To determine the prevalence of vitamin A deficiency (VAD) and serum concentrations of retinol, correlating them with IGF-1 concentrations in preschoolers with DS.
MethodsCross-sectional study was conducted on 47 children with DS aged 24 to 72 months, in Ribeirão Preto, Brazil. VAD was determined by the relative dose-response (RDR) test. Retinol serum concentration ≤ 0.70 µmol/L and IGF-1 serum concentration below the 3rd percentile for sex and age were considered to represent deficiency. C-reactive protein (CRP) was determined at the beginning of the study. Weight, height, and information about fever and/or diarrhea were obtained at the beginning of the study.
ResultsVAD prevalence was 25.5% (12/47), and 74.5% (35/47) of the children had deficient retinol before the intervention. CRP was not associated with VAD. Mean IGF-1 were 103.5 ng/mL (SD = 913) for the group with VAD and 116.3 ng/mL (SD = 54.9) for the group with no VAD (p-value = 0.85); 8.5% (4/47) of the children showed deficient IGF-1, but without VAD. No association was observed between VAD and IGF-1 deficiency. A moderate positive correlation was observed between pre-intervention retinol and IGF-1 (ρ = 0.37; p-value = 0.01).
Conclusiona high prevalence of VAD and deficient retinol was observed and there was a positive correlation between serum retinol and IGF-1.
Vitamin A deficiency (VAD) represents a global public health problem, affecting 190 million preschoolers each year in the world, and 4.4 million children have a diagnosis of xerophthalmia, mainly in developing countries.1 Subclinical VAD starts before the onset of ocular clinical signs and leads to a reduction of immune response and cell differentiation and multiplication. Thus, VAD has negative effects on the immunological response and is associated with greater morbidity-mortality due to infections, in addition to interfering with the processes of child growth and development.2,3
Retinoic acid can stimulate the secretion of growth hormone (GH) in vitro in rat pituitary cells.4 In humans, serum retinol concentrations are positively correlated with nocturnal GH secretion. In addition, some studies have observed a positive correlation between the serum concentrations of retinol and insulin-like growth factor 1 (IGF-1), including in newborns and infants,5,6 whose production is influenced by GH and by nutritional status. These components of the GH-IGF system axis, together with genetic inheritance, directly determine growth and development.6
The nutritional status related to vitamin A needs to be screened and monitored, especially in populations at higher risk of VAD, such as preschoolers and pregnant and nursing women, because of the greater nutrient demand,7 so that preventive and early diagnosis strategies may be effective. Children with Down Syndrome (DS) are also at a higher risk to develop VAD. In a Venezuelan study, Chávez et al.8 observed an 18.4% prevalence of VAD in preschoolers with DS aged 2 to 6 years, as opposed to a prevalence of 4% among children without the syndrome. Several hypotheses have been raised regarding VAD in children with DS. The increased gene expression of the enzyme superoxide dismutase (SOD) due to the third chromosome 21 increases the production of oxygen free radicals, which represents a risk factor for the reduction of serum retinol concentrations.9 Children with DS have orofacial muscle hypotonia and oral motor dysfunction which impairs the mandibular movements. In addition, a narrow palate and tongue protrusion also cause suction, chew and swallowing difficulties, possibly contributing to a low intake of micronutrients, among them vitamin A.10 In parallel, children with DS have more infectious episodes than persons without the trisomy.11 VAD may increase the severity of the infection, which in turn may reduce the intake and accelerate the body losses of vitamin A.1 Also, an increase in acute phase proteins, a phenomenon commonly occurring during infectious episodes, reduces the serum concentrations of retinol.11
Short stature and delayed growth are some of the main characteristics of persons with DS and it seems to be associated with disorders of GH secretion and IGF-1.12 There are scarce literature reports about the prevalence of this nutritional deficiency among children with DS and its relationship with IGF-1. The objectives of the present study were 1) to determine the prevalence of VAD and serum concentrations of retinol and IGF-1 among preschoolers with DS, and 2) to verify the correlation between serum concentrations of retinol and IGF-1 and to compare the mean serum concentrations of IGF-1 between the groups with and without VAD.
