboratory, which is under quality control of the National Reference Laboratory, a World Health Organization (WHO) collaborating center.
Definition of risk factorsThe International Diabetes Federation (IDF) definition of MetS for children and adolescents was used.15 Overweight and obesity were defined as BMI between 85th and 95th percentiles and BMI equal to or higher than 95th percentile, respectively. WC above the age- and gender-specific 90th percentile was considered as abdominal obesity.16 Abnormal serum lipids were defined as total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), or triglycerides (TG) higher than the level corresponding to the age- and gender-specific 95th percentile; or high-density lipoprotein cholesterol (HDL-C) lower than the age- and gender-specific 5th percentile.17 High fasting blood glucose (FBG) was considered as equal to or higher than 100mg/dL.16 Mean SBP or DBP above the age- and gender-specific 90th percentile was considered as elevated BP.18 Alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) were considered elevated if their levels were at or above the 90th percentile value calculated for children and adolescents.19
Definition of HW phenotypeThe HW phenotype was defined by the co-existence of WC above the age- and gender-specific 90th percentile and serum triglycerides levels higher than the age- and gender-specific 95th percentile.10
Statistical analysisContinuous and categorical variables were expressed as means±standard deviation (SD) and percentages, respectively. Independent t-test was used for comparing continuous variables, and the chi-squared test was used for categorical data. Binary logistic regression analyses were used to evaluate the association of HW with cardiometabolic risk factors, in each model, as possible confounders. Analyses were performed with SPSS version 16.0 statistical package for Windows (SPSS Inc., Chicago, USA). Two-tailed p-values were reported. p-values lower than 0.05 were considered as statistically significant.
ResultsIn this study, 5625 school students were included: 2824 (50.2%) males and 2801 (49.8) females, respectively. The mean age of studied population was 14.73 ± 2.41 years. The prevalence of HW (3% males and 3.5% females) and MetS (3% males and 5.1% females) was 3.3% and 4%, respectively. Sixty-nine (71.1%) participants with HW had MetS.
As some selected students did not provide a blood sample and some biochemical and laboratory measurements were not performed properly, some cases were lost.
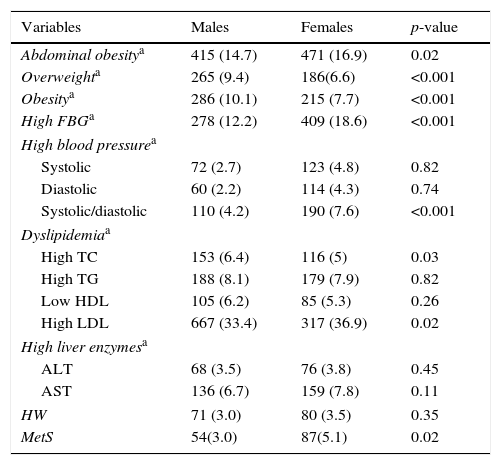
Means and prevalence of anthropometric variables, liver enzymes, and cardiometabolic risk factors according to gender and age groups, are presented in Table 1. Mean levels of liver enzymes, WC, SBP, DBP, and FBG were significantly higher in females than in males (p<0.001). The mean BMI, total cholesterol, TG, and LDL-C were significantly higher in males than in females (p<0.001). The prevalence of MetS, abdominal obesity, elevated FBG, high levels of both SBP and DBP, as well as high levels of LDL-C were significantly higher in females than in males (p<0.05). The prevalence of overweight/obesity and hypercholesterolemia were significantly higher in males than in females (p<0.05).
Frequency of cardiometabolic risk factors and liver enzymes in children and adolescents according to the gender: the CASPIAN-III study.
