The literature indicates a single universal cut-off point for weight loss after birth for the risk of hypernatremia, without considering other factors. The aim of this study was to construct and internally validate cut-off points for the percentage weight loss associated with the risk of hypernatremia, taking into account risk factors.
MethodsA prospective study with a three-day follow-up was conducted in 165 neonates with a gestational age ≥35 weeks. The main outcome variable was mild or moderate hypernatremia (serum sodium≥145mmol/L). Secondary variables (risk factors) were maternal and infant variables. A multivariate logistic regression model was constructed to predict hypernatremia, obtaining its probability and the optimal discriminant cut-off point for hypernatremia (receiver operating characteristic analysis). Based on this point, threshold weight loss values were obtained according to the other variables. These values were internally validated by bootstrapping.
ResultsThere were 51 cases (30.9%) of hypernatremia. The mean percentage weight loss for hypernatremic infants was 8.6% and 6.0% for the rest. Associated variables in the multivariate model included greater weight loss, male gender, higher education level, multiparity, and cesarean delivery. The model had an area under the receiver operating characteristic curve of 0.84 (sensitivity=77.6%; specificity=73.2%). Similar values were obtained in the bootstrapping validation. The lowest percentage weight loss was 4.77%, for cesarean delivery in male infants of mothers with a higher education level.
ConclusionsThe weight loss percentage values depended on the type of delivery, parity, newborn gender, and level of maternal education. External studies are required to validate these values.
A literatura indica um único ponto de corte universal na perda de peso após o nascimento para risco de hipernatremia, sem considerar outros fatores. Nosso objetivo foi criar e validar internamente pontos de corte para o percentual de perda de peso associado ao risco de hipernatremia considerando fatores de risco.
MétodosFoi feito um estudo prospectivo que incluiu 165 neonatos com idade gestacional ≥ 35 semanas, acompanhados por três dias. A principal variável de resultado foi hipernatremia leve ou moderada (sódio sérico ≥ 145 mmol/L). As variáveis secundárias (fatores de risco) foram variáveis maternas e dos neonatos. Um modelo multivariado de regressão logística foi criado para diagnosticar hipernatremia, obteve sua probabilidade e o ponto de corte discriminativo ideal para hipernatremia (análise da Característica de Operação do Receptor). Com base nesse ponto, obtivemos então os valores limites de perda de peso de acordo com as outras variáveis. Esses valores foram internamente validados por.
ResultadosHá 51 casos (30,9%) de hipernatremia. O percentual de perda de peso para neonatos hipernatrêmicos foi 8,6% e 6,0% para o restante. As variáveis associadas no modelo multivariado incluíram maior perda de peso, sexo masculino, maior nível de escolaridade, multiparidade e cesárea. O modelo apresentou uma área sob a curva da Característica de Operação do Receptor de 0,84 (sensibilidade=77,6%; especificidade=73,2%). Valores semelhantes foram obtidos na validação da bootstrapping. O menor percentual de perda de peso foi 4,77% para cesárea em neonatos do sexo masculino de mães com maior nível de escolaridade.
ConclusõesOs valores percentuais de perda de peso dependem do tipo de parto, paridade, sexo do recém-nascido e nível de escolaridade materna. São necessários estudos externos para validar esses valores.
Neonatal hypernatremic dehydration is a potentially serious condition that can occur in healthy newborns, mainly in association with feeding problems, especially with breastfeeding. It is defined as serum sodium concentrations equal to or greater than 145mmol/L.1 Hypernatremic dehydration is considered mild when plasma sodium is between 145 and 149mmol/L and moderate between 150 and 160mmol/L.2 Severe forms can lead to short-, medium-, and long term-complications, especially neurological problems such as seizures, cerebral edema, or intracranial hemorrhage,3–6 with the hypernatremia itself almost always being the cause of harm to the newborn.
