Data on clinical practice in pediatrics on the use of analgesic, antipyretic, and nonsteroidal anti-inflammatory drugs considering the best available evidence and regulatory-agency approved use are uncertain. This study aimed to determine the frequency of prescription of these drugs according to the best scientific evidence and use approved by regulatory agencies.
MethodsThis was a cross-sectional study of 150 pediatric prescriptions containing analgesic, antipyretic, and nonsteroidal anti-inflammatory drugs, followed by interview with caregivers at 18 locations (nine private drugstores and nine Basic Health Units of the Brazilian Unified Health System). The assessed outcomes included recommended use or use with no contraindication, indications with benefit evidence, and health surveillance agency-approved use. Data were analyzed in electronic databases and the variables were summarized by simple frequency.
ResultsA total of 164 analgesic, antipyretic, and nonsteroidal anti-inflammatory drugs were prescribed to 150 children aged 1–4 years (38.6%). Dipyrone was included in 82 (54.6%) and ibuprofen in 40 (26.6%) prescriptions. Non-recommended uses were identified in 15% of prescriptions and contraindicated uses were observed in 13.3%. Nimesulide (1.5%) is still prescribed to children younger than 12 years. The dose was incorrect in 74.3% of prescriptions containing dipyrone. Of the 211 reported clinical indications, 56 (26.5%) had no evidence of benefit according to the best available scientific evidence and 66 (31.3%) had indications not approved by the regulatory agencies.
ConclusionThere are significant discrepancies between clinical practice and recommended use of analgesic, antipyretic, and nonsteroidal anti-inflammatory drugs in pediatrics.
Dados sobre a prática clínica em pediatria no uso de analgésicos, antipiréticos e anti-inflamatórios não esteroides considerando a melhor evidência disponível e uso aprovado por agências reguladoras são incertos. Este estudo tem como objetivo verificar a frequência de prescrição de tais medicamentos segundo a melhor evidência científica e o uso aprovado por agências reguladoras.
MétodoEstudo transversal de 150 prescrições pediátricas, contendo analgésicos, antipiréticos e anti-inflamatórios não esteroides, seguido de entrevista aos cuidadores, em dezoito locais (nove drogarias privadas e nove Unidades de Saúde do SUS). Os desfechos avaliados incluíram uso recomendado ou sem contraindicação, indicações com evidência de benefício e o uso autorizado por agências de vigilância sanitária. Os dados foram analisados em banco eletrônico e as variáveis sumarizadas por frequência simples.
ResultadosForam prescritos 164 analgésicos, antipiréticos e anti-inflamatórios não esteroides para as 150 crianças com idade entre 1 e 4 anos (38,6%). Dipirona constou em 82 (54,6%) e ibuprofeno em 40 (26,6%). Usos não recomendados foram encontrados em 15% das receitas e usos contraindicados em 13,3%. Nimesulida (1,5%) ainda é utilizada em crianças com menos de 12 anos. Em 74,3% das prescrições contendo dipirona a dose estava incorreta. Das 211 indicações clínicas referidas 56 (26,5%) não tinham evidências de benefício segundo a melhor prova científica disponível, 66 (31,3%) eram indicações não aprovadas em agências de vigilância sanitária.
ConclusãoExistem importantes discrepâncias entre prática clínica e recomendações de uso de analgésicos, antipiréticos e anti-inflamatórios não esteroides em pediatria.
In Brazil, as in other developing countries, regulatory policies and regulations on the sales and prescription of medications for the pediatric age range are still insufficient for the sector to be free of risks related to inadequate drug prescriptions and uses.
Analgesics, antipyretics and nonsteroidal anti-inflammatory drugs (NSAIDs) are the most often prescribed medications in the pediatric age group.1 Predominantly naproxen, ketoprofen, and ibuprofen, which are over-the-counter (OTC) medications regulated by RDC No. 138/2003.2 Nimesulide and other drugs of the same group, although not included in the OTC list, can be purchased in any pharmacy in Brazil without a prescription.
Although these drugs have potential adverse effects, they are widely sold in pharmacies, disregarding restrictions of use, indications, toxicity, and contraindicated drug interactions. They are often prescribed without a defined therapeutic goal, generating unnecessary costs.
