To evaluate the incidence of small bowel bacterial overgrowth (SBBO) in children treated with omeprazole, and to test whether probiotics influence the incidence.
MethodsA double-blinded, placebo-controlled trial was performed in 70 children treated orally during four weeks with 20mg omeprazole per day. Lactobacillus rhamnosus R0011 (1.9×109 cfu) and Lactobacillus acidophillus R0052 (0.1×109 cfu) were simultaneously given daily to 36 subjects (probiotic group), while 34 subjects received placebo (placebo group). The diagnosis of SBBO was based on the development of suggestive symptoms, in combination with a positive glucose breath test.
ResultsAfter one month of proton pump inhibitor (PPI) treatment, 30% (21/70) had a positive breath test suggesting SBBO; of these 62% were symptomatic. Five children developed SBBO-like symptoms, but had a negative breath test; and 44 (63%) were symptom free and had a negative breath test. There was no difference in the incidence of positive breath tests in the probiotic versus the placebo group (33% vs 26.5%; p=0.13).
ConclusionsSince symptoms suggesting SBBO developed in 26% of PPI-treated children, and since the glucose breath test was abnormal in 72% of these, this side-effect should be more frequently considered. The probiotic tested did not decrease the risk to develop SBBO.
Avaliar a incidência de SBID em crianças tratadas com omeprazol e testar se os probióticos influenciam essa incidência.
MétodosUm ensaio duplo-cego controlado por placebo foi realizado em 70 crianças tratadas oralmente, durante 4 semanas, com 20mg de omeprazol por dia. Desses, 36 indivíduos receberam diária e simultaneamente Lactobacillus rhamnosus R0011 (1,9×109 cfu) e Lactobacillus acidophillus R0052 (0,1×109 cfu) (grupo probiótico), enquanto 34 receberam placebo (grupo placebo). O diagnóstico de SBID teve como base o desenvolvimento de sintomas sugestivos em combinação com um teste respiratório com glicose positivo.
ResultadosApós um mês de tratamento com IBP, 30% (21/70) apresentaram um teste respiratório positivo sugerindo SBID; desses, 62% foram sintomáticos. Cinco crianças desenvolveram sintomas parecidos com os de SBID, mas apresentaram um teste respiratório negativo; 44 (63%) não apresentavam sintomas e tiveram teste respiratório negativo. Não houve diferença na incidência de testes respiratórios positivos no grupo probiótico em comparação ao grupo placebo (33% em comparação a 26,5%; p: 0,13).
ConclusõesComo houve sintomas sugestivos de SBID em 26% das crianças tratadas com IBP e o teste respiratório com glicose deu resultados anormais em 72% delas, esse efeito colateral deve ser levado em consideração com mais frequência. O probiótico testado não reduziu o risco de desenvolver SBID.
Proton pump inhibitors (PPIs) such as omeprazole are administered in gastrointestinal diseases such as gastroesophageal reflux disease, gastric or duodenal ulcer, Zollinger-Ellison syndrome, and eradication therapy for Helicobacter pylori.1–3 The gastric acid secretory inhibitor effect of PPIs is much more potent than the effect of histamine receptor antagonists.2,4
Small bowel bacterial overgrowth (SBBO) is a condition marked by an increased number of intestinal bacteria and a change in bacterial composition in the gastrointestinal tract.5,6 Risk factors for SBBO are long term hypo/achlorhydria (as induced by PPI), intestinal anatomical abnormalities (such as diverticulum, fistula, stricture, adhesion, removal of ileocecal flap), hypomotility (such as in diabetic neuropathy, scleroderma), and severe immunodeficiency.5,6 The diagnosis of SBBO is based on clinical manifestations and on the results of a glucose breath test. Clinical manifestations of SBBO are marked by symptoms such as abdominal pain, flatulence, frequent flatus, diarrhea or constipation, and steatorhea.5–7 In chronic conditions, SBBO can cause anemia, failure to thrive, neuropathy, tetany, and paresthesia.5–7 The golden standard to diagnose SBBO is to culture intestinal aspirate. However, the latter is not performed in routine practice because of its invasive nature, its difficulty, its relatively high cost and time consumption, and its inability to diagnose SBBO in the distal area of the intestine.5–9 The Rome Consensus of 2009 recommended the glucose hydrogen breath test as the best diagnostic tool for SBBO, since it is non-invasive, easy to perform, has direct results, and has a good diagnostic accuracy.8
Probiotics are living microorganisms which, if ingested in adequate amounts, will result in a health benefit for the host.10 A role for probiotics in many diseases such as infectious diarrhea, antibiotic associated diarrhea, atopy, and constipation has been suggested.11–13 Efficacy has also been reported for some strains, such as Lactobacillus shirota, in SBBO.14 Probiotics’ mechanisms of action consist of competition with harmful bacteria, synthesis of antimicrobial conjugate (bacteriocin, lactic acid, organic acid, microsin, reuterin, and volatile fatty acid), stimulation of the immune response, and stimulation of the intestinal epithelium through production of short chain fatty acids.15–19 Until now, studies on the role of probiotics in prevention and therapy of SBBO in children are very limited.