The true incidence of hypernatremic dehydration is difficult to determine due to the cultural and methodological differences in the various studies, as well as to the particular aims of these studies. Nevertheless, it has been verified that the incidence has been increasing over the last 20 years, during which time breastfeeding has been implemented as the predominant form of newborn feeding.7 The incidence of hypernatremic dehydration found in the different studies ranges from four to 150 cases per 10,000 live births.8–10
Almost all neonates lose weight after birth, with a 10% loss of birth weight accepted as the maximum risk limit. The current trend is to reduce this threshold to strengthen the monitoring of children at greater risk of dehydration. The American Academy of Pediatrics recommends increased monitoring in those neonates who lose more than 7% of their birth weight, as this may be indicative of a failure in feeding, with a more intensive assessment of breastfeeding being recommended in these cases.11 Numerous studies have demonstrated a significant association between weight loss greater than 10% and the diagnosis of hypernatremia,3,9,12–15 noting a positive correlation between the degree of weight loss and sodium levels.12 However, other authors have demonstrated an association between weight loss greater than 7% and hypernatremia.16
The nadir of the normal course of weight loss occurs at 3–4 days of life.17 It is therefore advisable to weigh children during these first days of life to introduce preventive measures if necessary, considering that a delayed diagnosis may increase rehospitalization and neurological risk.2
Multiple studies have analyzed the relationship between different maternal, perinatal, and neonatal risk factors and hypernatremia of the newborn in the first days of life. Among these, the main factors are lower maternal education level,18 more advanced maternal age,19 the mother having had no previous experience with breastfeeding, or if she had breastfed, it was a negative experience.20 Perinatal factors include epidural anesthesia and cesarean delivery,21 the latter apparently having a greater influence on the possibility of the neonate suffering hypernatremia.22 Birth weight is considered by van Dommelen et al. to be a relevant factor for weight loss.19 Since these factors have shown a clear association with hypernatremia and because the scientific literature only provides a single cut-off point for weight loss without considering these factors, the aim of this study was to construct and internally validate (bootstrapping) cut-off points based on these different factors. This would then enable screening for possible neonatal hypernatremia according to weight loss and the introduction of preventive measures to avoid possible severe hypernatremic dehydration.
MethodsDesignThis prospective observational study with a follow-up of three days aimed to determine the risk of hypernatremia based on weight loss and other risk factors. The study was approved by the Ethics Committee of the Vega Baja Hospital (Orihuela, Alicante, Spain) on December 17, 2013 and followed routine clinical practice. Written informed consent was obtained from the mothers of the newborns, and the data were used in a strictly anonymous and confidential manner.
SettingMother-infant pairs at the Obstetrics and Gynecology Service of the Vega Baja Hospital (Orihuela, Alicante, Spain) were invited to participate. The Vega Baja Hospital of Orihuela is within the public health network, which provides free, universal health care. This facility serves a population of 175,000 inhabitants, with approximately 1400 births per year.
SampleAll neonates with a gestational age ≥35 weeks whose mothers understood and signed the informed consent for participation in the study at the Obstetrics and Gynecology Department of the Vega Baja Hospital of Orihuela were enrolled. Newborns were excluded if they required admission to the neonatology unit for a reason other than dehydration, or if they had major congenital malformations or any chromosomal disorder.
Since the objective of the study was to determine cut-off points for the percentage weight loss according to other parameters, this was addressed through the construction of a multivariate binary logistic regression predictive model. The sample size for the construction of this model was based on the number of events per variable, which had to be at least ten.23 In this study there was a sample size with 51 events. It was possible, therefore, to construct a multivariate binary logistic regression predictive model with five explanatory variables.
MeasurementThe main outcome variable was the presence or absence of mild or moderate hypernatremia, which was defined as a serum sodium value at the third day of life≥145≤160mmol/L.4
The secondary variables and the main outcome variable were obtained from the clinical history and an interview with the mother before discharge. The variables pertaining to the mother were: (a) maternal age (<30, 30–35, or >35 years), education level (primary, secondary, or higher), primiparous (yes/no), gestational age (<39 or ≥39 weeks), delivery type (vaginal/cesarean), epidural anesthesia (yes/no), and with a stable partner (yes/no). The variables pertaining to the newborn were: percentage difference between birth weight and weight at the third day of life, gender (male/female), feeding method (exclusive breastfeeding, formula, or mixed), urea (<20 or ≥20mg/dL), and serum bilirubin (<8, 8–12, or >12mg/dL) at the third day of life. Previous studies have shown all these variables to be associated with hypernatremia,18–21 except for having a partner. This variable was included because the authors believe that factors such as mood or predisposition to breastfeeding could vary with family stability. The newborn was weighed at birth and on the third day of life (48–72h), always using the same scale (Seca electronic infant scale, model 354, with an accuracy of ±10g). From these data, the percentage weight loss was calculated. At this time, taking advantage of blood sampling for neonatal screening and within usual clinical practice, a venous blood sample was drawn and the following concentrations were measured: sodium (analyzed using the Dimension Vista® System that employs indirect potentiometry, with integrated multi-sensor V-LYTE®, which performs a quantitative measurement of sodium), urea (Dimension Vista® System using bichromatic kinetics), and plasma bilirubin (Dimension Vista® System through an in vitro diagnostic test). Gestational age was assessed by ultrasound or, if not available, according to the date of last period.
