to determine the rate of extrauterine growth restriction in very low birth weight infants and to evaluate the influence of perinatal variables, clinical practices, and neonatal morbidities on this outcome.
Methodsa longitudinal study was performed in four neonatal units in the city of Rio de Janeiro. 570 very low birth weight infants were analyzed. The study included perinatal variables, variables related to clinical practices, and incident morbidities in these preterm infants. Extrauterine growth restriction was defined using z-scores for weight or head circumference ≤ -2 for corrected age. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) and R software.
Resultsthis study comprised 570 infants, of which 49% were males, and 33% were small for gestational age (SGA). The mean weight and head circumference at birth were 1,113 ± 267g and 27 ± 2cm, respectively. The mean z-scores of birth weight and weight at discharge were -0.96 ± 0.78 and -1.54 ± 0.75, respectively; for head circumference, the mean z-scores at birth and at discharge were -0.63 ± 1.18 and -0.45 ± 0.94, respectively. The rate of extrauterine growth restriction considering the weight was 26% (149/570) and considering the head circumference, 5% (29/570). SGA was the variable with the greatest impact on both growth restriction for weight (PR = 4.33) and for head circumference (PR = 2.11) in adjusted analyses.
Conclusionextrauterine growth restriction was high in the population, especially for SGA newborns and those with neonatal morbidities.
determinar a frequência da restrição de crescimento extrauterino em recém-nascidos pré-termos de muito baixo peso e avaliar o impacto de variáveis perinatais, práticas clínicas e morbidades neonatais nesta morbidade.
Materiais e métodosfoi realizado um estudo longitudinal em 4 unidades neonatais do Rio de Janeiro. Foram analisados 570 recém-nascidos pré-termos de muito baixo peso. Foram incluídas no estudo variáveis perinatais, variáveis relacionadas às práticas clínicas e morbidades incidentes nestes recém-nascidos. A restrição de crescimento extrauterino foi definida pelos escores z de peso ou perímetro cefálico ≤ -2 para idade corrigida. Na análise estatística foram utilizados o software SPSS e o software R.
Resultadosforam analisados 570 recém-nascidos dos quais 49% eram do sexo masculino e 33% nasceram pequenos para idade gestacional. A média do peso e perímetro cefálico ao nascimento foi respectivamente 1113 ± 267g e 27 ± 2cm. As médias de escore z do peso ao nascimento e na alta foram respectivamente, -0,96 ± 0,78 e -1,54 ± 0,75 e as do perímetro cefálico foram -0,63 ± 1,18 e -0,45 ± 0,94. A frequência de restrição de crescimento extrauterino considerando o peso foi 26% do perímetro cefálico foi de 5%. Nascer pequeno para idade gestacional foi a variável de maior impacto na restrição de crescimento tanto para o peso (RP 4,33) quanto para o perímetro cefálico (RP 2,11) nas analises ajustadas.
Conclusãoa restrição de crescimento extrauterino foi alta na população, especialmente para os recém-nascidos PIG e com morbidades neonatais.
The growing advances in neonatology and in intensive care have been increasing the survival of progressively more premature infants, born at increasingly younger gestational ages and with lower birth weights.1 Consequently, this fact has increased the concern of professionals working in long-term follow-up of these children regarding their quality of life, considering the different aspects involved, whether somatic growth or psychomotor development.2
Currently, in spite of improvements in the nutritional support of preterm infants with very low birth weight through aggressive and early parenteral and enteral nutrition, growth restriction in the postnatal period is often observed, with growth rates that are significantly lower than the intrauterine rates in fetuses of the same gestational age, a situation termed extrauterine growth restriction (EUGR).3
Thus, premature birth places infants at high nutritional risk, as it interrupts the growth phase at its fastest stage. Furthermore, many of these infants develop chronic diseases precisely at the initial period of their lives, when rapid growth is expected, with consequent high caloric requirements.3 Postnatal growth failure in very low birth weight infants is an almost universal phenomenon.4
Clark et al. showed significant EUGR for weight (28%), length (34%), and head circumference (16%) in preterm infants during hospitalization.5 Data from the neonatal research network of the National Institute of Child and Human Development (NICHD) demonstrated that 16% of preterm infants with very low birth weight were small for gestational age (SGA) at birth; however, when they reached 36 weeks of corrected age, 89% of this same population of preterm infants had postnatal growth failure.6 The impact of this growth restriction and nutritional problems at such early age can influence the future quality of life, as it can affect brain growth and, consequently, development, and contribute to the onset of chronic adult diseases such as hypertension, diabetes, obesity, and hypercholesterolemia.7–10
The aim of this study was to determine the frequency of EUGR in very low birth weight infants and to evaluate the impact of perinatal variables, clinical practices, and neonatal morbidities on this outcome.