For mild to moderate pain, in general, analgesics without anti-inflammatory effect (low-dose acetylsalicylic acid and ibuprofen, or paracetamol) should be prescribed. NSAIDs have similar efficacy, but their selection should consider relative toxicity, cost, and approved age group (based on safety and efficacy studies for the drug). NSAIDs have an “all or nothing” effect, i.e., increasing the dose does not increase therapeutic efficacy, but results in increased adverse effects.3
Although fever is a beneficial response in most cases, it is an important cause of anxiety for parents and physicians. The search for more efficient treatments has led to the use of antipyretic combinations in pediatrics, much appreciated by caregivers and healthcare professionals, but whose efficacy has been tested for only a few years in clinical trials.4–6 The new schemes consist of combinations of ibuprofen and paracetamol administered at varying times. The main concern with these treatments is safety, as they may increase the risk of kidney toxicity and Streptococcus infection.7,8 Therefore, it is not known whether these combinations are more effective than and as safe as monotherapy in children with fever.6
In developed countries, the indication of analgesics, antipyretics, and NSAIDs in pediatric patients is extremely limited. Currently, only two drugs are approved by the European Medicine Agency (EMEA) for the treatment of fever in children: paracetamol and ibuprofen.9 Millions of euros have been spent to raise awareness among prescribers regarding the rational use of drugs, seeking to modify inadequate prescription criteria and habits.10
Drug prescription is a legal document, for which the person prescribing the drug (physician) and the person dispensing it (pharmacist) are responsible and subject to sanitary control and surveillance legislation.
Children are considered “therapeutic orphans,” due to lack of clinical studies with this population. The treatments are based on extrapolations of doses developed for adults. In practice, the drug is often used at indications, doses, and frequencies for which it has not been approved, thus configuring “off label” use. This situation may contribute to children's exposure to adverse events, mainly due to inadequate drug use.1
Data on clinical practice in pediatrics regarding the use of these drugs, considering the best available evidence and use approved by regulatory agencies are uncertain. Therefore, this study aimed to verify the frequency of prescription of analgesics, antipyretics, and NSAIDs according to the best scientific evidence and use approved by regulatory agencies.
MethodsStudy design, location and periodThis was a cross-sectional study, based on the analysis of pediatric prescriptions and information provided by caregivers.
The authors chose to perform an exploratory, descriptive study aiming to identify, record, and analyze the characteristics to generate a hypothesis about the criteria used in pediatric prescription of analgesic, antipyretic, and NSAIDs. Although this subject can be a constant target of debate, it has not been explored in depth.
The study was initiated after approval by the Ethics and Research Committee, Universidade de Sorocaba (UNISO) (Document No. 037/08, 11/19/2008).
Selection of the study sites, criteria and case management proceduresData collection was carried out in nine private pharmacies and nine Basic Health Units (BHUs) from the Brazilian Unified Health System in the municipality of Sorocaba, SP, which were chosen by drawing lots, considering their geographical location. The field research was conducted for nine months. Volunteers (caregivers who had a pediatric prescription) were recruited to participate in the study according to order of arrival at the pharmacy. The research was conducted once a week at different hours. This study used two data sources: pediatric prescriptions and interviews with caregivers who had the prescriptions. Details on eligibility criteria, data collection, interviews, and the questionnaire used have been previously published by Ferreira et al.11
Indication classification according to the best available evidence and approval of regulatory agenciesFor the indication classification according to the best available clinical evidence of efficacy, theoretical data from Dynamed® (EBSCO, MA, USA),12 Clinical Evidence,13 and Drugdex® System Thomson Micromedex14 were used. To verify the approved indications, drug registration data from the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) and from the Food and Drug Administration (FDA) were used.
Drug indication was classified according to recommendation of use: use is not recommended (i.e., it can be used with precautions) and contraindicated use (absolutely prevents the use). Information on patient characteristics (age, comorbidity, among others) and the diagnosis reported by the caregiver were verified considering the recommended information on use found in the databases.
Data analysisContinuous variables were described by means and standard deviations or median, minimum, and maximum values, as appropriate, whereas binary variables were described by proportions. A descriptive exploratory analysis was employed.
The reported indications were classified as: (i) those with defined scientific evidence; (ii) those with no contraindications for use; (iii) those approved by a regulatory agency; or (iv) those without these properties.