Data collectionData were collected between December 2013 and June 2014 by a single researcher. After collection, data were computerized for their subsequent statistical treatment.
Data analysisQualitative variables were described by calculating frequencies (absolute and relative), while the mean and standard deviation were used to describe the only quantitative variable in the study (percentage weight loss). Furthermore, Spearman's correlation coefficient was calculated between weight loss and serum sodium level. The aim was to construct a multivariate binary logistic regression model with a maximum of five explanatory variables. Given that there were a total of 12 variables (bilirubin, maternal age, and education level groupings were considered as discrete quantitative linear variables, as they did not show a quadratic tendency), there were 2379 possibilities. To select the combination for the model, the area under the ROC curve (AUC) was calculated for all the possibilities, and the combination with the highest AUC was selected; i.e., the one with the highest discriminant capacity. The AUC value ranges from 0 to 1, with 1 representing maximum discrimination. A value greater than 0.80 is very satisfactory.24 The goodness of fit of the model was determined using the likelihood ratio test. On the ROC curve of the probabilities of the model, the optimal point was selected, i.e., the one that would minimize the square root of (1-sensitivity)2+(1-specificity)2. At this cut-off point, the percentage weight loss was determined according to the different parameters of the model. These parameters were evaluated for their possible values, and the cut-off points were obtained for the percentage weight loss. Finally, these cut-off points were internally validated through bootstrapping (1000 samples), by calculating the AUC, sensitivity, and specificity.25 All analyses were performed with a 5% significance level and the associated confidence interval (CI) was obtained for each relevant parameter. The statistical packages used were SPSS (IBM SPSS Statistics for Windows, version 19.0. NY, USA) and R 2.13.2 (R Development Core Team, 2008).
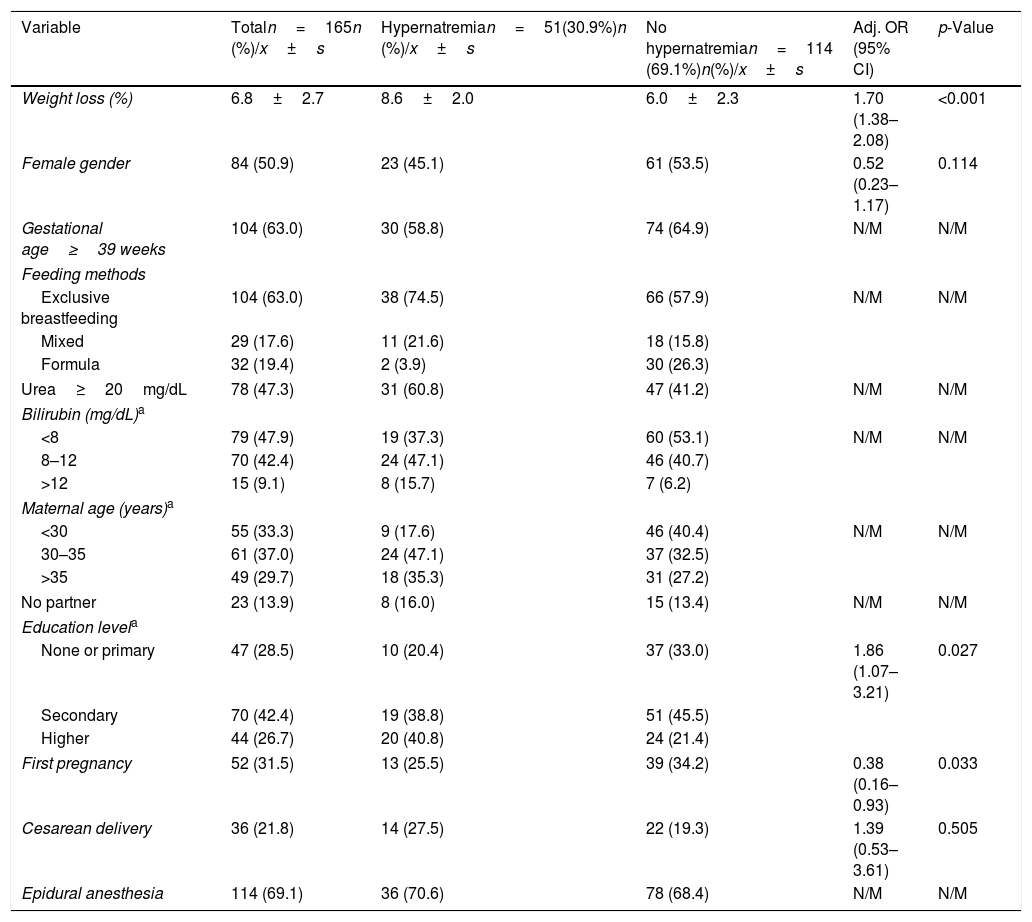
ResultsDuring the six months of the study, 460 children were born in this hospital, 86 of whom were excluded under the exclusion criteria, mainly hospital admissions. In addition, a total of 47 mothers were unable to sign the informed consent because they did not know enough Spanish. Of the remaining 327 neonates, the mothers of 141 (43.1%) did not sign the informed consent. Another 21 children were lost to