To evaluate a child development surveillance tool proposal to be used in primary care, with simultaneous use of the Denver II scale.
MethodsThis was a cross-sectional study of 282 infants aged up to 36 months, enrolled in a public daycare in a countryside community in Rio Grande do Sul/Brazil. Child development was assessed using the surveillance tool and the Denver II scale.
ResultsThe prevalence of probable developmental delay was 53%; most of these cases were in the alert group and 24% had normal development, but with risk factors. At the Denver scale, the prevalence of suspected developmental delay was 32%. When risk factors and sociodemographic variables were assessed, no significant difference was observed.
ConclusionThe evaluation of this surveillance tool resulted in objective and comparable data, which were adequate for a screening test. It is easily applicable as a screening tool, even though it was originally designed as a surveillance tool. The inclusion of risk factors to the scoring system is an innovation that allows for the identification of children with suspected delay in addition to developmental milestones, although the definition of parameters and choice of indicators should be thoroughly studied.
Avaliar uma proposta de um instrumento de vigilância em desenvolvimento para utilização na atenção primária, e a aplicação simultânea da escala de Denver II.
Métodosestudo transversal com uma amostra de 282 crianças até 36 meses da rede pública escolar, numa comunidade do RS. Foi avaliado o desenvolvimento infantil utilizando o instrumento de vigilância proposto e o Denver II.
ResultadosA prevalência de Provável Atraso no Desenvolvimento foi de 53%, sendo a maioria desses na condição de Alerta e 24% com desenvolvimento normal, mas com fatores de risco. No Denver a prevalência foi de 32% com suspeita para o atraso no desenvolvimento. Os fatores de risco e as variáveis sócio-demográficas avaliadas não apresentaram diferenças significativas.
ConclusãoA avaliação deste instrumento de vigilância trouxe dados objetivos e comparativos, nos moldes preconizados para um teste de triagem. É um instrumento de fácil aplicabilidade como triagem, sendo originalmente como vigilância. A inclusão dos fatores de risco no sistema de escore é uma inovação que possibilita o aumento da identificação de crianças com suspeita de atraso além dos marcos do desenvolvimento, ainda que a definição dos parâmetros e escolha dos indicadores deva ser melhor construída.
Child development is a continuous and dynamic process that promotes changes in several areas: physical, social, emotional, and cognitive, in a complex interaction among these changes and the environment where each stage is constructed, based on the previous steps.1,2 Development must be understood within the eco-bio-developmental model, which expands from biology and the environment to a broader concept, including epigenetics and neuroscience.1,3
Several studies have shown different prevalence rates of delay according to the evaluation method and age group, reaching up to 18%.4–8 In studies using only screening tests, the prevalence was higher, showing great variation.4,9,10
The early detection of children with possible developmental delays is one of the objectives of routine pediatric consultations.5 It is widely established in the literature that the cost of the evaluation and early intervention in child development is up to 100 times lower than that of treating a child with a late diagnosis.11
Recent studies show that investments in the first four years of life have a positive annual rate of return, whereas some late recovery programs show null and often negative returns.12–14 Surveillance is a continuous process that occurs during consultations and allows for the early detection of developmental problems,7 while screening is part of this process and characterized by being usually discrete and using a standardized tool. The systematic use of surveillance and screening is critical for pediatricians to identify potential risk factors and/or delays and promote interventions.5,7,11,15,16
The American Academy of Pediatrics recommends applying a screening tool in the first three years of life, even in the absence of risk factors, to increase the ability to identify possible delays,11,15,17 as, in the absence of a surveillance process, only 30% of the children will be detected as having delays before they reach school age.11 Recent studies have shown an increase in the use of tools to assess development, but they are still unfrequently used in pediatric services, whether public or private.7,17,18
Some tools are self-administered questionnaires, others are to be used by professionals in search for developmental information, and others that assess the main areas of development.7,11,17 The limitations of screening tests are inherent to the tool and age range. Although there are several tools, there is not a unique tool that is universally used for all populations.8,19 Historically, the Denver II Developmental Screening Test has been the most often used screening tool worldwide, especially in Brazil, as there is no tool for that purpose. In addition to being easy and quick to apply, the tool validity has been established by the accuracy obtained in the different percentiles in which each task was established for each assessed age.