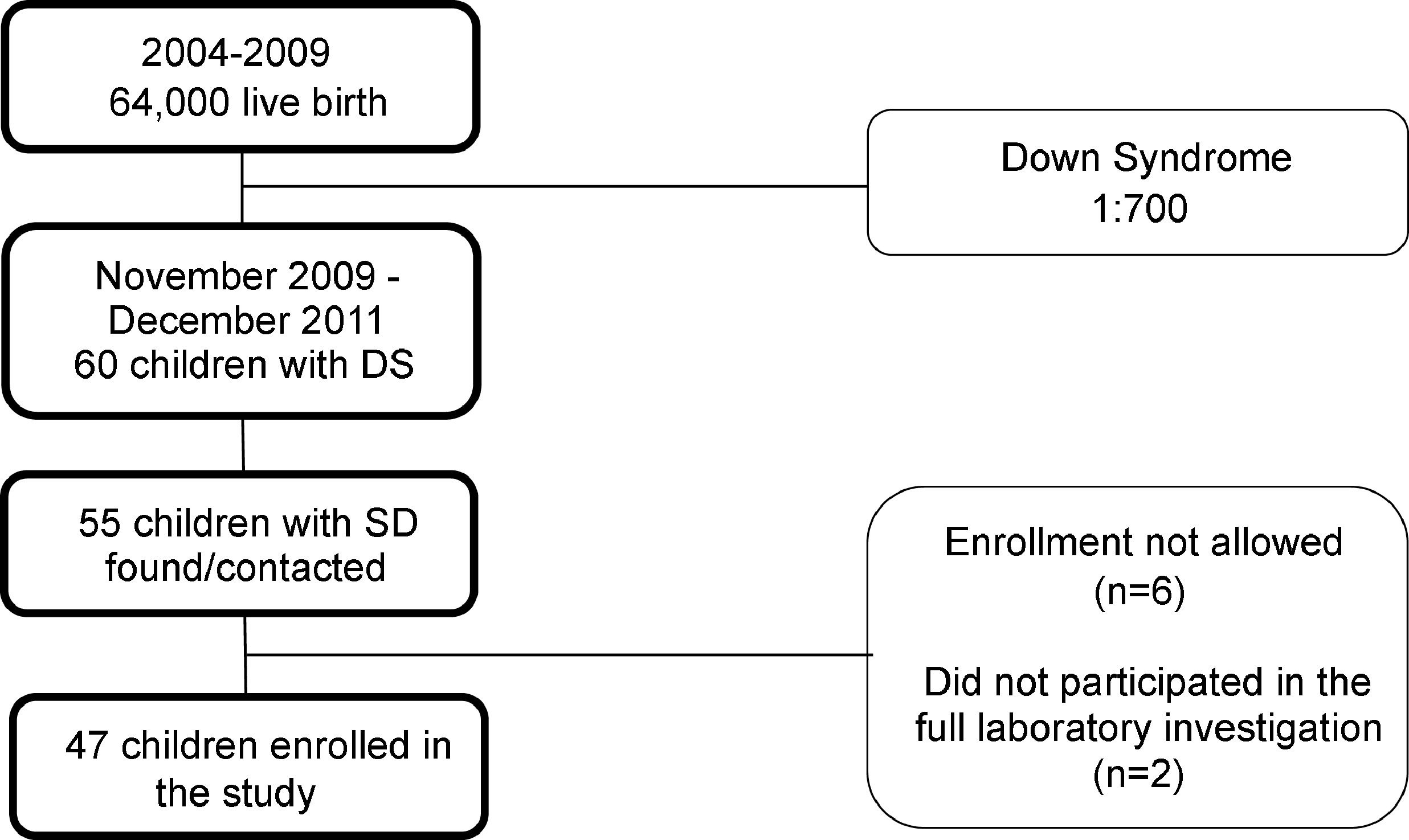
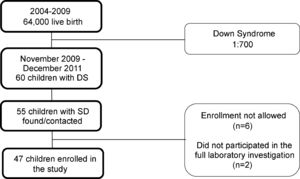
Subjects and methodsStudy design and populationThis was a cross-sectional, observational, and analytical study of prevalence. All preschool children with DS aged 24 to 72 months, living in the city of Ribeirão Preto (São Paulo, Brazil) during the period from November 2009 to December 2011 were eligible for the study. To estimate the study population, the number of live-born infants registered in Ribeirão Preto in the year 2009, a total of 10.744, was obtained. Based on the proportion of one baby born with the trisomy per 700 live births and considering the babies born in the city but no longer living in it, as well as babies that might have died due to comorbidities linked to the trisomy, the authors estimated a population of approximately 60 children with DS aged two to six years during the study period. An active search was conducted in genetics and cardiology outpatient clinics and in institutions dedicated to the care of children with developmental delay in the city. This process permitted us to locate 55 subjects eligible for the study. On this basis, the authors estimate that they could have possibly contemplated the entire population of children with DS aged two to six years living in the city of Ribeirão Preto during the period from November 2009 to December 2011. The parents or guardians of six children did not permit their inclusion in the study. Two children did not participate in the blood collection to complete the relative dose-response (RDR) test and were excluded from the study. Thus, 47 children participated in the present investigation. The flow diagram of subject inclusion in the study is presented in Fig. 1.
