To describe the epidemiological profile and the survival rate of patients with acute myeloid leukemia (AML) in a state reference pediatric hospital.
MethodClinical–epidemiological, observational, retrospective, descriptive study. The study included new cases of patients with AML, diagnosed between 2004 and 2012, younger than 15 years.
ResultsOf the 51 patients studied, 84% were white; 45% were females and 55%, males. Regarding age, 8% were younger than 1 year, 47% were aged between 1 and 10 years, and 45% were older than 10 years. The main signs/symptoms were fever (41.1%), asthenia/lack of appetite (35.2%), and hemorrhagic manifestations (27.4%). The most affected extra-medullary site was the central nervous system (14%). In 47% of patients, the white blood cell (WBC) count was below 10,000/mm3 at diagnosis. The minimal residual disease (MRD) was less than 0.1%, on the 15th day of treatment in 16% of the sample. Medullary relapse occurred in 14% of cases. When comparing the bone marrow MRD with the vital status, it was observed that 71.42% of the patients with type M3 AML were alive, as were 54.05% of those with non-M3 AML. The death rate was 43% and the main proximate cause was septic shock (63.6%).
ConclusionsIn this study, the majority of patients were male, white, and older than 1 year. Most patients with WBC count <10,000/mm3 at diagnosis lived. Overall survival was higher in patients with MRD <0.1%. The prognosis was better in patients with AML-M3.
Descrever o perfil epidemiológico e a taxa de sobrevida dos pacientes com Leucemia Mielóide Aguda (LMA), em um hospital pediátrico de referência estadual.
MétodoEstudo clínico-epidemiológico, observacional, retrospectivo e descritivo. Foram incluídos casos novos de pacientes com LMA, diagnosticados entre os anos de 2004 e 2012, com idade <15 anos.
ResultadosEntre os 51 pacientes estudados, 84% eram da raça branca, 45% do sexo feminino e 55% do masculino. Quanto a faixa etária, 8% tinham idade <1 ano, 47% entre 1 e 10 anos e, 45% >10 anos. Os principais sinais/sintomas ao diagnóstico foram febre (41,1%), astenia/inapetência (35,2%) e manifestações hemorrágicas (27,4%). O Sistema Nervoso Central foi o local extra-medular mais acometido (14%). Em 47% dos pacientes a leucometria ao diagnóstico foi <10.000/mm3. A doença residual mínima (DRM) no 15° dia de tratamento foi <0,1% em 16% da casuística. Recidiva medular ocorreu em 14% dos casos. Ao se comparar a DRM da medula óssea com o status vital, observou-se que estavam vivos 71,42% dos pacientes com LMA tipo M3 e 54,05% daqueles com LMA não-M3. A taxa de óbito foi de 43% e a principal causa imediata foi o choque séptico (63,6%).
ConclusõesNeste estudo, a maioria dos pacientes é do sexo masculino, raça branca, maiores que 1 ano de idade. A maioria dos pacientes com leucometria <10.000/mm3 ao diagnóstico está viva. A sobrevida global é maior nos pacientes com DRM <0,1%. O prognóstico é melhor nos pacientes com LMA-M3.
Acute myeloid leukemia (AML) is a clonal disease of hematopoietic tissue characterized by abnormal proliferation of progenitor cells of myeloid lineage, resulting in the insufficient generation of normal mature blood cells.1
It represents approximately 15–20% of acute leukemia cases in childhood,1 and accounts for about 30% of deaths in this age range.2 In Brazil, its estimated incidence is 400 cases per year.3
Hemorrhagic manifestations, fever, and pallor are frequent symptoms of the disease at diagnosis. The main causes of death are infections, bleeding episodes, leukostasis, and tumor lysis syndrome.4
The main factors affecting the mortality rate of these patients are those resulting from therapy intensification and disease relapse.5,6
AML prognosis improvement has been made possible by disease stratification into risk groups based on cytogenetics, the evaluation of early response to treatment, and the identification of chemotherapy-induction failure. Currently, the likelihood of AML cure in developed countries is around 60%.7,8
Considering that in recent decades there has been progress in the introduction of new chemotherapy treatment protocols for AML and that the information in the pediatric population and its clinical outcomes are still scarce, this study aimed to analyze the eight-year experience in a pediatric oncology service, in the South of Brazil.