| Variables | Males | Females | p-value |
|---|---|---|---|
| Abdominal obesitya | 415 (14.7) | 471 (16.9) | 0.02 |
| Overweighta | 265 (9.4) | 186(6.6) | <0.001 |
| Obesitya | 286 (10.1) | 215 (7.7) | <0.001 |
| High FBGa | 278 (12.2) | 409 (18.6) | <0.001 |
| High blood pressurea | |||
| Systolic | 72 (2.7) | 123 (4.8) | 0.82 |
| Diastolic | 60 (2.2) | 114 (4.3) | 0.74 |
| Systolic/diastolic | 110 (4.2) | 190 (7.6) | <0.001 |
| Dyslipidemiaa | |||
| High TC | 153 (6.4) | 116 (5) | 0.03 |
| High TG | 188 (8.1) | 179 (7.9) | 0.82 |
| Low HDL | 105 (6.2) | 85 (5.3) | 0.26 |
| High LDL | 667 (33.4) | 317 (36.9) | 0.02 |
| High liver enzymesa | |||
| ALT | 68 (3.5) | 76 (3.8) | 0.45 |
| AST | 136 (6.7) | 159 (7.8) | 0.11 |
| HW | 71 (3.0) | 80 (3.5) | 0.35 |
| MetS | 54(3.0) | 87(5.1) | 0.02 |
CASPIAN, childhood and adolescence surveillance and prevention of adult non-communicable disease; BMI, body mass index; FBG, fasting blood glucose; HW, hypertriglyceridemic waist; MetS, metabolic syndrome.
Abdominal obesity: waist circumference above the age- and gender-specific 90th percentile; overweight: 85th<BMI<95th percentile; obesity: BMI≥95th percentile; high FBG≥100mg/dL; dyslipidemia, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), and triglycerides (TG), higher than the level corresponding to the age- and gender-specific 95th percentile, and/or high-density lipoprotein cholesterol (HDL-C) lower than the age- and gender-specific 5th percentile; high blood pressure, systolic and diastolic blood pressure above the 90th percentile for that age and gender; high liver enzyme, alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) above the 90th percentile value for Iranian children and adolescents.
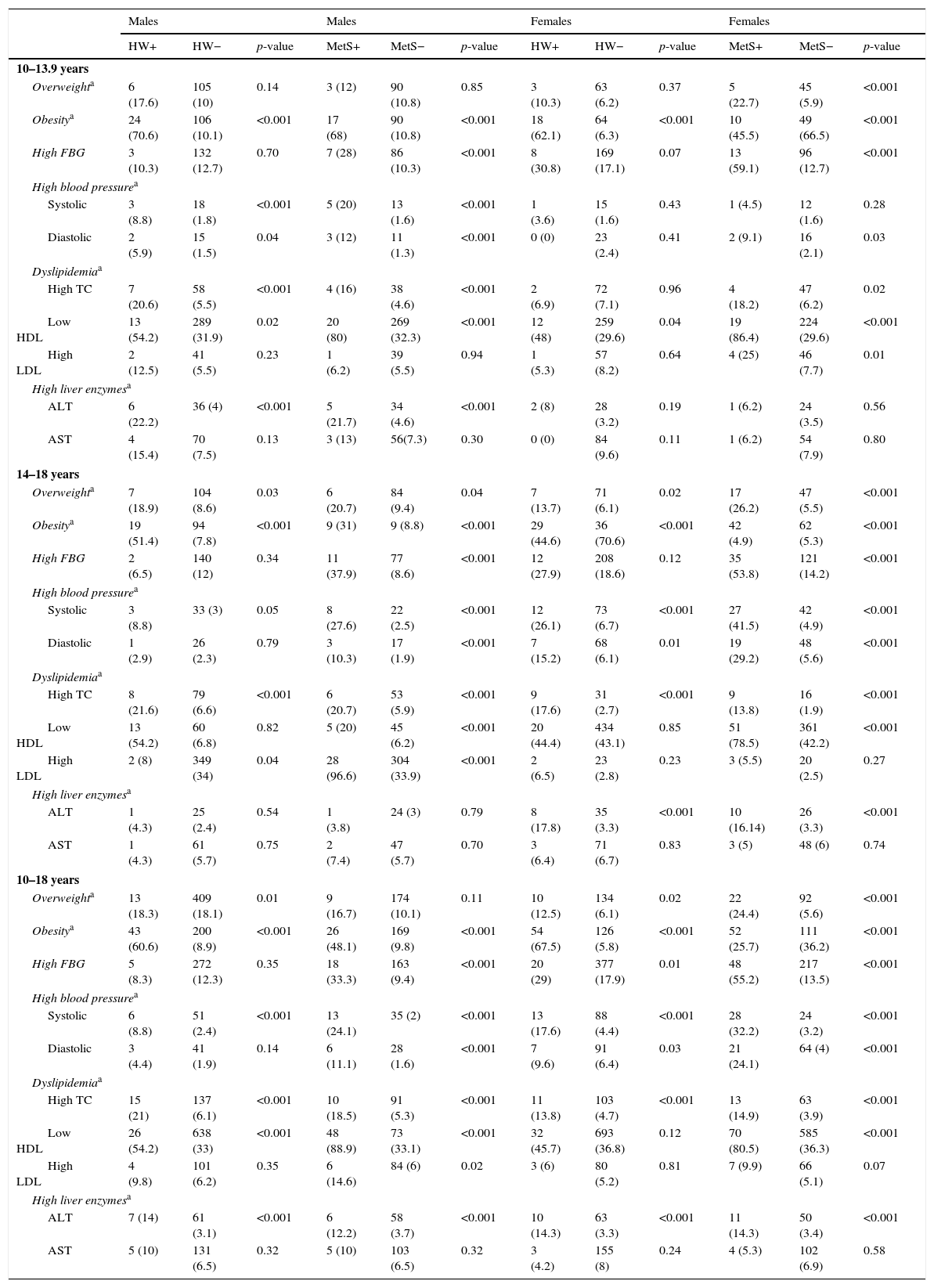
Table 2 presents the frequency of cardiometabolic risk factors and elevated liver enzymes in participants with and without HW and MetS by gender and age groups. The frequency of obesity, elevated SBP, htypercholestrolemia, and high levels of ALT were significantly higher in subjects with HW phenotype and MetS than in their peers (p<0.05).
Frequency of metabolic risk factors and elevated liver enzymes in children and adolescents with and without hypertriglyceridemic-waist and metabolic syndrome: the CASPIAN-III study.