The aims of this study were to test whether PPIs induce SBBO in children, and to evaluate whether the probiotic strains tested can prevent the development of SBBO.
MethodsStudy design and subjectsThis double-blinded, placebo-controlled randomized clinical trial was conducted in children ≥ 5 years old seen mainly because of complaints of epigastric pain in the Outpatient Clinic of the Cipto Mangunkusumo Hospital. When it was decided, on clinical grounds, that the presenting symptoms justified a therapeutic trial with omeprazole, and in the absence of exclusion criteria, a glucose breath test was performed to rule out SBBO prior to PPI-therapy.
All the subjects were treated with 20mg of oral omeprazole daily for four weeks. Patients swallowed the intact omeprazole capsule; patients with difficulties to swallow the capsule were allowed to open it and swallow the microgranules in media such as orange juice or berry juice. Furthermore, the patients were randomized in two groups: group A (probiotic group) received one probiotic capsule per day during four weeks, while group B (control group) was given a placebo capsule. The probiotic used is Lacidofil®, which contains 1.9×109 cfu Lactobacillus rhamnosus R0011 and 0.1×109 cfu Lactobacillus acidophillus R0052. A cold chain for the administration of the probiotic was preserved, as the probiotics were stored in a cooler and transported using a cold chain bag with gel ice packs inside.
The following patients were excluded: known SBBO; treatment with anti-acid agents or antibiotics during the past two weeks; immune suppression (steroid treatment, antituberculosis therapy, antiretroviral, or cytostatics); or warfarin, phenytoin or diazepam use.
Observation for medication compliance (PPI and probiotic/placebo) was performed twice a week through phone, by asking the parents about compliance. Subjects who did not take their medication during at least three days during the four-week intervention period were considered as “poor compliant”. A second glucose breath test was performed four to ten days after the end of therapy.
Hydrogen breath test8,9,20The patients fasted for ten to 12hours, and brushed their teeth in the morning prior to the examination. The breath test started by measuring the baseline hydrogen; the latter had to be<10ppm to be considered as normal (Gastrolyzer2, Bedfont Scientific Ltd.; distributor for Indonesia: CV Andalan Batara, Jakarta). A normal baseline hydrogen value was necessary to be eligible for inclusion. The patients ingested 2g/kg body weight glucose (maximum of 50g), which was diluted in 10mL/kg body weight water (maximum 250mL). The hydrogen level was measured every 15minutes up to two hours after ingestion of the glucose. Every clinical symptom or complaint during the breath test was recorded. A positive breath test was defined as an increase of>10ppm from baseline.21
Patients, parents, and all study participants were blinded. Study evaluators were blinded by omission of the name of patient on the hydrogen breath test data. The placebo was made by the Pharmacy Department of the Faculty of Medicine of the University of Indonesia.
Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS) version 17. The protocol was approved by the local ethical committee, and all parents signed an informed consent.