As with the other screening tools, the Denver II has no hypothesis construct, such as for instance an intelligence test, it defines the age at which a child performs a certain task. Although it has borderline sensitivity and specificity rates, it continues to be used in comparison studies.6,7,9,10
The use of a tool for child development surveillance began to be implemented by the Brazilian Ministry of Health (MOH) in 2002.20 The Integrated Management of Childhood Illness (IMCI) program, developed by the World Health Organization (WHO) and by the United Nations Children's Fund (UNICEF), served as the basis for use in child development surveillance. Subsequently, a manual was published for this purpose and a development surveillance table was adapted and has been used in the Child Health Handbook of the MOH21 in the primary care network. This proposal comprises, in addition to the developmental milestones, more relevant risk factors associated with developmental delays.2,22
The aim of this study was to evaluate a new proposal for a child development surveillance model that can be applied as a screening tool at certain points of child development together with the Denver II test, identifying possible associations between sociodemographic variables (income, parental level of schooling, number of siblings) and possible developmental delays.
MethodsA cross-sectional study was carried out with a school-based sample, having as inclusion criteria all children aged 0–36 months of age attending public pre-schools of the town of Igrejinha, state of Rio Grande do Sul, Brazil. The exclusion criteria comprised all children enrolled through the school inclusion program or with a diagnosis of any developmental problems.
Considering a conservative prevalence of 10% for developmental delays and power of 80%, with an alpha error of 5%, it would be necessary to assess 350 children. At the time, 357 children from public schools met the inclusion criteria; therefore, all were invited to participate.
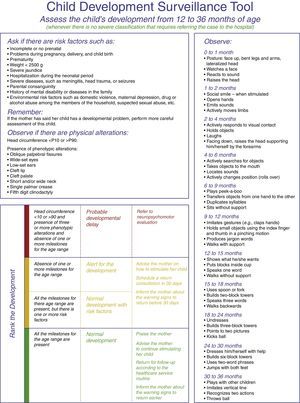
The adapted tool, here termed Surveillance Algorithm (SA), was derived from the developmental surveillance tool that has been used by the MOH in the primary care network, published in the Child Health Handbook and in the manual published by Figueiras.2 The proposed modification was related to the developmental assessment criterion for “probable delay”, which used the absence of milestones for the current age range and not the previous range. The assessed milestones correspond to the skills that 90/100 children perform at this age range. According to the manual, children aged 0–36 months were divided into age subgroups in months. Risk factors and phenotypic signs of genetic disorders were investigated, which are described in Table 1 and Fig. 1, as well as head circumference (HC) and milestones corresponding to gross and fine motor skills, and personal–social and language areas.
Percentage of delays in the SA according to the risk factors.
| Assessed risk factors a | Altered developmental milestones (%) | Normal developmental milestones (%) | Total (%) |
|---|---|---|---|
| Absence of prenatal care | 1 (0.35) | 2 (0.7) | 3 (1) |
| Problems during pregnancy | 12 (4.2) | 20 (7) | 32 (11) |
| Delivery problems | 3 (1) | 9 (3.1) | 12 (4.2) |
| Preterm birth | 7 (2.5) | 10 (3.5) | 17 (6) |
| Low birth weight | 5 (1.7) | 5 (1.7) | 10 (3.5) |
| Severe neonatal jaundice | 3 (1) | 11 (3.9) | 14 (4.9) |
| Neonatal hospitalization | 11 (3.9) | 14 (4.9) | 25 (8.8) |
| Severe diseases | 7 (2.5) | 3 (1) | 10 (3.5) |
| Mental disability or illness in the family | 12 (4.2) | 15 (5.3) | 27 (9.5) |
| Environmental risk factors | 18 (6.3) | 29 (10.2) | 47 (16.6) |
SA, surveillance algorithm.
The score of this tool uses the following classification: normal development, alert (with two subgroups: normal with risk factors, and absence of one or more milestones for the age range), and probable delay in child development (which includes altered HC and phenotypic changes and the absence of one or more milestones for the age range; Fig. 1).
The socio-demographic variables gender, age, and number of siblings were analyzed using central tendency and dispersion measures. Parental level of schooling and family income were stratified into groups and shown as absolute and relative frequencies. Risk factors and HC were analyzed dichotomously, where HC was considered to be altered when it was >90th and <10th percentile, using the curves developed by the National Center for Health Statistics of the Center for Disease Control (CDC), United States, in 2000.
The Denver II is a screening tool for child development that evaluates children aged 0–6 years old and includes items from the gross and fine-adaptive motor, social–personal and language areas. The possible outcomes were normal (absence of failures or just one alert), suspected delay (two or more alerts, or one or more failure), and non-testable (refusal to perform the testing).23,24
Invitation letters were sent to the parents and, according to initial acceptance, and informed consent form was signed.