MethodsThis was a clinical–epidemiological, observational, retrospective, descriptive study approved by the Ethics Committee on Human Research of Hospital Infantil Joana de Gusmão (HIJG).
The study included all new cases of AML patients younger than 15 years treated in HIJG between January 2004 and August 2012.
Exclusion criteria were loss to follow-up; patient transfer to another service; insufficient data at the hospital cancer registry and medical records and statistics department of HIJG.
The diagnosis of AML was based on morphology, cytochemistry, and immunophenotyping by flow cytometry of bone marrow aspirate and/or peripheral blood, performed with EuroFlow 8-color antibody panels (EuroFlow-ESLHO, Rotterdam, NL). The minimal residual disease (MRD) was analyzed by flow cytometry in the bone marrow after 15 days of induction treatment. The treatment protocols used were from the International Berlin-Frankfurt-Münster (BFM) Study Group AML-83 (n=6), AML-98 (n=22), and AML-2004 (n=23).
The analyzed variables were age at diagnosis (age range stratified as <1 year, 1 year to 10 years, and >10 years); gender; ethnicity/skin color, as established by the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística – IBGE) as white, black, brown, yellow, and indigenous; origin, according to the mesoregions of the state of Santa Catarina established by IBGE (city of Florianópolis, Northern Santa Catarina, Western Santa Catarina, Mountain Region, Southern Santa Catarina, Valley of Itajaí) and others (from other states); predominant signs and symptoms at diagnosis (fever, bone pain, abdominal pain, enlarged lymph nodes, hemorrhagic manifestations, gingival hypertrophy, pallor, asthenia/lack of appetite, swelling of soft tissue, and respiratory symptoms); laboratory findings at diagnosis (white blood cell count, neutropenia, and platelet count); extramedullary involvement at diagnosis (CNS or other sites); AML classification according to the French–American–British group (FAB)1; presence and type of laboratory genetic alterations; presence or absence of clinical and laboratory remission after the first chemotherapy induction, according to the MRD value on the 15th day of induction, obtained by flow cytometry; vital status (alive or deceased); death (in remission or not from neoplastic disease), immediate cause of death; presence and location of disease recurrence (bone marrow, extramedullary, or combined); and bone marrow transplantation.
The statistical procedures used in the study were descriptive measures, frequency tables, and Pearson's chi-squared test, with 95% of significance (p<0.05) to analyze, as an exploratory characteristic, the association between two variables. Associations were verified between the variables vital status and white blood cell count, vital status and type M3-AML MRD, and vital status and non-M-AML MRD.
ResultsOf the 51 patients, 55% (n=28) were males and 45% (n=23) females, at a ratio of 1.2:1. Whites predominated, accounting for 84% of patients. Brown and black patients represented 16% of the sample.
Mean age at diagnosis was 7.3 years (SD±4.8 years) with a median of 9 years. The youngest age at diagnosis was 19 days and the oldest, 14 years. Regarding the age range, 8% of the sample were aged <1 year, 47% were between 1 and 10 years, and 45% were >10 years.
As for the origin, according to the mesoregions of Santa Catarina by IBGE, 29% were from the city of Florianópolis (n=15), 24% were from the Western Region (n=12), 20% from the Mountain Region (n=10), 10% from the Northern Region (n=5), 8% from the Southern Region (n=4), 8% from the Itajai Valley (n=4), and 2% (n=1) were from another state, Paraná.
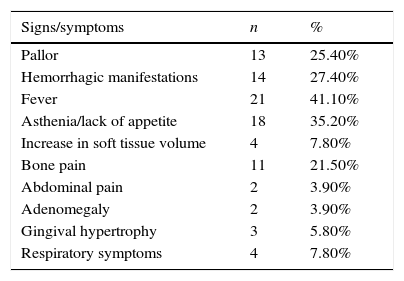
The main signs and symptoms at diagnosis are described in Table 1.