MethodsThis was a longitudinal study, which analyzed a cohort of 570 newborns (NBs) with very low birth weight admitted in four neonatal units of the Perinatal Network (Rio de Janeiro) from January of 2007 to December of 2011. The four neonatal units have similar infrastructure, and clinical and nutritional practices are standardized in clinical protocols with equal levels of adherence. These guidelines recommend early parenteral nutrition and use of the mother's own milk, fortified, or formula for preterm infants in the absence of breast milk for this population.
All infants admitted during the study period were included in the study, using a convenience sample. Infants with congenital malformations, who died, or who were transfered during hospitalization were excluded.
The information was obtained from the database of the Vermont Oxford Network of the Perinatal Network. This database contains perinatal variables, demographic characteristics of the mothers and very low birth weight infants, as well as variables related to clinical practices and incident morbidities in these preterm infants.11
The variables included in the study were: use of antenatal corticosteroids, maternal hypertension, weight and head circumference (HC) at birth and at discharge, gestational age at birth and corrected at discharge, gender, weight gain (g/kg/day), HC growth (cm/week), weight classification for gestational age (adequate for gestational age AGA and SGA), length of hospital stay, respiratory distress syndrome (RDS), use of invasive mechanical ventilation (IMV) and oxygen at 36 weeks, patent ductus arteriosus (PDA), proven sepsis, necrotizing enterocolitis (NEC), and EUGR.
To evaluate the adequacy of birth weight for gestational age, the z-score of birth weight (BW) was used to classify the NBs. Infants with BW z-score > -1.29 (10th percentile) were classified as AGA, and those with a BW z-score ≤ -1.29 (10th percentile) were considered SGA. Fenton growth chart calculation spreadsheets were used for the calculation of z-scores.12,13
The intrauterine growth restrictions (IUGR) and EUGR were defined by the weight z-scores or HC ≤ -2 for corrected gestational age at birth for IUGR and at hospital for EUGR. The EUGR variable was used as the outcome for the statistical analyses.14 Exploratory analysis of the database was performed with the Statistical Package for Social Sciences (SPSS) software release 20.0.15
Aiming to identify possible associations between the explanatory variables and outcomes, a univariate analysis was performed containing each of the independent variables. After this step, the variables were included in the Poisson regression analysis with robust variance to estimate the adjusted prevalence ratio. A significance level of 5% was used to adjust variables. Statistical analyses were performed using R software, release 2.15.1.16
The study was approved by the Ethics Committee on Human Research of the Instituto Fernandes Figueira / Fiocruz, protocol No. CAEE 0078.1.008.000-11.
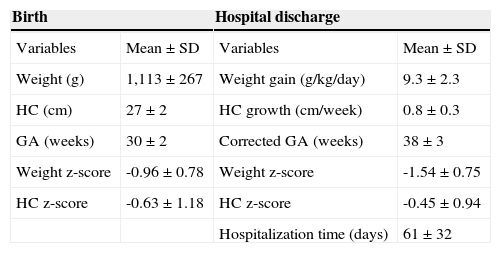
ResultsDuring the study period, 712 infants were considered eligible, of which 16 were not included in the study due to congenital malformations, 12 died, and 114 were transferred to other units; therefore, the study included 570 preterm infants. The demographic characteristics of the study population at birth and at hospital discharge are shown in Table 1. Of the population studied, 49% were males, 67% of the NBs were AGA, and 33% were SGA.
Demographic characteristics at birth and at hospital discharge.
| Birth | Hospital discharge | ||
|---|---|---|---|
| Variables | Mean ± SD | Variables | Mean ± SD |
| Weight (g) | 1,113 ± 267 | Weight gain (g/kg/day) | 9.3 ± 2.3 |
| HC (cm) | 27 ± 2 | HC growth (cm/week) | 0.8 ± 0.3 |
| GA (weeks) | 30 ± 2 | Corrected GA (weeks) | 38 ± 3 |
| Weight z-score | -0.96 ± 0.78 | Weight z-score | -1.54 ± 0.75 |
| HC z-score | -0.63 ± 1.18 | HC z-score | -0.45 ± 0.94 |
| Hospitalization time (days) | 61 ± 32 | ||
GA, gestational age; HC, head circumference; SD, standard deviation.