The research protocol followed the Declaration of Helsinki and was approved by the Research Ethics Committee of Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCRP n°. 9573/2009) and persons responsible for the participants gave written informed consent to participate in the study.
Data collection and analysisData were collected at an academic primary health care center (“Vila Lobato” Community Social Medical Center – Ribeirão Preto, SP, Brazil). Vitamin A was determined using the relative dose-response (RDR) test. For the test, a venous blood sample was obtained from each child after a six-hour fast (A0). Children with fever and/or diarrhea at the time of collection were sent home and invited to return two weeks after the end of the episode to start participating in the study. Immediately after blood collection (A0) the child received an oral solution containing 600 μg retinyl palmitate and a new blood sample was obtained five hours after this intervention (A5). At each collection (A0 and A5), the tubes for the determination of serum retinol concentration were protected from light with aluminum foil to minimize vitamin A photodegradation. The samples were then taken to the laboratory, where they were centrifuged at 2500 rpm for 15 minutes. Serum was stored at -70°C in Eppendorf® tubes also protected from light until the time for analysis in the Nutrition and Metabolism Laboratory, Department of Internal Medicine (Ribeirão Preto Medical School, University of São Paulo). Serum retinol concentration was determined by high-performance liquid chromatography (HPLC) before (A0) and after (A5) supplementation according to a standard technique of the laboratory itself based on the method of Arnaud et al..13 The detection limits of the method are 0.016 and 13.7 µmol/l. RDR was calculated by the formula (A5-A0/A5) x 100. Individual results of 20% or more are indicative of low hepatic reserves of vitamin A,14 i.e., VAD. The adoption of a serum retinol concentration of 1.05 µmol/l as the cut-off value to consider a child as having VAD would result in a high proportion of deficiency even in a population of well-nourished preschoolers;14 therefore, in the present study, the authors used the cutoff point recommended by the WHO as indicative of VAD, that is, ≤ 0.70 µmol/L.15
Serum IGF-1 and C-reactive protein (CRP) concentrations were determined in the blood sample before supplementation with retinyl palmitate (A0). For the determination of serum IGF-1 concentrations, the blood samples were centrifuged at 2500 rpm for 15 minutes and the serum was stored at -70°C until the time for determination in the Endocrinology Laboratory, Department of Internal Medicine (Ribeirão Preto Medical School, University of São Paulo). Serum IGF-1 concentration was determined by solid-phase immunometric immunoassay labeled by chemoluminescence using the commercial kit IMMULITE 2000 SIEMENS® (UK) for IGF-1. Serum IGF-1 concentrations below the 3rd percentile for sex and age were considered deficient.
For CRP determination the blood samples were centrifuged at 2500 rpm and serum was stored at -70°C until the time for analysis in the biochemistry laboratory of the university. CRP was determined by turbidimetric immunoassay and serum concentrations above 0.5 mg/dL were considered abnormal.
The nutritional status of the participants was determined by measuring weight and height at the beginning of the study and classified according to the growth curves for children with DS. A socioeconomic questionnaire was applied to determine family income, number of household residents and parents’ schooling.
The occurrence of fever and diarrhea was considered a clinical marker of infection/inflammation. The parents/guardians of the children were asked if the child had experienced at least one episode of axillary temperature higher than 37°C measured with a thermometer during a period of 15 days prior to entering the study. Similarly, the parents/guardians of the children were asked if the child had shown a pattern of evacuation characterized by three or more episodes of the passage of liquid or semi-liquid feces over a period of 24 hours, or passage of liquid feces with blood visible to the naked eye at least once during 15 days prior to entering the study.