Signs and symptoms at diagnosis in number (n) and percentage (%) of patients with acute myeloid leukemia treated in Hospital Infantil Joana de Gusmão (HIJG), from 2004 to 2012.
| Signs/symptoms | n | % |
|---|---|---|
| Pallor | 13 | 25.40% |
| Hemorrhagic manifestations | 14 | 27.40% |
| Fever | 21 | 41.10% |
| Asthenia/lack of appetite | 18 | 35.20% |
| Increase in soft tissue volume | 4 | 7.80% |
| Bone pain | 11 | 21.50% |
| Abdominal pain | 2 | 3.90% |
| Adenomegaly | 2 | 3.90% |
| Gingival hypertrophy | 3 | 5.80% |
| Respiratory symptoms | 4 | 7.80% |
At diagnosis, 24% of patients had extramedullary disease (n=12), and the most often affected site was the central nervous system (CNS) in 14% (n=7). The other 10% had skin involvement (n=2), bone involvement, and soft tissue edema (n=3).
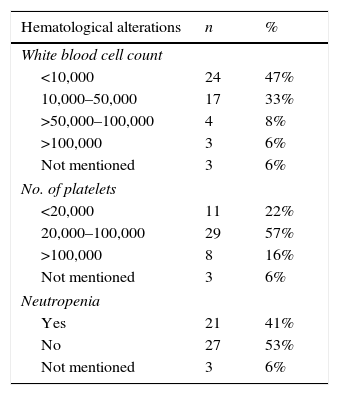
Laboratory data at AML diagnosis (leukocytes, platelets, and neutropenia) and morphological FAB classification are described in Table 2.
Hematological alterations and French–American–British (FAB) classification at diagnosis of patients with acute myeloid leukemia treated at Hospital Infantil Joana de Gusmão (HIJG), from 2004 to 2012.
| Hematological alterations | n | % |
|---|---|---|
| White blood cell count | ||
| <10,000 | 24 | 47% |
| 10,000–50,000 | 17 | 33% |
| >50,000–100,000 | 4 | 8% |
| >100,000 | 3 | 6% |
| Not mentioned | 3 | 6% |
| No. of platelets | ||
| <20,000 | 11 | 22% |
| 20,000–100,000 | 29 | 57% |
| >100,000 | 8 | 16% |
| Not mentioned | 3 | 6% |
| Neutropenia | ||
| Yes | 21 | 41% |
| No | 27 | 53% |
| Not mentioned | 3 | 6% |
| FAB | n | % |
|---|---|---|
| M0 | 6 | 12% |
| M1 | 1 | 2% |
| M2 | 14 | 27% |
| M3 | 14 | 27% |
| M4 | 8 | 16% |
| M5 | 4 | 8% |
| M6 | 1 | 2% |
| M7 | 3 | 6% |
When correlating WBC count at diagnosis with vital status (p=0.014), it was observed that 79.2% of patients with WBC count <10,000 lived, whereas 29.4% of those with WBC counts between 10,000 and 50,000 lived. The three patients (6%) that had WBC count >100,000 died.
The study of genetic disorders was documented in 51% of patients (n=26), showing alterations in 46.15% of cases (n=12). The most frequent alteration was t(15;17), found in six patients (23%). One of these patients had an association of t(15;17) with FLT3-ITD mutation. The other genetic disorders found were polyploidy, t(8;21), presence of MLL rearrangement, t(3;5) deletion of chromosome 18, trisomy of chromosome 21, trisomy of chromosome 8, and tetrasomy of chromosome 8.
MRD analysis in 45% of patients (n=23) was <0.1%; in 16% (n=8) it was between 0.1% and 1.0%; in 25% (n=13) it was >1.0%; 14% (n=7) of the cases lacked the test result in the medical record.