follow-up. Consequently, it was possible to analyze 165 cases. The median age of the infants at discharge was 2.9 days, and all the measurements were performed before discharge (48–72h). Three newborns remained hospitalized due to hypernatremia or excessive weight loss values. The description of the variables studied in the whole sample is shown in Table 1. It is worth noting that there were 51 cases (30.9%, 95% CI: 23.9–38.0%) of mild or moderate hypernatremia (sodium≥145mmol/L). Five of these had moderate hypernatremia (sodium≥150mmol/L; 3.0%, 95% CI: 0.9–6.9%); thus, the low number meant we were unable to carry out further analysis with this parameter. The highest sodium value was 155mmol/L. The mean percentage weight loss on the third day of life was 6.8±2.7%. Spearman's correlation coefficient between weight loss and serum sodium level was 0.54 (p<0.001).
Analysis of hypernatremia in newborns in the province of Alicante.
| Variable | Totaln=165n (%)/x±s | Hypernatremian=51(30.9%)n (%)/x±s | No hypernatremian=114 (69.1%)n(%)/x±s | Adj. OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Weight loss (%) | 6.8±2.7 | 8.6±2.0 | 6.0±2.3 | 1.70 (1.38–2.08) | <0.001 |
| Female gender | 84 (50.9) | 23 (45.1) | 61 (53.5) | 0.52 (0.23–1.17) | 0.114 |
| Gestational age≥39 weeks | 104 (63.0) | 30 (58.8) | 74 (64.9) | N/M | N/M |
| Feeding methods | |||||
| Exclusive breastfeeding | 104 (63.0) | 38 (74.5) | 66 (57.9) | N/M | N/M |
| Mixed | 29 (17.6) | 11 (21.6) | 18 (15.8) | ||
| Formula | 32 (19.4) | 2 (3.9) | 30 (26.3) | ||
| Urea≥20mg/dL | 78 (47.3) | 31 (60.8) | 47 (41.2) | N/M | N/M |
| Bilirubin (mg/dL)a | |||||
| <8 | 79 (47.9) | 19 (37.3) | 60 (53.1) | N/M | N/M |
| 8–12 | 70 (42.4) | 24 (47.1) | 46 (40.7) | ||
| >12 | 15 (9.1) | 8 (15.7) | 7 (6.2) | ||
| Maternal age (years)a | |||||
| <30 | 55 (33.3) | 9 (17.6) | 46 (40.4) | N/M | N/M |
| 30–35 | 61 (37.0) | 24 (47.1) | 37 (32.5) | ||
| >35 | 49 (29.7) | 18 (35.3) | 31 (27.2) | ||
| No partner | 23 (13.9) | 8 (16.0) | 15 (13.4) | N/M | N/M |
| Education levela | |||||
| None or primary | 47 (28.5) | 10 (20.4) | 37 (33.0) | 1.86 (1.07–3.21) | 0.027 |
| Secondary | 70 (42.4) | 19 (38.8) | 51 (45.5) | ||
| Higher | 44 (26.7) | 20 (40.8) | 24 (21.4) | ||
| First pregnancy | 52 (31.5) | 13 (25.5) | 39 (34.2) | 0.38 (0.16–0.93) | 0.033 |
| Cesarean delivery | 36 (21.8) | 14 (27.5) | 22 (19.3) | 1.39 (0.53–3.61) | 0.505 |
| Epidural anesthesia | 114 (69.1) | 36 (70.6) | 78 (68.4) | N/M | N/M |
n (%), absolute frequency (relative frequency); x±s, mean±standard deviation; Adj. OR, adjusted odds ratio; CI, confidence interval; N/M, not in the model.