The SA was applied to all children, followed by the Denver Test II. All preterm children had their age corrected until the age of 2 years for the application of both tests were, according to the criteria of the Denver II manual, considering as term pregnancy those of 38 weeks or more; for pregnancies below 38 weeks, the correction uses 40 weeks for the calculation.23
For quality control purposes, the tools were applied by different examiners for each test, who were blinded to the previous testing results. To minimize the learning effect, when performing the same tests at two consecutive times, the order of application was reversed in the second half of the sample.
The training phase was carried out with the two tools; this phase was filmed for improvement and standardization of HC measurement (measuring tape around the frontal and occipital bone) among the four examiners. A pilot study was performed with children of the same age and who were not part of the sample, showing at the end a 90% agreement between the examiners.
By drawing lots, 5% of the interviews were repeated by the coordinator to ensure the reliability of the collected data.
The data were typed in duplicate in the database to identify possible discrepancies in the entered data.
The study was evaluated and approved by the Research Ethics Committee of Universidade Federal de Ciências da Saúde de Porto Alegre (Opinion No. 332.335/2013).
At the comparative assessment, Denver II was used as a reference to estimate sensitivity, specificity, positive (PPV) and negative predictive values (NPV), and accuracy. The chi-squared test was used to verify a possible association between sociodemographic variables for the difference in proportions, with a 5% significance level.
Statistical analyses were performed using SPSS (SPSS for Windows, Version 10.0. USA).25
ResultsOf the 357 children aged 0–36 months enrolled in public pre-schools during that period, the study 282 were assessed. In 48 cases, there was no response to the invitation to participate in the study, 16 children left the preschool at that period, and 11 cases refused to return for retesting. The mean age of the sample was 28 months, 136 (48%) of whom were aged between 30 and 36 months, with 56% of males (Table 2).
Twenty-seven children included in the study completed 36 months of age during the study. A separate analysis was performed with these children; as no statistically significant differences regarding tool properties were observed, they remained in the study.
At the SA assessment, a little over half of the sample had a result indicating probable delay, with most of those in the alert condition, and 68 cases (24%) were classified as normal development with risk factors (Table 3). Regarding the Denver test, 91 cases (32%) showed suspected developmental delay.
Test properties according to the proposed categories – %.
| P | S | Sp | PPV | NPV | A | |
|---|---|---|---|---|---|---|
| Probable delay (absent milestones+altered HC+phenotypic alterations) | 53 | 70 | 56 | 42 | 79 | 60 |
| Alert (absent milestones only) | 39 | 57 | 70 | 47 | 77 | 66 |
| Normal development with risk factors (present milestones with risk factors) | 24 | – | – | – | – | – |
| Grouped alert (grouping risk factors+absent milestones) | 63 | 78 | 45 | 40 | 80 | 55 |
HC, head circumference; P, prevalence; S, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; A, accuracy.
When exploring the possibilities of comparison between the two tools regarding sensitivity, the SA showed different results according to the three proposed categories: probable delay (70%), alert (57%), and normal development with risk factors (21%; Table 3), with a specificity of 56%, 70%, and 74%, respectively.
For probable delay, PPV was 42% and NPV was 79%; for alert, PPV was 47% and NPV was 77%.
When assessing the risk factors, there were no significant statistical differences associated with absence of developmental milestones (Table 1).
Regarding the phenotypic alterations assessed in the SA, the frequencies found were very low or nonexistent; regarding altered HC, almost all cases were higher than the 90th percentile.
DiscussionIn this sample, the prevalence of suspected delay in the SA was 53% for probable delay and 39% for alert, with a significant difference due to the presence of altered HC in the probable delay group.
Originally, the surveillance tool used the milestones of the previous age range as the score criterion for probable delay, while the alert score uses the current age range. In this study, the authors chose to perform the assessment using the milestones of the expected age range for both, because, using the milestones of the previous age range and considering what has been discussed about the cutoff point, the delay would have already been evident.7,18
At the Denver II test, the prevalence was 32% of suspected delay, consistent with other studies, although with variations in the prevalence due to the scoring method used and cultural differences.4,9,10
The presence of physical alterations and risk factors in the scoring criteria of this surveillance tool is a peculiarity in relation to other tools found in literature,7,11,14,17,18 but some of them should be reviewed, despite not being significant in this sample.
The physical alteration regarding HC alone, as the tool was originally proposed, already determines a probable delay in the SA; HC above the 90th percentile was prevalent (93%) among those with altered HC. This was probably due to a genetic factor of macrocephaly and tall stature without associated cranial pathology, causing an increase in sensitivity and decreasing the PPV.26 It may be related to the ethnic characteristics and, additionally, it could be explained by a sample fluctuation. Daymont26 concluded that the HC has low sensitivity and PPV for the diagnosis of macrocephaly-associated pathologies.