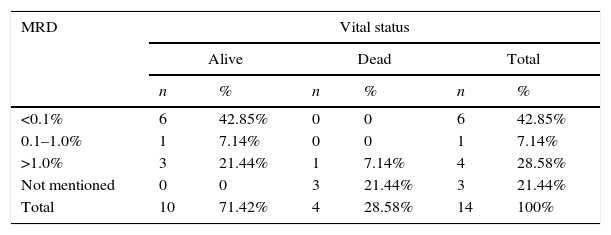
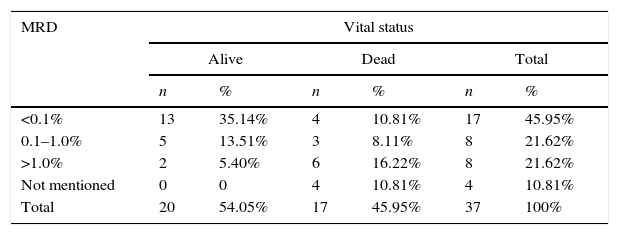
The comparison between the bone marrow MRD, on the 15th day of treatment and the vital status of patients with acute promyelocytic leukemia (M3-AML) and acute non-promyelocytic leukemia (non-M-AML) is described in Tables 3 and 4.
Minimal residual disease (MRD) in bone marrow on the 15th day of treatment of acute promyelocytic leukemia (M3-AML) and vital status of patients treated at Hospital Infantil Joana de Gusmão (HIJG), from 2004 to 2012 (p=0.025).
| MRD | Vital status | |||||
|---|---|---|---|---|---|---|
| Alive | Dead | Total | ||||
| n | % | n | % | n | % | |
| <0.1% | 6 | 42.85% | 0 | 0 | 6 | 42.85% |
| 0.1–1.0% | 1 | 7.14% | 0 | 0 | 1 | 7.14% |
| >1.0% | 3 | 21.44% | 1 | 7.14% | 4 | 28.58% |
| Not mentioned | 0 | 0 | 3 | 21.44% | 3 | 21.44% |
| Total | 10 | 71.42% | 4 | 28.58% | 14 | 100% |
Minimal residual disease (MRD) in bone marrow on the 15th day of treatment of non-acute promyelocytic leukemia (non-M3-AML) and vital status of patients treated at Hospital Infantil Joana de Gusmão (HIJG), from 2004 to 2012 (p=0.019).
| MRD | Vital status | |||||
|---|---|---|---|---|---|---|
| Alive | Dead | Total | ||||
| n | % | n | % | n | % | |
| <0.1% | 13 | 35.14% | 4 | 10.81% | 17 | 45.95% |
| 0.1–1.0% | 5 | 13.51% | 3 | 8.11% | 8 | 21.62% |
| >1.0% | 2 | 5.40% | 6 | 16.22% | 8 | 21.62% |
| Not mentioned | 0 | 0 | 4 | 10.81% | 4 | 10.81% |
| Total | 20 | 54.05% | 17 | 45.95% | 37 | 100% |
Overall survival was 57% in this study. By correlating vital status with M3-AML and non-M-AML, it was observed that 71.42% and 54.05% of the patients were alive, respectively, at the end of the study.
Of the patients who died (n−22), 64% (n=14) were not in complete clinical remission of leukemia at the end of chemotherapy induction. The main immediate cause of death was septic shock in 63.6% of patients (n=14). Of the deaths, three occurred within the first 15 days of treatment, one from intracranial hemorrhage (M3-AML) and the other two, in addition to sepsis, had pulmonary leukostasis and intracranial hemorrhage with spinal fluid infiltrate at diagnosis, respectively.
Regarding the deaths that occurred after the 16th day of induction treatment (n=19), 12 occurred due to septic shock. Of these, six occurred after chemotherapy re-induction for recurrence.
Of patients with WBC count >100,000, one patient had M3-AML, whose immediate cause of death was septic shock, another had M0-AML with pulmonary leukostasis associated with septic shock, and the other had M5-AML with acute renal failure.
Regarding the patients (n=6) that had t(15;17) mutations, two died. The patient that had t(15;17) and FLT3-ITD mutations was alive and in remission until the date of completion of this study.
Among the assessed patients, 39% (n=20) were submitted to radiotherapy and 16% (n=8) to bone marrow transplantation (BMT). Of the patients who underwent BMT, two died and the others are alive and in complete remission of leukemia.
In 14% of cases (n=7) there was disease recurrence in the bone marrow and, of these, six patients died.
DiscussionIn this study, most patients were male and older than 1 year. Most of those that had WBC count <10,000/mm3 at diagnosis are alive.
Overall survival is higher in patients with MRD <0.1% and the prognosis is better in patients with M3-AML.
In other studies,9,10 male gender also showed a slight predominance to females (1.2:1).