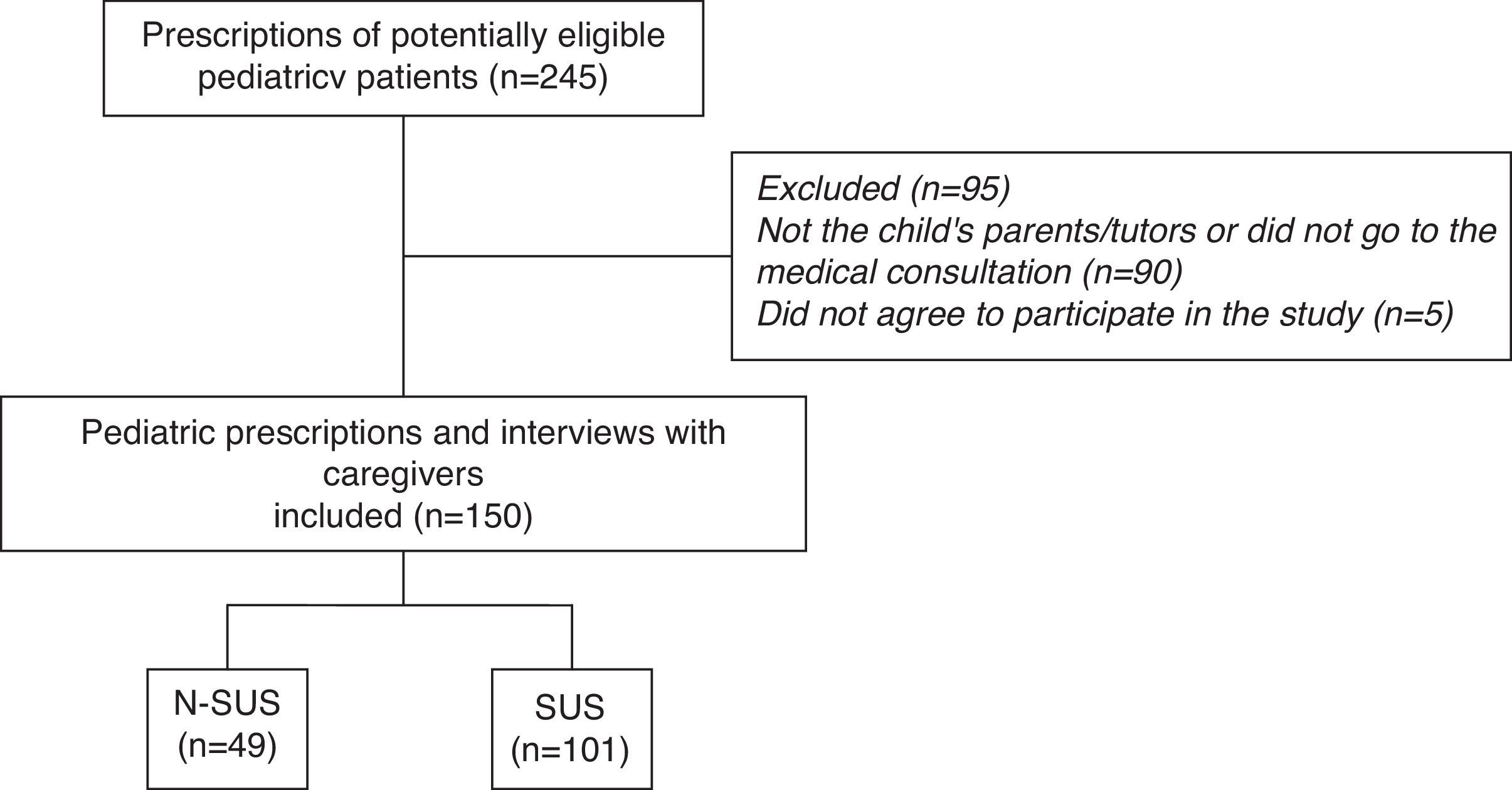
ResultsSample composition is described in Fig. 1.
Sample characteristics were described in the study by Ferreira et al.11 There was a higher prevalence of three or more drugs per prescription, found in the age group of 1–4 years (interquartile range, 3.5–8.7), and 60% of the prescriptions failed to mention the medical specialty. In 51.3% of cases, the mothers were the caregivers who took the prescription to be filled.