It was observed that although the mean z-score for HC increased during the hospitalization period (-0.63 to -0.45), the mean weight z-score showed worsening (-0.96 to -1.54).
Of the 570 NBs evaluated, 26% presented growth restriction at discharge, considering weight, and 5% when the evaluated variable was HC. The preterm infants included in the present study were discharged with a mean corrected gestational age of 38 ± 3 weeks; the mean hospital stay was 61 ± 32 days.
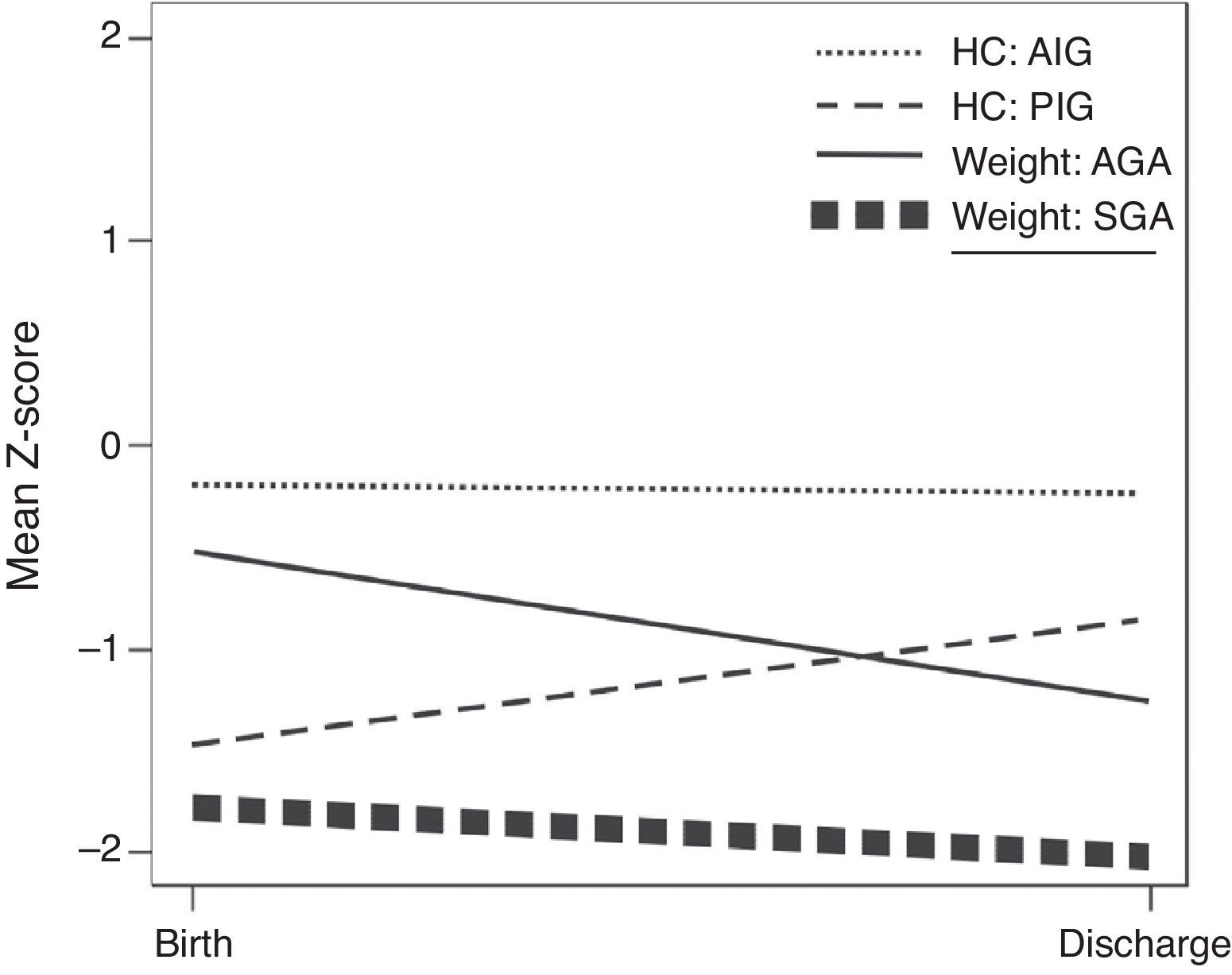
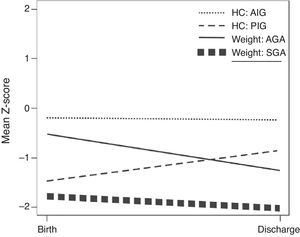
The evolution of the mean z-scores for weight and HC for the SGA and AGA infants between birth and hospital discharge is shown in Fig. 1.