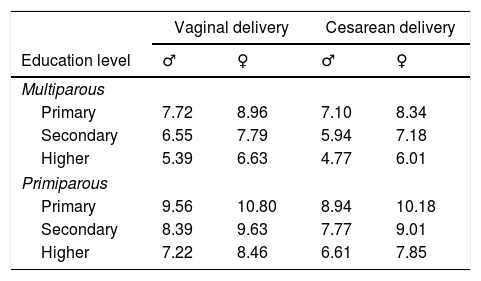
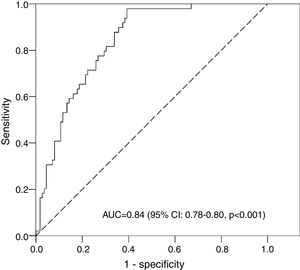
The optimum multivariate mathematical model (with the highest AUC) to predict mild or moderate hypernatremia contained the following associated variables: greater weight loss, male gender, higher education level, multiparity, and cesarean delivery (Table 1). This model had an AUC of 0.84 (95% CI: 0.78–0.90, p<0.001; Fig. 1). The optimum cut point for the model probabilities was 0.311, which had a sensitivity of 77.6% and a specificity of 73.2%. By dividing the closed formula of the mathematical model with this probability value, the cut-off points for the percentage weight loss were obtained according to the model parameters (gender, education level, type of delivery, and multiparity; Table 2). To see how they apply, an example has been provided: suppose there is a boy who was born by cesarean section and whose mother has a higher education, this being her first delivery. The cut-off point for weight loss on the third day is 6.61% (Table 2), so if the child has lost this percentage or more, he is at risk for hypernatremia.
Cut-off points for weight loss (%) for risk of hypernatremia according to gender, maternal education level, first pregnancy, and type of delivery.
| Vaginal delivery | Cesarean delivery | |||
|---|---|---|---|---|
| Education level | ♂ | ♀ | ♂ | ♀ |
| Multiparous | ||||
| Primary | 7.72 | 8.96 | 7.10 | 8.34 |
| Secondary | 6.55 | 7.79 | 5.94 | 7.18 |
| Higher | 5.39 | 6.63 | 4.77 | 6.01 |
| Primiparous | ||||
| Primary | 9.56 | 10.80 | 8.94 | 10.18 |
| Secondary | 8.39 | 9.63 | 7.77 | 9.01 |
| Higher | 7.22 | 8.46 | 6.61 | 7.85 |
When validating these cut-off points by bootstrapping, the following values were obtained for the parameters analyzed: AUC, 0.84 (95% CI: 0.77–0.89); sensitivity, 78.0% (95% CI: 66.0–88.9%); and specificity, 73.2% (95% CI: 65.2–81.3%). These results were very similar to those of the original sample. In other words, the cut-off points were internally validated.
DiscussionThis study constructed cut-off points for the percentage weight loss at the third day of life to detect mild or moderate hypernatremia. These cut-off points depend on the newborn gender, maternal education level, parity, and type of delivery; they can be applied independently of the newborn feeding method. Finally, satisfactory results were obtained in the internal validation by bootstrapping.
The fundamental strength of this study is the novel objective to establish cut-off points for percentage weight loss to detect mild or moderate hypernatremia, taking into account the associated factors. The authors found no studies evaluating these very relevant factors in routine clinical practice. The aim of previous studies was to find a universal cut-off point for weight loss.2,17,26 However, this is inadequate, as it has been shown that different factors influence hypernatremia. The internal validation of the cut-off points was carried out through 1000 bootstrap samples, obtaining very similar results to those of the original sample. Additionally, when selecting the most relevant factors in the development of hypernatremia, this study followed an algorithm that examined approximately 2500 multivariate models, selecting the model that had a maximum predictive capacity, giving more validity to the results.23
Although the fact that non-significant factors were used in the multivariate model that was constructed might be considered a limitation, it should be considered that the objective was to obtain a mathematical model that, overall (all factors together), attained a good prediction. This was achieved with the optimum model, as the likelihood ratio test was very significant and the AUC was higher than 80%. In other words, this is not a limitation. Regarding sample size, it was only possible to introduce five explanatory variables in the model, which had a good predictive capacity. With a larger sample size, a greater number of predictors could have been introduced, consequently improving the accuracy in predicting mild, moderate, and severe hypernatremia. Clearly, the study sample could have been skewed, as only about half of all the mother-infant pairs initially available (n=374) could be used. The others were not included in the study for specific reasons (lack of understanding Spanish, not wishing to participate, or lost to follow-up). This possible bias must therefore be considered the main limitation of the study. Nevertheless, this study is more focused on discussing the addition of other risk factors to be considered when calculating the cut-off points, with the idea of other researchers applying this method to determine whether the results are externally valid. Information bias was minimized by using reliable and validated measurement methods, as well as rigorous data collection. Finally, confounding bias was reduced by using approximately 2500 multivariate models.