As in the study by Kavcic,10 it was observed that AML predominated in whites. In Santa Catarina, as a result of the white descent of the majority of the population, white ethnicity was predominant, making it impossible to establish comparisons between the ethnic groups.
Corroborating other studies,10–12 this study showed a higher prevalence of AML in the age range >1 year old.
This study, as in the literature, also demonstrated that the signs and symptoms of AML result from bone marrow infiltration, with hemorrhagic manifestations, fever, asthenia, pallor, and bone pain.2
In previously published studies, the percentage of CNS involvement at diagnosis ranged from 5% to 15%.2,13 In this study, 14% of patients had CNS involvement at diagnosis, similarly to what was described by Rubnitz et al.13
Studies have shown that the percentage of patients with WBC counts >100,000/mm3 is between 12% and 15%.6,9,14 In this series, only 6% of cases had this elevated WBC count at diagnosis. This difference can be explained by the lack of data available in the medical records of some patients, the size of this sample, or the earlier disease diagnosis.
WBC count is the most important risk factor for treatment failure, due to the possibility of causing bleeding complications or respiratory failure due to leukostasis.9,15 The present study showed that 80% of patients had WBC count <50,000/mm3 at diagnosis, similar to the study by Imamura et al.14
The probability of event-free survival (EFS) in five years was only 23% in patients with WBC count >100,000/mm3 according to the analysis of the BFM-83 and BFM-87 treatment protocols.9 This study showed that 79.2% of the patients (p=0.014) with WBC count <10,000/mm3 at diagnosis lived, whereas among those with WBC counts between 10,000 and 50,000/mm3, only 29.4% lived. Only three patients in this study had a WBC count >100,000/mm3 at diagnosis, thus making the statistical analysis impossible.
The FAB morphological classification for AML can define treatment and risk group stratification.3 In previously published studies9,14,16 the most frequently identified subtype was M2, ranging from 33.6% to 37%. In the present study, the most prevalent morphological types were M2 (27%) and M3 (27%), similar to the findings of Xiao-Jun Xu et al.6
After the advent of all-trans-retinoic acid (TRA), M3-AML became the FAB subtype with the best prognosis, by inducing the differentiation of leukemic precursors into mature cells, controlled by this drug.1,17 The present study also found good therapeutic response to this medication, considering that 71.42% of patients with M3-AML and 54.05% of those with non-M3-AML were alive at the end of this study.
AML is a heterogeneous disease from the molecular point of view, including somatic and epigenetic alterations that contribute to myeloid leukemogenesis. These cytogenetic abnormalities, somatic mutations, and the induction therapy response are important information for risk stratification and adequate therapy allocation.18
Chromosomal abnormalities in AML include aberrations described as gain or loss of whole chromosomes structural abnormalities or balanced translocations.19 The literature reports that the translocation t(8;21) is the most prevalent, varying between 12% and 23%,12–14 whereas t(15;17) is observed in 3.4–10% of cases.12,13
According to the present results, 46% of the assessed patients have some cytogenetic alteration, and translocation t(15;17) was most prevalent (23%), which is justified by the number of cases of M3-AML in this study. As this alteration has a favorable prognosis, it confirms the findings of increased survival in patients with M3-AML. It is noteworthy that the cytogenetic analysis of patients in this study was documented in only 51% of cases, which might have underestimated the results.
A recent study15 stated that survival is poor in patients with translocation t(15;17) and FLT3-ITD mutation association, thus representing an independent prognostic predictor, with a progression-free survival rate of 16%. In the present series, only one patient with M3-AML had the translocation t(15;17) and FLT3-ITD association, and was alive and without signs of relapse at the end of the study.