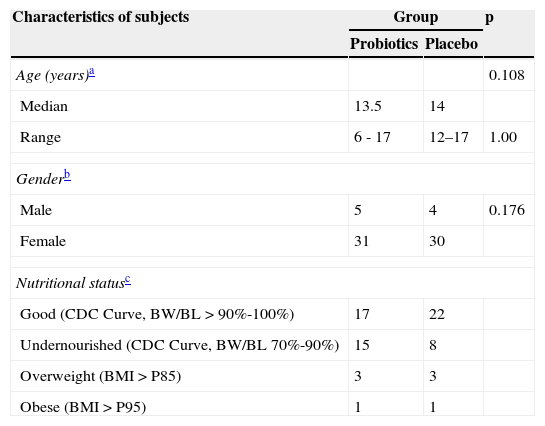
ResultsInitially, 73 patients were included in the study. However, home visits found that three patients did not have a refrigerator at home. Since it was not known whether they were in the probiotic or the placebo group, and since the probiotic needed to be conserved in a cool environment, these patients were considered as dropout. Thus, data of 70 patients were available for analysis. Most of the subjects were female (61/70), with a good nutritional status (39/70) (Table 1).
Characteristics of subjects and its distribution between groups.
| Characteristics of subjects | Group | p | |
|---|---|---|---|
| Probiotics | Placebo | ||
| Age (years)a | 0.108 | ||
| Median | 13.5 | 14 | |
| Range | 6 - 17 | 12–17 | 1.00 |
| Genderb | |||
| Male | 5 | 4 | 0.176 |
| Female | 31 | 30 | |
| Nutritional statusc | |||
| Good (CDC Curve, BW/BL>90%-100%) | 17 | 22 | |
| Undernourished (CDC Curve, BW/BL 70%-90%) | 15 | 8 | |
| Overweight (BMI>P85) | 3 | 3 | |
| Obese (BMI>P95) | 1 | 1 | |
BMI, body mass index; BW/BL, birth weight/birth length; CDC, Centers for Disease Control and Prevention.
The mean age was 13.5 years (range 6-17 years). Of the 70 subjects, 36 were included in the probiotic and 34 in the placebo group. Medication compliance was rather low since “good compliance” was only observed in 41/70 patients (59%). Compliance was “poor” in 29 patients; 11 complied poorly with the probiotic or placebo, and 18 complied poorly with the PPI. Nevertheless, 66/70 (94%) of the patients said to be symptom-free or to have a clinically significant improvement of their symptoms after the intervention. Only 4/70 (6%) said to have not improved.
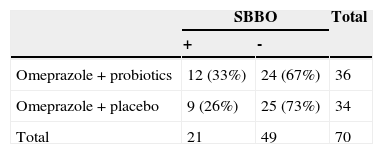
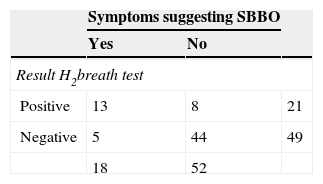
In total, 18 subjects developed symptoms suggesting possible SBBO; in 13 of these, the second breath test suggested SBBO. 16 of these 18 patients had a “good PPI compliance”. According to the results of the breath test (regardless of the presence/absence of symptoms), SBBO was found in 21/70 (30%) of the patients, with a slight trend of more SBBO in the probiotic group (33%; 12/36) than in the placebo group (26%; 9/34) (p=0.13, Table 2). From the 21 subjects with positive hydrogen breath test, 13 subjects presented recurrent SBBO symptoms during therapy (Table 3), while eight were asymptomatic. Finally, 44/70 (63%) patients were asymptomatic and had a negative glucose breath test under PPI treatment. Five patients had symptoms suggesting SBBO, but had a normal breath test result. 13/21 (62%) developed at least one symptom compatible with SBBO during PPI therapy; four (19% of the total group, or 31% of the symptomatic group) presented more than one symptom (Table 4). Two subjects developed extensive SBBO with diarrhea, abdominal pain, and flatulence.
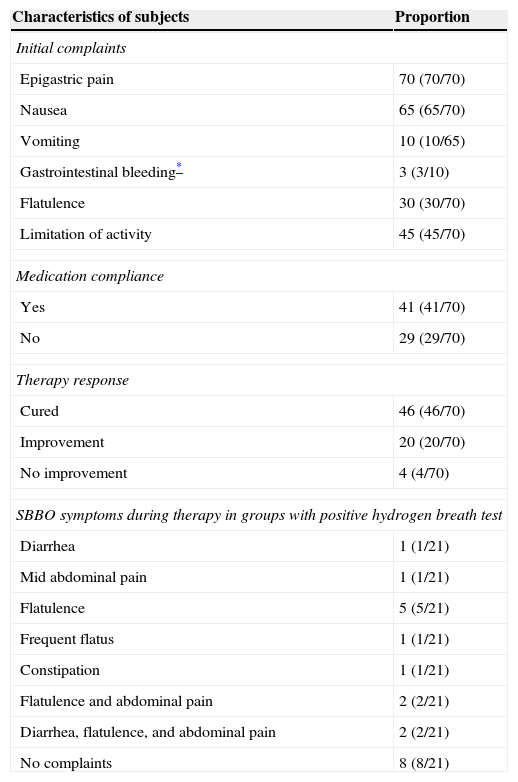
Complaints and therapy response.