The results show that the cut-off point for weight loss at the third day of life established in the literature (7–10%) does not predict all cases of mild or moderate hypernatremia. Indeed, some cases very prominently remain outside this level, resulting in neonates who are at risk of hypernatremia, even though they may have a percentage weight loss below the classically established limits. In order of increasing risk, these include the following: having a mother who is multiparous, who has a higher education level, being a male newborn, and being born by cesarean delivery (Table 2). According to this study's findings, for example, a weight loss value above 4.8% should be used for a male newborn born by cesarean delivery to a multiparous mother with a higher education level.
Regarding the methodology used in the literature, it is noteworthy that most studies do not mention three types of newborn feeding, focusing exclusively on breastfed newborns.22 The present study included all newborns and grouped them according to their type of nutrition (exclusive breastfeeding, formula, or mixed feeding), observing that the presence of hypernatremia among children with exclusive breastfeeding and mixed feeding was very similar.
The assessment of the percentage weight loss at the third day of life is of interest for two reasons. Firstly, in population studies, it is the time of maximum weight loss, and secondly, important weight loss leads to a higher prevalence of hypernatremia. Given that when hypernatremia presents it increases from this age,19 it is important to confirm the diagnosis to allow adequate nutritional intervention. The findings agree with the literature that breastfeeding, cesarean delivery, and male gender are all risk factors for early hypernatremia.22,27 Notably, though, in this study hypernatremia was more frequent among mothers with a high education level in comparison to what others have reported.26 However, the present study differs from previous research in that the data were treated using a multivariate logistic regression analysis, which eliminates the influence of other confounding variables. The first factor obtained was male gender, which can be explained by the fact that male neonates have less organ maturity than female neonates, as well as higher oxidative stress.28 It was found that multiparous and better-educated women have a higher risk of hypernatremia, in contrast to the scientific literature.22 The authors believe that this discrepancy could be due to the fact that a period of three days was assessed, while the rest of the studies covered a much longer period.22 Seeking a clinical explanation for this, it may be that primiparous mothers receive more clinical care during the immediate neonatal period and that more educated mothers are more likely to exclusively breastfeed in this area for the first three days, which is a known risk factor.22 However, these are hypotheses and assessments that need to be confirmed by qualitative studies.
Although this study found a higher prevalence of hypernatremia than the scientific literature, it is important to bear in mind that only the first three days of life and mainly mild hypernatremia were evaluated, when other studies evaluated established hypernatremia.22
The methodology used was shown to be effective in determining cut-off points for the risk of hypernatremia with respect to various risk variables and it could be used for other disorders, as well as in future studies with larger sample sizes and for validation of these cut-off points in other populations (external validation).
Considering these results, the authors suggest that screening for early hypernatremia in clinical practice should include tables of cut-off points based on recognized risk variables rather than single cut-off points for the percentage weight loss at the third day of life.
The cut-off points for percentage weight loss indicating mild or moderate early neonatal hypernatremia depend on the type of delivery, parity, gender, and maternal education level. In other words, there are other factors to be considered with the percentage weight loss to detect hypernatremia. Accordingly, the authors have constructed tables for these cut-off points depending on the intersection of the variables obtained. Finally, the methodology used has been described in detail and can be applied in other populations, or in the analysis of other outcomes in pediatric populations.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Maria Repice and Ian Johnstone for their help with the English version of this text.
Please cite this article as: Ferrández-González M, Bosch-Giménez V, López-Lozano J, Moreno-López N, Palazón-Bru A, Cortés-Castell E. Weight loss thresholds to detect early hypernatremia in newborns. J Pediatr (Rio J). 2019;95:689–95.