| Males | Males | Females | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HW+ | HW− | p-value | MetS+ | MetS− | p-value | HW+ | HW− | p-value | MetS+ | MetS− | p-value | |
| 10–13.9 years | ||||||||||||
| Overweighta | 6 (17.6) | 105 (10) | 0.14 | 3 (12) | 90 (10.8) | 0.85 | 3 (10.3) | 63 (6.2) | 0.37 | 5 (22.7) | 45 (5.9) | <0.001 |
| Obesitya | 24 (70.6) | 106 (10.1) | <0.001 | 17 (68) | 90 (10.8) | <0.001 | 18 (62.1) | 64 (6.3) | <0.001 | 10 (45.5) | 49 (66.5) | <0.001 |
| High FBG | 3 (10.3) | 132 (12.7) | 0.70 | 7 (28) | 86 (10.3) | <0.001 | 8 (30.8) | 169 (17.1) | 0.07 | 13 (59.1) | 96 (12.7) | <0.001 |
| High blood pressurea | ||||||||||||
| Systolic | 3 (8.8) | 18 (1.8) | <0.001 | 5 (20) | 13 (1.6) | <0.001 | 1 (3.6) | 15 (1.6) | 0.43 | 1 (4.5) | 12 (1.6) | 0.28 |
| Diastolic | 2 (5.9) | 15 (1.5) | 0.04 | 3 (12) | 11 (1.3) | <0.001 | 0 (0) | 23 (2.4) | 0.41 | 2 (9.1) | 16 (2.1) | 0.03 |
| Dyslipidemiaa | ||||||||||||
| High TC | 7 (20.6) | 58 (5.5) | <0.001 | 4 (16) | 38 (4.6) | <0.001 | 2 (6.9) | 72 (7.1) | 0.96 | 4 (18.2) | 47 (6.2) | 0.02 |
| Low HDL | 13 (54.2) | 289 (31.9) | 0.02 | 20 (80) | 269 (32.3) | <0.001 | 12 (48) | 259 (29.6) | 0.04 | 19 (86.4) | 224 (29.6) | <0.001 |
| High LDL | 2 (12.5) | 41 (5.5) | 0.23 | 1 (6.2) | 39 (5.5) | 0.94 | 1 (5.3) | 57 (8.2) | 0.64 | 4 (25) | 46 (7.7) | 0.01 |
| High liver enzymesa | ||||||||||||
| ALT | 6 (22.2) | 36 (4) | <0.001 | 5 (21.7) | 34 (4.6) | <0.001 | 2 (8) | 28 (3.2) | 0.19 | 1 (6.2) | 24 (3.5) | 0.56 |
| AST | 4 (15.4) | 70 (7.5) | 0.13 | 3 (13) | 56(7.3) | 0.30 | 0 (0) | 84 (9.6) | 0.11 | 1 (6.2) | 54 (7.9) | 0.80 |
| 14–18 years | ||||||||||||
| Overweighta | 7 (18.9) | 104 (8.6) | 0.03 | 6 (20.7) | 84 (9.4) | 0.04 | 7 (13.7) | 71 (6.1) | 0.02 | 17 (26.2) | 47 (5.5) | <0.001 |
| Obesitya | 19 (51.4) | 94 (7.8) | <0.001 | 9 (31) | 9 (8.8) | <0.001 | 29 (44.6) | 36 (70.6) | <0.001 | 42 (4.9) | 62 (5.3) | <0.001 |
| High FBG | 2 (6.5) | 140 (12) | 0.34 | 11 (37.9) | 77 (8.6) | <0.001 | 12 (27.9) | 208 (18.6) | 0.12 | 35 (53.8) | 121 (14.2) | <0.001 |
| High blood pressurea | ||||||||||||
| Systolic | 3 (8.8) | 33 (3) | 0.05 | 8 (27.6) | 22 (2.5) | <0.001 | 12 (26.1) | 73 (6.7) | <0.001 | 27 (41.5) | 42 (4.9) | <0.001 |
| Diastolic | 1 (2.9) | 26 (2.3) | 0.79 | 3 (10.3) | 17 (1.9) | <0.001 | 7 (15.2) | 68 (6.1) | 0.01 | 19 (29.2) | 48 (5.6) | <0.001 |
| Dyslipidemiaa | ||||||||||||