Low birth weight, often associated with developmental delays,2,6,9,27,28 was little prevalent and showed no statistical association in the sample. One possible explanation is the 2500g cutoff used in the SA, which reduces the specificity by adding late preterm infants with adequate weight for gestational age (GA).9,28 A change in the weight and proportionality criteria of the newborn might be more useful for the risk factor definition.28
Regarding prematurity, there are differences when GA is below 32 weeks, when compared to late and moderately preterm infants,29 even with correction for GA. Perhaps it would be better to assess the nutritional status and weight/length proportionality at birth, which can offer more discriminative parameters regarding developmental delays.9 The use of information about jaundice in the neonatal period is very vague and difficult to interpret, as it is based solely on subjective information.
In relation to maternal depression, it is a well-established risk factor in the literature30 and potentially modifiable, when an early intervention is performed. Using a standard tool for its detection is required, in addition to the information on psychotherapy and/or drug treatment.15
The simultaneous use of the Denver scale allowed for an inevitable comparison between the two tools, even with the limitations regarding the absence of a gold standard and the epidemiological exercise with a screening tool that is already well established worldwide and a surveillance tool that provides objective measures. The sensitivity obtained with the tested tool in comparison with Denver II was 70% in the probable delay group and 57% in the alert group, whereas the specificity was 56% and 70%, respectively. Considering these properties, the sensitivity for the probable delay group is acceptable. Regarding the alert group, the results were the opposite.
One possible explanation for these results may be related to the criteria used for group classification, as although it did not represent an associated pathology, the HC above the 90th percentile was considered as a risk factor.7,18
Similarly, in the alert group, the sensitivity may be related to the distribution of age ranges. The fact that some children were at the initial age of the range and the necessity to use a proposed cutoff of the 90th percentile may have caused a differential misclassification, by classifying children with typical development as having developmental delay.
Regarding the PPV obtained in SA, the results were 42% and 47% for probable delay and alert, respectively, meaning that approximately half of the cases of suspected delay will not be confirmed in subsequent evaluations. Conversely, the NPV of 79% and 77% for the probable delay and alert, respectively, makes the tool more effective when the result is negative.
During the analysis, to explore the potentials of the SA, the subjects from the alert group were grouped by associating risk factors. Thus, this new category had is sensitivity increased to nearly 80%, which is desirable in a screening tool (Table 3).
This study had limitations regarding its sample, which has the characteristics of a town in the southern region of Brazil and, thus, does not translate the epidemiological profile of the Brazilian population. Although the entire child population enrolled in the town's preschools was assessed, the losses may also have affected the results.
Assessing child development is a complex task that requires continued surveillance in the early years of life and knowledge of child development normality. Although there are several screening tools, there is not a unique tool that can be used for all age groups. Additionally, the best tools still have a significant number of false positives, approximately 25%, and do not indicate those children situated between 1 and 2 standard deviations as being still at risk for possible delays.7,8 Because of limitations regarding the choice of a comparison standard, due to the lack of a tool with Brazilian standards, it was decided to use the Denver II screening test, which has been adapted and used in several other national studies on child development and is still widely used in other countries.6,9,10,28
Obviously, the misidentification of a developmental delay may cause a consequent increase in costs of specialized assessments and supplementary tests, in addition to family anxiety. However, in the reverse situation, the time of the intervention can be missed and the costs will increase, as treatments will be longer and perhaps permanent.12,13
A recent study raises this question and reinforces the need for systematic monitoring, assessment of risk factors and of parental perception for suspicion of possible developmental and behavioral problems as sufficient to direct them to diagnostic testing.8
SA is easy to learn and to apply, allowing healthcare teams and the family to take an active role in child monitoring. The presence of risk factors in the scoring system is an innovation that provided increased sensitivity of the tool, even though the definition of parameters and the choice of the best indicators should be thoroughly studied.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the interns Lais Duarte, Juliana Fritsch, Fabiana Adams, and Maiane Braun for their dedication and competence in the field work. Also, they would like to thank all children, parents, and teachers of pre-schools in Igrejinha-RS, who sincerely cooperated with this research.
Please cite this article as: Coelho R, Ferreira JP, Sukiennik R, Halpern R. Child development in primary care: a surveillance proposal. J Pediatr (Rio J). 2016;92:505–11.
Study conducted at the Post-Graduate Program in Health Sciences, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA); and Child Development Outpatient Clinic, Hospital da Criança Santo Antônio (HCSA), Santa Casa de Porto Alegre, RS, Brazil.