Statistical analysisAs described, there is no sample size calculation in this study because the authors reached the whole eligible population, except those who did not agree to participate. Confidence intervals (95%CI) for the prevalence of VAD among the whole population and in subgroups of children were calculated by the Clopper-Pearson exact method. The difference in mean serum retinol concentrations before and after supplementation was determined by the Student t-test for paired data. Mean serum IGF-1 concentrations were compared between children with and without VAD by multiple linear regression, with adjustment for age and serum retinol concentrations before supplementation. Residual analysis was used to examine the assumptions of this regression model, including checks for linearity, normality, and homoscedasticity of the residuals. Spearman correlation coefficient was used to determine the correlation of pre-supplementation serum retinol concentrations with serum IGF-1 concentrations. The statistical analyses were carried out using SAS 9.2 and R 2.15.10 software, with the level of significance set at 5%.
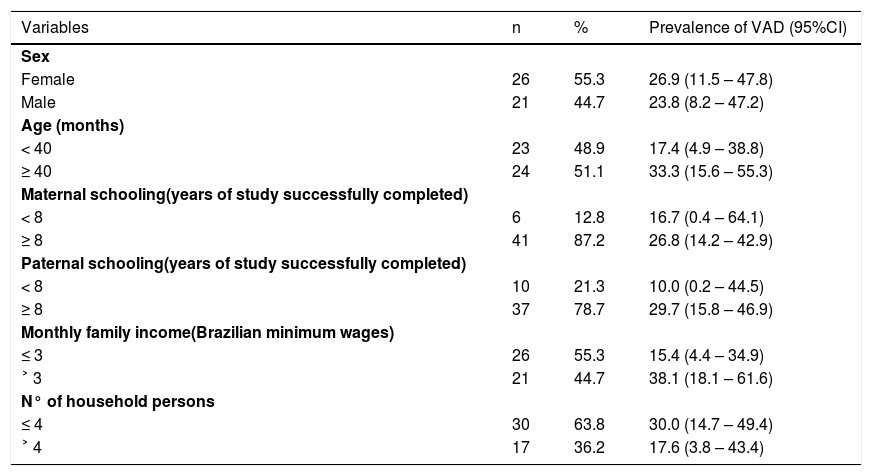
ResultsThe mean age of the participants was 42.8 [Standard Deviation (SD) 14.9] months (range 25-69 months) and 55.3% (n = 26) were females. Only one child was above the 95th percentile in the weight/age indicator. This child had no VAD and had adequate serum IGF-1 concentrations. All the other children (n = 46) were classified between the 5th and 95th percentile for weight/age and height/age. The parents of most of the children had an education of over eight years, an income of less than three Brazilian minimum wages, and the children lived in a household with less than four people. Table 1 shows the socioeconomic characteristics of the study population and prevalence of VAD (determined by the RDR test) for each class category of these variables.
Socioeconomic characteristics of preschoolers with Down Syndrome, and prevalence of VAD determined by the RDR test. Ribeirão Preto 2009-2011. Brazil.
VAD, vitamin A deficiency; RDR, relative dose-response; 95%CI, 95% confidence interval.
The prevalence of VAD determined by the RDR test was 25.5% (n = 12; 95%CI: 13.9-40.3). The mean age of the children with and without VAD was 47.0 (SD 16.8) and 41.4 (SD 14.2) months, respectively. Mean pre- (A0) and post-supplementation (A5) retinol concentrations were 0.64 µmol/l (SD 0.24) and 0.70 µmol/L (SD 0.24), respectively, with a significant difference between them (mean difference: 0.06; 95%CI: 0.02-0.10; p-value < 0.01). 74.5% (n = 35; 95%CI: 42.3-89.1) of the children had pre-supplementation serum retinol concentrations below 0.70 µmol/l. After intervention with the oral administration of 600 µg retinol palmitate for the RDR test, 57.4% (n = 27; 95%CI: 34.2-76.3) of the children still had serum retinol concentrations below 0.70 µmol/L.