AGA infants presented greater decline in weight z-score(-0.53 to -1.27), although the HC remained constant (-0.21 to -0.25). In SGA infants, the decrease in weight z-score was lower (-1.83 to -2.07) when compared to AGA infants, and the HC z-score showed an increase (-1.47 to -0.85).
Of the 33% (190/570) infants born SGA, 54.2% (103/190) had EUGR at discharge considering weight and 7.4% (14/190) considering HC. The rates for AGA infants were 12.3% (46/374) considering weight and 4% (15/374) considering HC. These differences were statistically significant for weight (p-value = 0.000), but not for HC (p-value = 0.10). Regarding growth restriction, it was observed that, at birth, 11% of the newborns studied had IUGR, considering weight. Upon discharge, this rate of growth restriction had increased to 77.4%. Regarding HC, the rate of growth restriction at birth was 10% and at discharge, 9.7%.
At the univariate analysis, the variables that showed statistical significance when the outcome variable was the weight z-score at hospital discharge were: maternal hypertension (PR = 1.36; CI: 1.03-1.79), male gender (PR = 0.70; CI: 0.53-0.93), SGA birth (PR = 4.41; CI: 3.56-5.95), RDS (PR = 0.69; CI: 0.53-0.91), and hospital length of stay (PR = 1.01; CI: 1.01-1.02). For the z = score of HC at discharge, these variables were: mechanical ventilation (PR = 2.92; CI: 1.21-7.07), oxygen use at 36 weeks (PR = 7.90; CI: 15.77), PDA (PR = 3.35; CI: 1.55-7.22), and hospital length of stay (PR = 1.03; CI: 1.03-1.04). Proven sepsis and NEC presented low frequency in the population, 4.6% and 1.2%, respectively, and were not included in the univariate analysis.
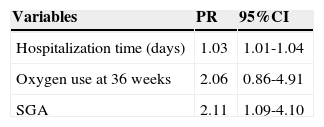
At the regression analysis, using as outcome the HC z-score ≤ -2 corrected for gestational age at discharge, the variables that remained in the final model were: hospital length of stay, oxygen use at 36 weeks, and SGA birth (Table 2).
Variables in the final model of Poisson regression for HC z-score at hospital discharge.
| Variables | PR | 95%CI |
|---|---|---|
| Hospitalization time (days) | 1.03 | 1.01-1.04 |
| Oxygen use at 36 weeks | 2.06 | 0.86-4.91 |
| SGA | 2.11 | 1.09-4.10 |
CI, confidence interval; HC, head circumference; PR, Prevalence Ratio; SGA, small for gestational age.
Variables included in the analysis: gender, SGA, use of antenatal steroids, maternal hypertension, patent ductus arteriosus (PDA), respiratory distress syndrome (RDS), ventilation, oxygen use at 36 weeks, time of hospitalization.
It was observed that adding one day to the time of hospitalization increased by 3% the chance of growth restriction at discharge, and SGA birth increased this risk by 2.1 times.
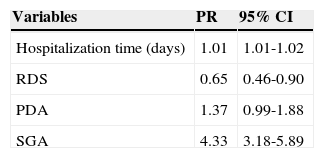
When the outcome was weight z-score ≤ -2 for corrected age at discharge, the variables that remained in the final model were: length of hospital stay, RDS, PDA, and SGA birth (Table 3).
Variables of the final model of Poisson regression for weight z-score at hospital discharge.
| Variables | PR | 95% CI |
|---|---|---|
| Hospitalization time (days) | 1.01 | 1.01-1.02 |
| RDS | 0.65 | 0.46-0.90 |
| PDA | 1.37 | 0.99-1.88 |
| SGA | 4.33 | 3.18-5.89 |
CI, confidence interval; PDA, patent ductus arteriosus; PR, prevalence ratio; RDS, respiratory distress syndrome; SGA, small for gestational age.
Variables included in the analysis: gender, SGA, use of antenatal steroids, maternal hypertension, PDA, RDS, ventilation, oxygen use at 36 weeks, time of hospitalization.