The 150 patients were taking 506 drugs, of which 431 (85.2%) were prescribed. However, caregivers of 58 children reported that they were also using other medications, of which 75 (14.8%) were not included in the analyzed prescription and were therefore the result of other prescriptions or self-medication. Ninety-one patients did not use any drugs other than those listed in the assessed prescription (data not shown).
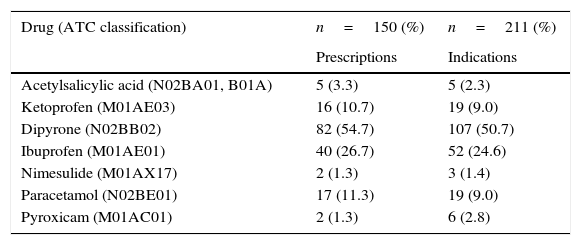
In the 150 prescriptions, the seven analgesics, antipyretics, and NSAIDs identified were prescribed 164 times for 211 indications. This means there were prescriptions with more than one drug from this group. The most commonly observed drug was dipyrone in 82 cases (54.7%), followed by ibuprofen, in 40 (26.7%; Table 1). According to the International Classification of Diseases (ICD 10) the most frequent clinical indications for dipyrone, in 68 cases, (63.5%) were for the treatment of symptoms of flu, colds, influenza-related infections, tonsillitis, pharyngitis, and other respiratory diseases (J00-J11.9). The most frequent indications for ibuprofen, in 17 cases, (32.7%) were to treat unspecified symptoms and signs, such as pain, fever, headache, and others (R50-R52).
Reported clinical indications.
| Drug (ATC classification) | n=150 (%) | n=211 (%) |
|---|---|---|
| Prescriptions | Indications | |
| Acetylsalicylic acid (N02BA01, B01A) | 5 (3.3) | 5 (2.3) |
| Ketoprofen (M01AE03) | 16 (10.7) | 19 (9.0) |
| Dipyrone (N02BB02) | 82 (54.7) | 107 (50.7) |
| Ibuprofen (M01AE01) | 40 (26.7) | 52 (24.6) |
| Nimesulide (M01AX17) | 2 (1.3) | 3 (1.4) |
| Paracetamol (N02BE01) | 17 (11.3) | 19 (9.0) |
| Pyroxicam (M01AC01) | 2 (1.3) | 6 (2.8) |
ATC, Anatomical Therapeutic Chemical Code.
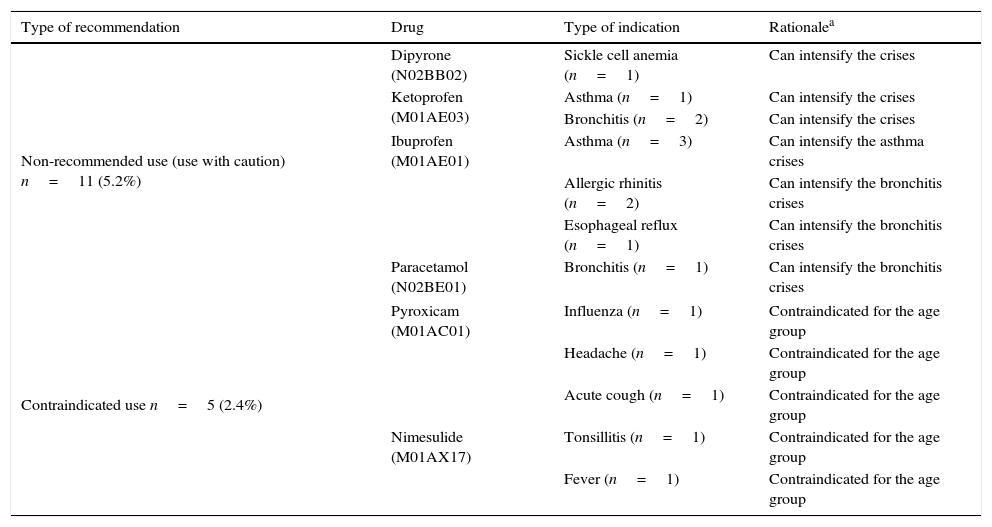
Table 2 shows the non-recommended and contraindicated use of analgesics, antipyretics, and NSAIDs. In this sample, six (2.84%) cases were indications to treat symptoms associated with allergic conditions (asthma or rhinitis), which is not recommended due to the possibility of disease exacerbation. Additionally, nimesulide (M01AX17) and piroxicam (M01AC01) were indicated for pain and fever management in children younger than 12 years.