| Characteristics of subjects | Proportion |
|---|---|
| Initial complaints | |
| Epigastric pain | 70 (70/70) |
| Nausea | 65 (65/70) |
| Vomiting | 10 (10/65) |
| Gastrointestinal bleeding* | 3 (3/10) |
| Flatulence | 30 (30/70) |
| Limitation of activity | 45 (45/70) |
| Medication compliance | |
| Yes | 41 (41/70) |
| No | 29 (29/70) |
| Therapy response | |
| Cured | 46 (46/70) |
| Improvement | 20 (20/70) |
| No improvement | 4 (4/70) |
| SBBO symptoms during therapy in groups with positive hydrogen breath test | |
| Diarrhea | 1 (1/21) |
| Mid abdominal pain | 1 (1/21) |
| Flatulence | 5 (5/21) |
| Frequent flatus | 1 (1/21) |
| Constipation | 1 (1/21) |
| Flatulence and abdominal pain | 2 (2/21) |
| Diarrhea, flatulence, and abdominal pain | 2 (2/21) |
| No complaints | 8 (8/21) |
SBBO, small bowel bacterial overgrowth.
This was the first study conducted in children that focused on SBBO incidence with PPI treatment as single risk factor, and also evaluated the role of probiotics in prevention of SBBO. The results suggest a placebo-effect of the PPI, since 18/70 were not compliant; only four reported no significant improvement, all of which in the non-compliant group. A cohort study by Boissieu et al. in 53 children, aged between 2 months and 12 years, with complaints of chronic diarrhea, abdominal pain, or both, reported SBBO as a frequent cause (34%), especially before the age of 2 years.22 Scarpellini et al. reported the prevalence of SBBO to be as high as 65% in children aged 3 to 16 years with irritable bowel syndrome (IBS).23 Ratuapli et al. published recently a large retrospective analysis on the effect of PPI on SBBO in adults.21 Overall, the authors reported that PPI did not induce SBBO; HBT-positivity was associated with older age and use of antidiarrheal products.21 However, Del Pioano almost simultaneously confirmed a strong bacterial overgrowth in the stomach and duodenum of adults treated with PPI, increasing with treatment duration.24 The glucose breath test at baseline was normal in all children included in the present study.
In the present study, the subjects involved were children with a mean age of 13.5 years, who presented with the single chief complaint of epigastric pain. The proportion of girls in this study was much higher than boys, but gender was normally distributed in both groups (p=1.00). This difference can be explained by coincidence, by the fact that there are more girls than boys in this age group in Jakarta, and/or by the fact that functional gastrointestinal complaints might be more frequent in girls. However, this striking difference needs further investigation.