In the BFM study, bone marrow MRD measured after the first treatment course was the best event-free survival predictor when compared to the risk classification scheme by FAB subtype, cytogenetics, and the presence of blasts in peripheral blood morphology on the 15th day of anti-neoplastic treatment.15 The presence of high MRD after the first course of induction was significantly associated with an adverse outcome. The recurrence rate was particularly high (49%) in patients with MRD >1%, while in patients with lower MRD (0.1–1%), the rate was only 17%.15
The study by Al-Mawali et al.20 also demonstrated that MRD >0.15% was an independent predictor of poor prognosis after chemotherapy induction. Other studies have also demonstrated a correlation between MRD <0.1% and improved event-free survival, such as the study by Rubnitz et al.21 and Inaba et al.22,23
Although MRD is a strong prognostic factor, approximately 25% of patients with low MRD will have disease recurrence. The study by Karol et al.24 identified, among the risk factors for recurrence, certain 11q23 abnormalities such as t(6;11) and t(10;11), megakaryocytic leukemia without t(1:22), and age ≥10 years.24
In this study, when comparing the MRD <0.1% on the 15th day of treatment with vital status, it was observed that 100% of patients with M3-AML and 76.5% of patients with non-M3-AML were alive, respectively. When MRD was >1.0%, it was observed that 75% of M3-AML patients and 25% of non-M3-AML patients were alive. There was a statistical significance between bone marrow MRD and vital status (p=0.022), as well as between M3-AML and vital status (p=0.025) and non-M3-AML and vital status (p=0.019). These data corroborate the prognostic importance of MRD quantification.
Regarding recurrence, the bone marrow is the most frequent site of AML relapse.15 In this series, all recurrences occurred at this site.
Recent antineoplastic therapies, such as new chemotherapeutic agents, monoclonal antibodies, and immunomodulators, are challenges in the assessment of the impact on the child's immune function and of infectious complications.19
The literature reports that the leading cause of death in these patients is infection, and that it is mainly related to chemotherapy intensification.5,6,9
A study by Silva et al.,25 analyzing mortality trends in Brazil in children with leukemia in the 1980–2010 period, reports that the deaths associated to the treatment of acute myeloid leukemia in less developed countries can reach up to 33%, as the intensive chemotherapy regimen predisposes to severe neutropenia and exposes patients to infections.
Pediatric patients treated for AML are at high risk for infectious complications, predominantly bacterial and fungal. Some institutions have implemented prophylaxis with antibiotic and anti-fungal agents, aimed at reducing these infections. However, the systematic evaluation of antibiotic prophylaxis studies indicates that there is insufficient data to define guidelines for their use. Therefore, additional studies are needed to accurately identify the best antibiotic regimen, as well as for which population of children they would be indicated.26,27
The present study also showed that septic shock (63.6%) was the most prevalent cause of death. Of the 19 patients that died after the 15th day of the induction therapy, six had relapsed. The deaths that occurred in this period were probably due to infections after aplasia induction.
In recent decades, in spite of the improved AML prognosis, approximately one-third of patients had recurrence. This population has a poor prognosis, with long-term survival probability of approximately 35%, despite the use of intensive chemotherapy and allogeneic bone marrow transplantation. The toxicity and mortality resulting from this treatment are also significant.28
Currently, studies with targeted therapies are being developed, focusing primarily on the functional pathways of leukemic cells, such as cell surface receptors, specific intracellular kinases, proteins that regulate cell death and gene expression modulators, aiming to improve AML patient survival.29
AML treatment should be based on a clinical structure capable of supporting the therapeutic aggressiveness, in addition to a laboratory structure capable of discriminating the risk groups and contributing to the identification of relevant prognostic factors, as well as providing supportive measures for the care of these patients.
In this series, selection bias was considered due to the inclusion of all cases diagnosed at the service. These results may have been influenced by characteristics of the assessed population; the different treatment protocols used (BFM); the available medical and hospital resources in this service; and the sample size. However, the results observed in this study show the need to perform national, multicenter, analytical studies, aiming at confirmation of the observed associations and thus, establishing prognostic factors for AML.
In this study, the comparison of bone marrow MRD on the 15th day of induction treatment with vital status showed that 71.42% of patients with type M3 AML and 54.05% of those with non-M3-AML were alive. The death rate was 43% and the main immediate cause was septic shock (63.6%).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Hospital Infantil Joana de Gusmão, Florianópolis, SC, Brazil.
Please cite this article as: de Lima MC, da Silva DB, Freund AP, Dacoregio JS, Costa TE, Costa I, et al. Acute Myeloid Leukemia: analysis of epidemiological profile and survival rate. J Pediatr (Rio J). 2016;92:283–9.