Characterization of non-recommended use (use with caution) and contraindicated use of AA and NSAIDs found in the prescriptions, considering patient characteristics and clinical indication (reported diagnosis).
| Type of recommendation | Drug | Type of indication | Rationalea |
|---|---|---|---|
| Non-recommended use (use with caution) n=11 (5.2%) | Dipyrone (N02BB02) | Sickle cell anemia (n=1) | Can intensify the crises |
| Ketoprofen (M01AE03) | Asthma (n=1) | Can intensify the crises | |
| Bronchitis (n=2) | Can intensify the crises | ||
| Ibuprofen (M01AE01) | Asthma (n=3) | Can intensify the asthma crises | |
| Allergic rhinitis (n=2) | Can intensify the bronchitis crises | ||
| Esophageal reflux (n=1) | Can intensify the bronchitis crises | ||
| Paracetamol (N02BE01) | Bronchitis (n=1) | Can intensify the bronchitis crises | |
| Contraindicated use n=5 (2.4%) | Pyroxicam (M01AC01) | Influenza (n=1) | Contraindicated for the age group |
| Headache (n=1) | Contraindicated for the age group | ||
| Acute cough (n=1) | Contraindicated for the age group | ||
| Nimesulide (M01AX17) | Tonsillitis (n=1) | Contraindicated for the age group | |
| Fever (n=1) | Contraindicated for the age group | ||
AA, analgesic and antipyretic; NSAIDs, non-steroidal anti-inflammatory drugs.
According to Dynamed (https://dynamed.ebscohost.com), Clinical Evidence (http://clinicalevidence.bmj.com/x/index.html), Drugdex® System. Thomson Micromedex, Greenwood Village, Colorado, USA (http://www.micromedex.com).
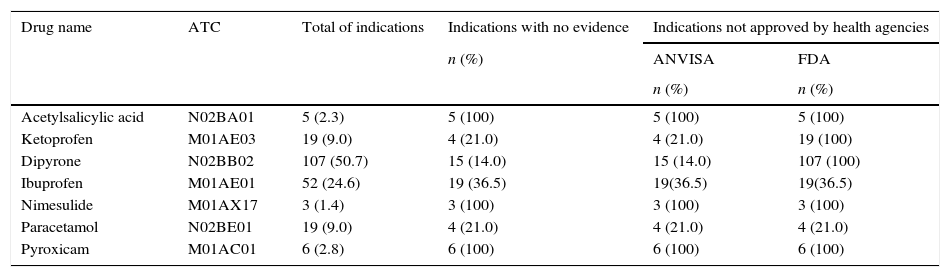
Table 3 shows the indications with no evidence of benefit according to the best scientific evidence and the number of indications not approved by ANVISA or the FDA. It was observed that 100% of the reported indications for acetylsalicylic acid (N02BA01, B01A), dipyrone (N02BB02), nimesulide (M01AX17), and piroxicam (M01AC01) had no clinical studies to support their use.
Frequency of reported indications, with no scientific evidence of benefit and use not approved by health agencies.
| Drug name | ATC | Total of indications | Indications with no evidence | Indications not approved by health agencies | |
|---|---|---|---|---|---|
| n (%) | ANVISA | FDA | |||
| n (%) | n (%) | ||||
| Acetylsalicylic acid | N02BA01 | 5 (2.3) | 5 (100) | 5 (100) | 5 (100) |
| Ketoprofen | M01AE03 | 19 (9.0) | 4 (21.0) | 4 (21.0) | 19 (100) |
| Dipyrone | N02BB02 | 107 (50.7) | 15 (14.0) | 15 (14.0) | 107 (100) |
| Ibuprofen | M01AE01 | 52 (24.6) | 19 (36.5) | 19(36.5) | 19(36.5) |
| Nimesulide | M01AX17 | 3 (1.4) | 3 (100) | 3 (100) | 3 (100) |
| Paracetamol | N02BE01 | 19 (9.0) | 4 (21.0) | 4 (21.0) | 4 (21.0) |
| Pyroxicam | M01AC01 | 6 (2.8) | 6 (100) | 6 (100) | 6 (100) |
ATC, Anatomical Therapeutic Chemical Code; ANVISA, Brazilian Health Surveillance Agency; FDA, Food and Drug Administration.