It was observed that 27.6% (n = 13) of the children studied had serum CRP concentrations considered to be high, with only three of these children (23%) having VAD. There was no association between pre-supplementation serum retinol and CRP concentrations (p-value = 0.99).
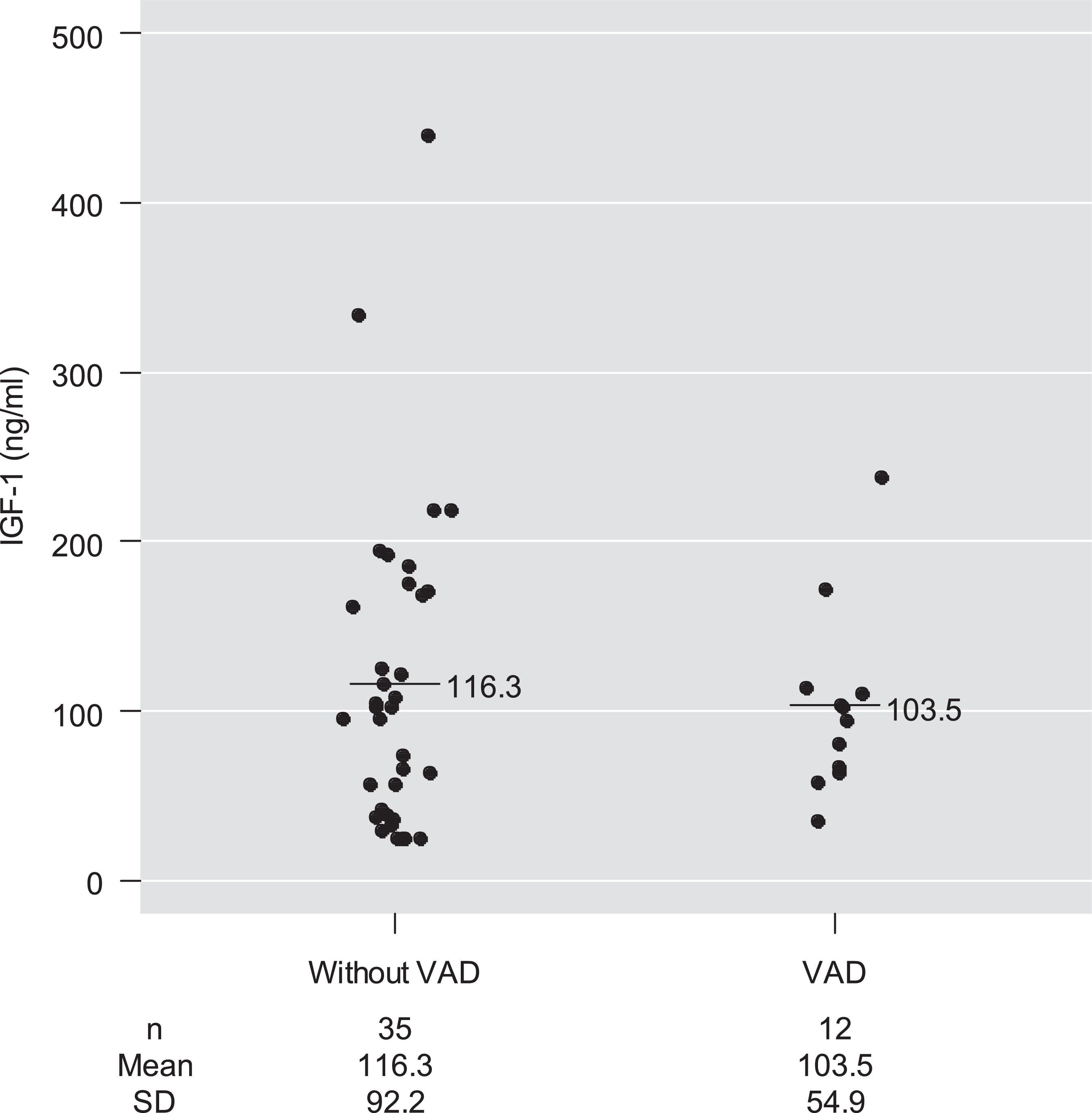
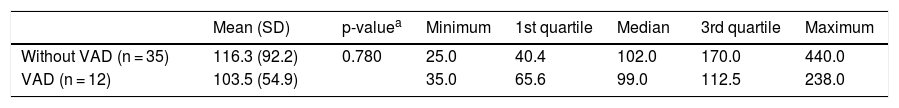
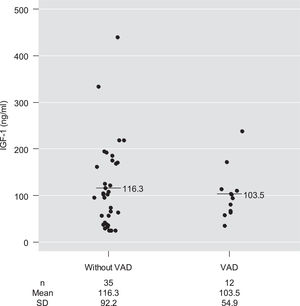
Mean serum IGF-1 concentrations among children with and without VAD were 103.5 ng/mL and 116.3 ng/mL, respectively, with no significant difference between them after adjustment for age and for pre-supplementation serum retinol concentrations (p-value = 0.78) (Table 2 and Fig. 2).