| High TC | 8 (21.6) | 79 (6.6) | <0.001 | 6 (20.7) | 53 (5.9) | <0.001 | 9 (17.6) | 31 (2.7) | <0.001 | 9 (13.8) | 16 (1.9) | <0.001 |
| Low HDL | 13 (54.2) | 60 (6.8) | 0.82 | 5 (20) | 45 (6.2) | <0.001 | 20 (44.4) | 434 (43.1) | 0.85 | 51 (78.5) | 361 (42.2) | <0.001 |
| High LDL | 2 (8) | 349 (34) | 0.04 | 28 (96.6) | 304 (33.9) | <0.001 | 2 (6.5) | 23 (2.8) | 0.23 | 3 (5.5) | 20 (2.5) | 0.27 |
| High liver enzymesa | ||||||||||||
| ALT | 1 (4.3) | 25 (2.4) | 0.54 | 1 (3.8) | 24 (3) | 0.79 | 8 (17.8) | 35 (3.3) | <0.001 | 10 (16.14) | 26 (3.3) | <0.001 |
| AST | 1 (4.3) | 61 (5.7) | 0.75 | 2 (7.4) | 47 (5.7) | 0.70 | 3 (6.4) | 71 (6.7) | 0.83 | 3 (5) | 48 (6) | 0.74 |
| 10–18 years | ||||||||||||
| Overweighta | 13 (18.3) | 409 (18.1) | 0.01 | 9 (16.7) | 174 (10.1) | 0.11 | 10 (12.5) | 134 (6.1) | 0.02 | 22 (24.4) | 92 (5.6) | <0.001 |
| Obesitya | 43 (60.6) | 200 (8.9) | <0.001 | 26 (48.1) | 169 (9.8) | <0.001 | 54 (67.5) | 126 (5.8) | <0.001 | 52 (25.7) | 111 (36.2) | <0.001 |
| High FBG | 5 (8.3) | 272 (12.3) | 0.35 | 18 (33.3) | 163 (9.4) | <0.001 | 20 (29) | 377 (17.9) | 0.01 | 48 (55.2) | 217 (13.5) | <0.001 |
| High blood pressurea | ||||||||||||
| Systolic | 6 (8.8) | 51 (2.4) | <0.001 | 13 (24.1) | 35 (2) | <0.001 | 13 (17.6) | 88 (4.4) | <0.001 | 28 (32.2) | 24 (3.2) | <0.001 |
| Diastolic | 3 (4.4) | 41 (1.9) | 0.14 | 6 (11.1) | 28 (1.6) | <0.001 | 7 (9.6) | 91 (6.4) | 0.03 | 21 (24.1) | 64 (4) | <0.001 |
| Dyslipidemiaa | ||||||||||||
| High TC | 15 (21) | 137 (6.1) | <0.001 | 10 (18.5) | 91 (5.3) | <0.001 | 11 (13.8) | 103 (4.7) | <0.001 | 13 (14.9) | 63 (3.9) | <0.001 |
| Low HDL | 26 (54.2) | 638 (33) | <0.001 | 48 (88.9) | 73 (33.1) | <0.001 | 32 (45.7) | 693 (36.8) | 0.12 | 70 (80.5) | 585 (36.3) | <0.001 |
| High LDL | 4 (9.8) | 101 (6.2) | 0.35 | 6 (14.6) | 84 (6) | 0.02 | 3 (6) | 80 (5.2) | 0.81 | 7 (9.9) | 66 (5.1) | 0.07 |
| High liver enzymesa | ||||||||||||
| ALT | 7 (14) | 61 (3.1) | <0.001 | 6 (12.2) | 58 (3.7) | <0.001 | 10 (14.3) | 63 (3.3) | <0.001 | 11 (14.3) | 50 (3.4) | <0.001 |
| AST | 5 (10) | 131 (6.5) | 0.32 | 5 (10) | 103 (6.5) | 0.32 | 3 (4.2) | 155 (8) | 0.24 | 4 (5.3) | 102 (6.9) | 0.58 |
CASPIAN, childhood and adolescence surveillance and prevention of adult non-communicable disease; BMI, body mass index; FBG, fasting blood glucose; HW, hypertriglyceridemic waist; MetS, netabolic syndrome.