Acetylsalicylic acid had five indications: four for the treatment of sickle cell anemia and one for tonsillitis, whose prescription curiously stated that a tablet should be diluted in half a glass of water for gargling every 8h for 5 days. These instructions are not approved by health agencies and have no recommended use based on scientific evidence.
In this sample, dipyrone was prescribed to 82 patients (54.6%) and was combined with other analgesics and antipyretics (AA) or NSAIDs in eight (9.7%) cases. The doses were higher than those recommended by regulatory agencies or even the drug leaflets in 41 (55.4%) prescriptions. Many of them included a recommended use that does not appear in any official protocol or consulted database.
Ibuprofen was prescribed for 52 clinical conditions; in 19 (36.5%), its use is not based in scientific evidence or authorized by any health agency. It is noteworthy that there were indications for patients with bronchitis, stomatitis, reflux, rhinitis-sinusitis, and cough.
Paracetamol was indicated for 19 clinical conditions, four of which (21%) without scientific evidence, including respiratory allergies, reflux, cough, and stomatitis.
DiscussionMain findingsThe prescriptions (150) containing the seven analgesic, antipyretic, NSAIDs (acetylsalicylic acid, ketoprofen, dipyrone, ibuprofen, nimesulide, paracetamol, and piroxicam) for pediatric use had 56 (26.5%) indications with no scientific evidence. Of the 211 reported indications, 14 (6.6%) were not authorized by any regulatory agency, 11 (5.2%) were not recommended or should be used with caution, and five (2.4%) had contraindicated use.
Among these seven drugs that had 100% indications not approved by the FDA or ANVISA, mainly because they were prescribed to children younger than 12 years, are ketoprofen (approved by ANVISA, but not by the FDA), nimesulide (approved by ANVISA, but not by the FDA), and piroxicam (not approved by either agency). Dipyrone is not approved for use by the FDA.
Comparison with other studiesIn the present study, the highest prevalence of use of analgesic, antipyretic, NSAIDs with no evidence of benefit was found for drugs prescribed to children younger than the recommended age. Several drugs whose use was approved in Brazil in 2009 (ketoprofen, nimesulide, and piroxicam) have use restrictions according to age, as specified by regulatory agencies in other countries.
It is noteworthy the case of nimesulide, which was never approved for pediatric use and whose sales have been suspended in several countries (Ireland, England, Australia, France, Finland, Portugal, and Spain)15 due to the possibility of liver damage, skin reactions, and fatal Reye's syndrome; it was initially approved for pediatric and adult use in Brazil. The approval of nimesulide for pediatric use in Brazil before 2007, without more conclusive studies regarding its safety in this population,14 was surprising and can explain the inadequate prescriptions of this medication still observed in the present sample. Currently, ANVISA requires leaflets to include: “This product is not suitable for children younger than 12 years.”
However, when accessing drug sales sites16 it is possible to identify in the Brazilian market at least 16 laboratories that manufacture nimesulide as oral solution at concentrations of 10mg/mL, 50mgmL, or 100mg/mL, whose drug leaflets still bring dose recommendation for children aged >1 year old of 1 drop/kg for the treatment of pain and injuries. Conversely, when the same query is carried out in ANVISA electronic drug information site, only four laboratories have registered the drug leaflet of nimesulide as oral solution, where the following orientations can be found: “Adult and pediatric use in children older than 12 years” and “This product is contraindicated for children younger than 12 years.”17
This discrepancy in information can confound prescribers, healthcare professionals, and consumers, increasing the risks of inappropriate use of this medication by the pediatric population.
The World Health Organization has twice issued warnings against the marketing of nimesulide; in 2003, it was placed in the category of special products under pharmacovigilance. In 2007, the EMEA started a systematic analysis of liver damage caused by this product and decided to maintain it in the market, with its use approved for children older than 12 years, providing they are under constant surveillance and limiting the use to a maximum of 15 consecutive days.18
A similar situation was observed with piroxicam in Brazil. Until 2009, drug presentations included oral solution/drops without age-restricted use. Currently, piroxicam is no longer found in this pharmaceutical form and presentation, and its indication is restricted to children older than 12 years.