Means, medians, and quartile distributions of serum IGF-1 concentrations (ng/mL) among preschool children with Down syndrome, according to vitamin A deficiency. Ribeirão Preto 2009-2011. Brazil.
| Mean (SD) | p-valuea | Minimum | 1st quartile | Median | 3rd quartile | Maximum | |
|---|---|---|---|---|---|---|---|
| Without VAD (n = 35) | 116.3 (92.2) | 0.780 | 25.0 | 40.4 | 102.0 | 170.0 | 440.0 |
| VAD (n = 12) | 103.5 (54.9) | 35.0 | 65.6 | 99.0 | 112.5 | 238.0 |
SD, standard deviation; VAD, vitamin A deficiency.
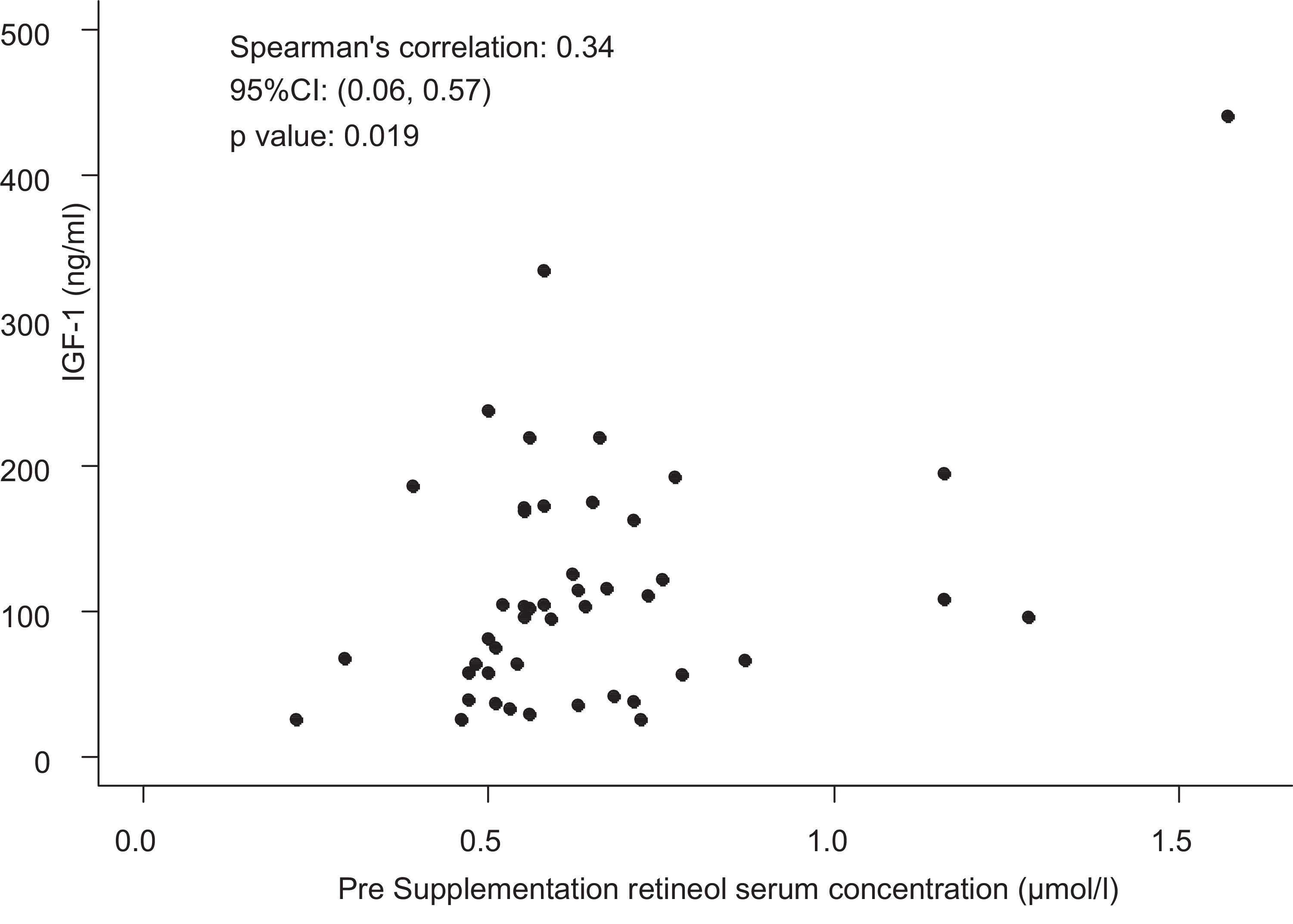
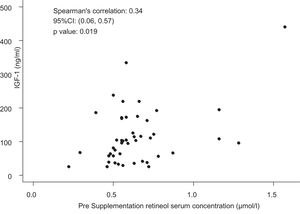
Only four children had IGF-1 deficiency (< 3rd percentile), although without VAD. Thus, the presence of VAD was not associated with IGF-1 deficiency (p-value = 0.56). However, all children with serum IGF-1 concentrations below the 25th percentile for sex and age had retinol values below 0.55 µmol/L. A moderate and significant positive correlation was observed between pre-supplementation serum retinol and IGF-1 concentrations (ρ = 0.34; p-value = 0.019; Fig. 3).
DiscussionThe present study was the first to investigate the prevalence of VAD and its association with serum IGF-1 concentrations in a population of preschoolers with DS. The prevalence of VAD among children with DS has been little investigated. Some studies have detected the presence of VAD in children with DS, but have not investigated its prevalence,16 while other studies did not even demonstrate the presence of VAD among these children.17 A prevalence of VAD of 25.5% was detected by the RDR test. Furthermore, 74.5% of the children had deficient serum retinol concentrations before the intervention (pre-supplementation – A0) required for the RDR test, i.e., oral supplementation with 600 µg retinol palmitate. In a study of Venezuelan children with DS aged 4 to 12 years, Chávez et al.8 detected an 18.4% prevalence of retinol concentrations < 0.70 µmol/L. However, it should be