Overweight: 85th<BMI<95th percentile, obesity: BMI≥95th percentile; dyslipidemia: total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG) higher than the level corresponding to the age- and gender-specific 95th percentile, and/or high density lipoprotein cholesterol (HDL-C) lower than the age- and gender-specific 5th percentile; elevated blood pressure: systolic and diastolic blood pressure above the 90th percentile for that age and gender; elevated liver enzyme, alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) above the 90th percentile value for Iranian children and adolescents.
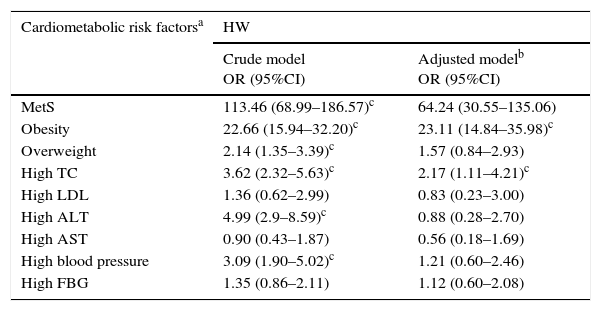
In Table 3, the relationship of the HW phenotype with cardiometabolic risk factors and liver enzymes is presented after adjustment for confounding variables, such as gender, socio-economic status, parental education level, family history of chronic diseases, sedentary life style, and BMI. Cardiometabolic risk factors were defined according to the Adult Treatment Panel III (ATP III) criteria modified for children and adolescents, as follows; over weight, BMI between the 85th–95th percentile; Obesity, BMI>95th percentile; Low HDL, <40mg/dL; high LDL, >130mg/dL; high TG, ≥150mg/dL; high TC, >200mg/dL; elevated FBG, >100mg/dL; high blood pressure, >95th percentile (adjusted by age, sex, and height).
Association of cardiometabolic risk factors with hypertriglyceridemic-waist in logistic regression model: the CASPIAN-III study.
| Cardiometabolic risk factorsa | HW | |
|---|---|---|
| Crude model OR (95%CI) | Adjusted modelb OR (95%CI) | |
| MetS | 113.46 (68.99–186.57)c | 64.24 (30.55–135.06) |
| Obesity | 22.66 (15.94–32.20)c | 23.11 (14.84–35.98)c |
| Overweight | 2.14 (1.35–3.39)c | 1.57 (0.84–2.93) |
| High TC | 3.62 (2.32–5.63)c | 2.17 (1.11–4.21)c |
| High LDL | 1.36 (0.62–2.99) | 0.83 (0.23–3.00) |
| High ALT | 4.99 (2.9–8.59)c | 0.88 (0.28–2.70) |
| High AST | 0.90 (0.43–1.87) | 0.56 (0.18–1.69) |
| High blood pressure | 3.09 (1.90–5.02)c | 1.21 (0.60–2.46) |
| High FBG | 1.35 (0.86–2.11) | 1.12 (0.60–2.08) |
CASPIAN, childhood and adolescence surveillance and prevention of adult non-communicable disease; FBG, fasting blood glucose; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; HW, hypertriglyceridemic waist; MetS, metabolic syndrome.