As for the ketoprofen, it appears that it is sold in Brazil in the pediatric formulation as a medication with analgesic and antipyretic properties at low doses, as well as anti-inflammatory properties at larger doses, indicated for the symptomatic relief of fever and/or pain in children older than 6 months. The leaflet contains directions for use in special populations, that is, children younger than 6 months, in whom drug safety and efficacy have not been established yet.19 The FDA does not approve its use in the pediatric population, but considers it effective in cases of fever (Class IIb, category B), osteoarthritis (class IIb, category C), pain (class IIa, category B) and rheumatoid arthritis (class IIb, category C).13
Ufer et al. confirmed the association between the use of drugs not approved for pediatric use and the prevalence of adverse effects.1 Wilton et al. observed that 20% of pediatric prescriptions in Sweden contained drugs recently introduced in the market, including a percentage of drugs with some contraindication for the age range.20 In this clinical–epidemiological scenario, it is believed that the number of medications considered inappropriate for pediatric use is higher than that disclosed by several studies.21,22
In 10% of the present sample, two AA or NSAIDs were included in the same prescription, to be used alternately. This indication lacks evidence, which increases the risk of liver damage and may create doubts for the caregiver about administration intervals.23,24 A study in Argentina, with 1600 pediatricians, showed that 59% of them alternate two antipyretics, and concluded that this practice is more common among physicians with less experience.25 It is odd that some clinical protocols of the Brazilian Ministry of Health indicate this practice; one noteworthy example is the treatment of dengue fever.
Considering the uncertainty surrounding the superiority or safety of combined antipyretic regimens when compared with monotherapy, paracetamol or ibuprofen alone should continue to be used. National Institute of Health and Clinical Excellence (NICE) guidelines state that paracetamol and ibuprofen should not be routinely administered together or used interchangeably. However, if the patient does not respond to one of these drugs, an alternative drug could be used.26
Another interesting finding in the present sample was related to the prescribed dose. Dipyrone, the drug with the highest prevalence of prescriptions at inadequate doses, had 55.4% of prescriptions above the recommended or approved doses. These data are different from those obtained by Ferreira et al.,27 who observed dipyrone use at lower doses administered by routes not indicated to children younger than 1 year. Alves et al.28 observed that children received doses higher than those recommended by the drug leaflet, increasing the risks of adverse events, including hypotension.
The calculation of the pediatric dose is still a major therapeutic problem. Dose calculation based on the patient's age is not always the best option, especially in infants, and can result in overdose. Patients of the same age may differ in body mass29; however, calculating the pediatric dose based on body weight of the subject is not indicated either, as it is known that children's maturation process occur gradually and does not correspond to the individual gain in stature.30 Studies with this age group are still necessary to establish the optimal dose. This fact may explain the observed dose variations in this sample.
Study strengths and limitationsThis study presented the first detailed data on use of analgesic, antipyretic, and NSAIDs in pediatric patients in Brazil. Patients were included shortly after the medical consultation, which reduced the confounding factor of recalling the reported indications or the child's signs and symptoms. Patients were identified throughout the year, in all four seasons, reducing possible seasonality biases. Additionally, concerned about the population representativeness, the authors selected 18 different locations, including patients treated at the public and the private sectors.
A detailed interview script was used by two trained interviewers, and the answers were cross-checked with the data contained in the prescriptions. Perhaps the main limitation of this study was its sample size, but regarding this aspect, a descriptive exploratory analysis was chosen, with no associations between variables.
Sources of evidence recommended by regulatory agencies and by the World Health Organization were used for data analysis (Dynamed, Clinical Evidence, Drugdex® System Thomson Micromedex).12–14
Practical implications and final considerationsDrug prescription should be based on the best available evidence of benefit and on the values and preferences of the individual that will be treated.
Addressing the parents’ anxiety and fears about fever and educating them on the immunological usefulness of fever and the risks associated with the overuse of antipyretics should remain a priority.
There is urgent need for intervention measures in drug dispensing, which will result in the rational use of these drugs, as well as a positive impact on health outcomes. Drug registration policies that consider the best available scientific evidence could decrease the advent of drugs with unclear use indications.
These findings show that important differences can be observed between clinical practice in pediatrics regarding the use of AA and NSAIDs and recommendations based on the best available scientific evidence and use approved by regulatory agencies.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ferreira TR, Lopes LC. Analysis of analgesic, antipyretic, and nonsteroidal anti-inflammatory drug use in pediatric prescriptions. J Pediatr (Rio J). 2016;92:81–7.