Sixty-two percent of the subjects with positive hydrogen breath test developed SBBO symptoms during therapy and during the breath test (Tables 2 and 3). This is in agreement with studies in children, which have showed SBBO to be a common cause of abdominal pain and diarrhea in children.22 16 of 18 children with SBBO symptoms had a “good compliance to PPI”, reinforcing the argument of PPIs as a cause of SBBO. To the authors’ knowledge, studies on SBBO incidence in children related to omeprazole therapy have not been performed; however, this incidence was reported to be as high as 45% to 56% in adult subjects.25–27 The difference in SBBO incidence in the present study (19%) with that of the adult studies can be explained by several factors, such as difference in dosage and duration of omeprazole treatment, different characteristics of the patients, and different diagnostic methods. This differs from the characteristics of adult studies, in which the mean age of the patients is over 50 years. SBBO incidence is higher later in life due to the decline in immunity and intestinal motility, as well as hypo/achlorhydria.5,6,21 The incidence of SBBO and hypo/achlohydria in a senior age group (mean age 84 years) was reported to be 80%.28 The high metabolism of omeprazole in certain age ranges (between 1 and 6 years and 13 and 16 years), which is in line with the subjects’ age in this study, may contribute to the lower incidence of SBBO in adolescents.29
The duration of omeprazole therapy is directly related with SBBO incidence.24 This finding is also supported by a study by Lombardo et al., which showed that SBBO incidence is significantly higher in the group with PPI therapy for over 13 months (p<0.001).30 In the present study, SBBO incidence was evaluated in children treated with 20mg of omeprazole daily for four weeks. The dosage and duration of therapy is smaller in the present study than in the reports in adults using 20 to 40mg of omeprazole for ≥ four weeks, up to 9.5 months.25–27
The probiotic strains administered did not decrease the development of SBBO. However, probiotics are a therapeutic option of potential benefit.31Lactobacillus shirota was shown to be effective in altering fermentation patterns in the small bowel, consistent with SBBO reduction.14 Del Piano et al. demonstrated that four probiotic strains with a marked antagonistic activity towards five E. coli bacteria and an effective amount of N-acetylcysteine (NAC) reduced bacterial overgrowth in long-term PPI-treated subjects.24 A significant decrease in fecal enterococci, total coliforms, E. coli, molds, and yeasts in subjects treated with PPIs was recorded at the end of the probiotic supplementation.24
The hydrogen breath test measures the amount of expired hydrogen in the expired air after fasting for ten to 12hours, followed by ingestion of glucose substrate. If the glucose is not absorbed, but metabolized by intestinal bacteria, intraluminal gasses such as hydrogen (H2), methane (CH4), and carbon dioxide (CO2) will be produced, which can be measured by the breath test.20 The glucose hydrogen breath test is reported to have a sensitivity, specificity, and diagnostic accuracy of 62.5%, 81.8%, and 71.7%, respectively. According to the Rome consensus of 2009, the glucose hydrogen breath test is the recommended diagnostic tool in patients with suspected SBBO.8
In the present study, five subjects developed SBBO-like symptoms but had a negative hydrogen breath test. The glucose breath test can be false negative due to intestinal colonization with non-hydrogen producing bacteria, or because the level of hydrogen production is not high enough to be detected, and SBBO is occurring in the distal part of ileum (where all the glucose has already been absorbed).20,32 According to literature, the prevalence of colonization with non-hydrogen producing bacteria varies from 2% to 43%.33 However, non-hydrogen producing bacteria produce methane, resulting in an increase of expired methane despite normal hydrogen. Levit et al. reported that 36.4% of adults (aged 18 to 88 years) with SBBO had a methane-producing gastrointestinal colonization.34 Since the device used in the present study only measures hydrogen and not methane, further information on this aspect cannot be provided. In some patients, SBBO-related symptoms are similar to the initial presenting symptoms. Although it cannot be excluded that in a few patients it was the initial symptoms that persisted, this is unlikely for different reasons: i) the initial symptoms had strong improvement; ii) symptoms were reported as being different; iii) there was an association with the positive breath test. Moreover, 16 of 18 children with symptoms suggesting SBBO were compliant to PPI treatment. The second breath test was performed four to ten days after stopping PPI therapy, when the potential acid rebound secretion period caused by PPI interruption was over.35
In conclusion, SBBO was found to be frequent in children treated with 20mg/day of omeprazole for four weeks (26% if only symptoms are considered; 30% if only results of hydrogen breath test are considered; 19% if both symptoms and positive hydrogen breath test are considered). The probiotic tested did not prevent the development of SBBO. Children who develop symptoms such as diarrhea, abdominal pain, and flatulence under PPI treatment should be investigated for SBBO.
FundingDexa Medica provided free samples of the probiotic and placebo.
Conflicts of interestYV is a consultant for Biocodex and United Pharmaceuticals. The other authors declare no conflicts of interest.
Please cite this article as: Hegar B, Hutapea EI, Advani N, Vandenplas Y. A double-blind placebo-controlled randomized trial on probiotics in small bowel bacterial overgrowth in children treated with omeprazole. J Pediatr (Rio J). 2013;89:381–7.