Cardiometabolic risk factors according to Adult Treatment Panel III (ATP III) criteria modified for children and adolescents: over weight, BMI between the 85th and 95th percentile; obesity, BMI>95th percentile; low HDL, <40mg/dL, high LDL, >130mg/dL; high TC, >200mg/dL; high FBG, >100mg/dL; high blood pressure, >95th percentile (adjusted by age, sex, height).
HW had significant association with elevated levels of cholesterol and ALT, as well as obesity and low HDL-C (p<0.05).
DiscussionThe findings of present study indicated that, in the pediatric age group, the HW phenotype is associated with elevated level of cholesterol and ALT, as well as obesity and low HDL-C.
Although the usefulness of HW phenotype in determining cardiometabolic risk factors has been studied in previous studies among adult population and in different groups of patients, few studies in this field were conducted in pediatric populations.8–10 The association of the HW phenotype with elevated liver enzymes was not studied in any previous studies in children and adolescents.
Evidence suggest that HW is a simple clinical phenotype that represents excess visceral adipose tissue. Visceral fat accumulation is strongly associated with cardiometabolic risk factors even in children.20
HW is considered a practical and simple tool that could be used as an alternative concept to MetS and may be used for screening high-risk populations.21 WC is an anthropometric indicator associated with some metabolic factors, including abdominal obesity, hyperinsulinemia, and increased levels of apolipoprotein. TG concentration, another component of HW, is mainly associated with low HDL-C and elevated LDL-C. High LDL-C could be predicted by hypertriglyceremia before its manifestation. An association between hypertriglyceridemia and presence of small dense particles of LDL-C has been suggested.18,22,23
In this study, the prevalence of both HW and MetS was investigated in children and adolescents. In this study, the prevalence of HW was 8.5%. The reported prevalence rate of HW in previous studies in the pediatric age group ranged between 6 and 8.5%: 7.3%, 7.2%, 6.4%, and 8.5% in studies from the United Kingdom,9 Brazil,24 Tehran-Iran,10 and a nationwide study in Iran,25 respectively.
Differences in lifestyle habits, genetic background, and ethnicity, as well as differences in laboratory measurements could explain, at least in part, the various prevalence rates of HW in the studied populations. In addition, the definition used for HW was not similar in the abovementioned studies.
The findings of the present study indicated that approximately 70% of adolescent with HW phenotype fulfilled the IDF criteria for MetS. As reported in a previous study, adolescents with the HW phenotype are more likely to have MetS and clustering of cardiovascular risk factors than those without this phenotype.10
In the present study, the association between HW and liver enzymes among adolescents was investigated was investigated. The results indicated that participants with HW had higher level of ALT than those without HW. A significant association was observed between HW phenotype and elevated ALT. No associations were observed between AST and HW phenotype, perhaps because ALT is considered as a better predictor of liver injury than AST, as AST is also produced in tissues other than the liver.26
Several studies demonstrated the correlation of MetS and non-alcoholic fatty liver disease (NAFLD),11,27,28 which has recently become as an important health problem in the pediatric population.28 Elevated liver enzymes are usually used as a non-invasive method to detect cases with NAFLD. Findings of studies in Iran and other regions confirmed a significant association between elevated liver enzymes and cardiometabolic risk factors among children and adolescents. These studies proposed that elevated ALT and AST could be considered as a cardiometabolic risk factor and an additional component of the MetS in the pediatric age group.11,27,29,30 The results of the present study could serve as confirmatory evidence for such suggestion.