pointed that the study by Chávez et al.8 included older children and not simply preschoolers – an age range at higher risk for VAD -, a fact that may have contributed to a lower prevalence of deficient serum retinol concentrations compared to the present study. It is interesting to point out that, in the cited study, children with DS aged two to six years had significantly lower mean retinol concentrations than older children. It is known that serum retinol concentrations are not necessarily consistent with hepatic vitamin A reserves and may be normal even when liver reserves are extremely low.18 In addition, due to homeostatic control, serum retinol concentrations remain constant within a broad range of hepatic reserves. And they may be influenced by some factors such as infection, inflammation and obesity.19 The most sensitive method for the assessment of vitamin A status in relation to the hepatic reserves is the isotope dilution method; however, this method is too expensive for the population assessment.19 Moreover, the changes in serum retinol concentrations due to a standard oral dose of vitamin A reflect the fact that when the hepatic reserves of vitamin A are reduced, the retinol-binding protein (RBP) accumulates in the liver before the retinol concentrations decrease. Thus, when a standard vitamin A dose is administered, retinol binds to the accumulated RBP and is rapidly released into the serum, a phenomenon that causes an increase in serum retinol concentrations.19 The RDR test is based on this physiological response of the RBP-retinol complex to identify persons with low hepatic reserves. Since the authors used a different method for the classification of vitamin A status (RDR) compared to the Venezuelan study, it is difficult to compare the observed prevalence. However, so far, no studies have been published in the literature regarding the determination of VAD prevalence among children with DS using the RDR test.