These findings are also consistent with a previous study that indicated a significant relationship between elevated ALT and cardiometabolic risk factors and HW phenotype among 6–18-year-old students.11,24
In the present study, adolescents with HW phenotype had higher frequency of overweight/obesity, as well as elevated SBP and total cholesterol. Elevated FBG and DBP were more prevalent in females with HW phenotype than in other participants; whereas low HDL-C was more prevalent among males with HW phenotype.
After adjustment for age, females with HW phenotype were more likely to have high cholesterol, high FBG, elevated BP, MetS, obesity, and elevated ALT. Further adjustment for factors including socio-economic status, parental education, family history of chronic diseases, and sedentary lifestyle showed that females with HW phenotype were more likely to have overweight/obesity, MetS, elevated BP, and increased ALT.
After adjustment for age, males were more likely to have high cholesterol, elevated BP, MetS, obesity, and elevated ALT. With additional adjustment for the abovementioned confounding factors, male were more likely to have high cholesterol and MetS.
Despite the limited experience on the performance of HW phenotype in identifying cardiometabolic risk factors among children and adolescents, some recent studies have investigated such relationship. Bailey et al. have evaluated the association between HW and cardiometabolic disorders in 234 adolescents aged 10–19 years in the United Kingdom. They indicated that adolescents with HW had higher levels of cholesterol, FBG, and DBP, as well as lower level of HDL-C, than those without HW. In their study, participants with HW phenotype had higher mean scores for clustered cardiometabolic risk scores. Adolescents with HW phenotype were at higher risk for low HDL-C, impaired fasting glucose, and >one and >two cardiometabolic risk factors including hypercholesterolemia, low HDL-C, elevated SBP or DBP, and impaired FBG than those without this phenotype. These authors concluded that HW could be a simple marker for identifying children and adolescents who are at high risk for cardiometabolic risk factors.9
Conceição-Machado et al. have investigated the prevalence of HW and its association with metabolic abnormalities in 1076 Brazilian adolescents aged 11–17 years. Accordingly, adolescents with HW phenotype had higher level of obesity, non-HDL cholesterol and LDL-C than those without it. These authors reported a significant association between the HW phenotype and atherogenic lipid profile. They did not find any association between FBG and HW phenotype.24
A study among Tehranian adolescents demonstrated that HW phenotype was associated with hyperlipidemia and elevated BP, but not with FBG.10
The present results regarding the lack of association between HW phenotype and FBG are consistent with those reported in Brazil23 and Iran.10 However, they are not in agreement with the results of the study conducted in the United Kingdom.8 The observed results may be due to the differences in dietary habits and ethnicity in the studied populations.
The findings of the present study are in line with the previous nationwide study by the authors, which indicated a higher rate of hyperlipidemia among adolescents with HW phenotype.24 These findings suggest that the HW phenotype could be used as a simple screening tool for identification of high-risk children and adolescents.
The main limitation of the current study was its cross-sectional nature. In addition, itw as not possible to determine the pubertal stage of participants, and because of the effects of puberty on lipid profile, especially among boys, the number of subjects with hypertriglyceridemia could be overestimated.31
Another limitation was the missing data=for each variable; however, as presented in the tables, the number of the missing data was only one.
The main strength of this study was the large, nationwide sample of a pediatric population. However, in spite of the large sample studied, the number of participants with HW and or MetS was low; without considering this large number of participants, an appropriate sample size of children and adolescents with HW or MetS would not have been reached. Future studies with larger number of participants with such disorders would reach more generalizable results.
The other strengths were using the age- and sex-specific percentiles for WC and TG levels, as well as studying liver enzymes in addition to cardiometabolic risk factors.
The findings of the present study suggest that the HW phenotype could be used as a screening tool for identifying children at high-risk for elevated ALT and some cardiometabolic risk factors. Due to its simplicity, low cost, and usefulness, HW can be used in primary care settings and large epidemiological studies instead of measuring all MetS components. Further longitudinal studies are necessary to verify the clinical implications of the present findings.
FundingThis study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interestThe authors declare no conflict of interest.
Please cite this article as: Kelishadi R, Jamshidi F, Qorbani M, Motlagh ME, Heshmat R, Ardalan G, et al. Association of hypertriglyceridemic-waist phenotype with liver enzymes and cardiometabolic risk factors in adolescents: the CASPIAN-III study. J Pediatr (Rio J). 2016;92:512–20.