Studies have shown that children with DS are more susceptible to infections.11 In the present study, although the children were apparently healthy without acute febrile or diarrheic episodes, more than one-quarter of them had high CRP concentrations. However, only three children with VAD had high CRP serum concentrations. Subclinical infectious or inflammatory processes may reduce serum retinol concentrations in the organism in a transitory manner, leading to an overestimate of the prevalence of values considered deficient. In response to inflammation or tissue injury, CRP concentrations increase within six to eight hours after the insult, peaking within 48 hours.20 Thus, children with infections or inflammatory processes during the period of incubation may not show increased serum CRP concentrations. It is also known that in infectious or inflammatory processes the serum concentrations of retinol start to decline before the occurrence of high increases in CRP.8,11. Finally, since RBP is a negative acute-phase protein, subclinical infectious or inflammatory processes may interfere with the release of the retinol-RBP complex by the liver even after supplementation with retinol palmitate, possibly influencing the RDR results. These phenomena, in addition to strict homeostatic control of serum retinol concentrations, even when liver stores are extremely low, may explain the difference in prevalence detected by RDR and by serum retinol concentrations.
The prevalence of deficient serum retinol concentration detected in the present population was higher than that observed in studies conducted on children without DS of similar age ranges when compared with Brazilian data21 and international data.22 On the other hand, two studies using tests of the response of the serum concentrations to a standard dose of vitamin A have reported a higher VAD prevalence than observed in the present study. Samba et al.,23 using the modified dose-response test (MRDR), observed a 30% VAD prevalence and a 50% prevalence of deficient serum retinol concentrations among preschoolers of sub-Saharan Africa. In two studies, conducted with healthy individuals in the same community investigated by the present study, there was a 74.5% prevalence of VAD among preschoolers without DS, as determined by the + S30DR method24 and, recently, insufficient concentrations of vitamin A were observed in breastmilk of lactating women.25
Inadequate vitamin A intake in response to the demands of the organism represents a common pathway towards the development of deficiency.1 On the other hand, in Brazil DS children receive periodic assistance since the first months of life in specialized institutions, as well as multidisciplinary medical monitoring with physiotherapists and nutritionists for the treatment of their complications. Furthermore, in some cases, their parents or tutors spend greater care on the diet and hygiene of these children. These observations may explain in part the differences in VAD prevalence detected in the present study compared to the values reported by other authors who studied children without the trisomy.
In the present study, children without VAD had higher mean serum IGF-1 concentrations than children with VAD, but the difference was not significant. The relatively small number of individuals studied could explain this finding. However, a moderate positive correlation was observed between the serum retinol and IGF-1 concentrations. Similar results were found among pregnant Nepalese women and healthy Japanese adults.5 Regarding children, Dijkhuizen et al.7 have also found a moderate correlation between the plasma concentration of retinol and IGF-1 among healthy infants in Indonesia. Du et al.6 also observed a moderate positive correlation between serum retinol and IGF-1 concentrations in Canadian newborn girls but not in boys. Although some studies have shown that vitamin A supplementation may improve the growth of children with VAD26 and associated with zinc supplementation, increase the serum concentrations of IGF-1,27 no studies of this kind have been conducted on children with DS. Thus, whether an improvement of serum retinol concentrations in populations with a high prevalence of deficiency might lead to an increase in IGF-1 concentrations of some clinical relevance in children with DS is a question proposed by the present study. All children of the present study who presented with serum IGF-1 concentrations below the 25th percentile for sex and age had serum retinol concentrations below 0.55 µmol/L. Despite the high proportion of deficient retinol values detected, only four children had deficient serum IGF-1 concentrations.
The present study has some limitations. Although the study group practically included the entire population of preschoolers with DS in the city, the number of subjects studied was relatively small, a fact that may have reduced the power of some analyses. Since it was a cross-sectional study, changes along time that might have occurred in the relationship between retinol and IGF-1 concentrations, especially during or in the absence of infectious episodes or inflammatory processes, could not be detected. Finally, another limitation was the lack of information about vitamin A intake that might have helped explain some findings.
ConclusionThe present population-based study detected an elevated prevalence of VAD among preschoolers with DS using the RDR method, as well as a high proportion of deficient serum retinol concentrations. A small proportion of children had deficient IGF-1 concentrations. No difference in mean IGF concentrations was observed between children with and without VAD. However, a positive correlation was observed between serum retinol and IGF-1.