Adding one day to the hospital length of stay resulted in a 2% increase in the chance of having growth restriction at discharge. The risk factor with the greatest impact was SGA birth, which resulted in a 4.33-fold increase in the chance of having growth restriction at discharge. Moreover, the presence of RDS reduced by 35% the chance of growth restriction at discharge. However, the rate of RDS was higher in AGA infants (76.8%) compared to SGA (23.2%), and the statistical power for this variable was 39.5%.
The power of the study was low for some of the analyzed variables considering the worst for the two outcomes: gender (3%), RDS (39.5%), use of antenatal corticosteroids (16.4%), and maternal hypertension (26.3%). The power of the study for the “SGA birth” variable was 75% for HC and 100% for weight.
DiscussionThe present results demonstrate that EUGR remains a significant problem for preterm infants. This postnatal growth failure in very low birth weight infants is a universal phenomenon.4,10 In this study, it was observed that 26% (149/570) of the NBs developed EUGR for weight and 5% (29/570) for HC. These rates, albeit high, are lower than those observed in other studies performed in Brazil and other countries. Gianni et al. performed a longitudinal study, in which 200 very low birth weight infants admitted to neonatal intensive care units in eight hospitals in the city of Rio de Janeiro were prospectively followed, and a prevalence of EUGR of 63.5% for weight was observed.17
Goulart et al. analyzed a cohort of 303 preterm infants born at Hospital São Paulo, whose birth weight was < 2,000g, and observed that the frequency of measurements < 10th percentile and -2 z-scores at 1 year of corrected age were respectively 43% and 24% for weight, and 16% and 5% for HC.18 Shan et al. analyzed 2,015 preterm infants with low birth weight born at four hospitals in Shanghai and found a frequency of 56.8% of EUGR for weight.19 These variations in EUGR frequency can be explained by the assessment of populations with different demographic characteristics, the cutoff used to define EUGR, as well as the use of different reference curves.
The present data demonstrated that weight z-score worsened during hospitalization. However, the HC z-score showed improvement (-0.63 ± 1.18 to -0.45 ± 0.94), and, on average, stayed above the 10th percentile. Previous studies observed similar weight and HC evolution during hospitalization in neonatal intensive care units.20–22
The expectation regarding growth in preterm infants is for maximum acceleration to take place between 36-40 weeks of corrected age. The weight gain observed was 9.3 ± 2.3g/kg/day, much lower than the rates of weight gain recommended for these infants (approximately 15g/kg/day).23 Therefore, better growth performance was expected at 38 weeks.24
The American Academy of Pediatrics (AAP) has recommended that, under optimum care and with nutritional support, preterm infants in neonatal units should grow in the same rate as fetuses of the same intrauterine gestational age.2 This recommendation is a controversial issue. Can the intrauterine growth rate be maintained in infants outside the womb?
Cooke has discussed this issue, and has observed that even with AAP recommendations, both AGA and SGA infants were underweight after gestational age was corrected, when compared to infants born at term of the same gestational age. He claims that the extrauterine growth is influenced by a complex interaction of factors and it is often difficult to establish adequate nutrition in critically ill and medically unstable newborns.10 Infants born prematurely generally display low intrauterine weight gain and, thus, are often born with growth restriction. Therefore, when the calorie-protein offer is calculated based on birth weight, the amount offered would be lower than what these patients should receive.25
In the present study, it was also observed that the very low birth weight preterm infants who already had intrauterine growth restriction were at higher risk for EUGR for both variables, weight and HC. There were also differences in growth evolution between SGA and AGA. Differences in the evolution of extrauterine growth of SGA and AGA preterm infants have also been described by other authors. Ornelas et al. compared 100 preterm SGA and AGA infants and observed that the SGA infants were well below the growth curves of the AGA infants up to the 40th week.26 Ehrenkranz et al., in a multicenter study, found a higher growth rate in infants that were born AGA and did not develop severe morbidities.23
The EUGR may be due to multiple factors. One of the most important factors that the physician can alter is insufficient nutritional support in neonatal morbidities, which not only increase the energy requirements of preterm infants, but also often impair the nutritional offer.27 In the present study, the univariate analysis showed associations among mechanical ventilation, oxygen at 36 weeks, PDA, and hospitalization time with EUGR at hospital discharge, as measured by HC z-score. The presence of maternal hypertension, SGA, and hospitalization time were variables that significantly influenced growth restriction assessed by the z-score of weight.
Hospitalization time can be considered as an indicator of severity in preterm infants, and it is probably reflected as weight gain.28 The present results demonstrated that the length of hospitalization was a risk factor for EUGR both in weight and HC assessments. RDS was associated with growth restriction for z-score of weight in the univariate analysis, and remained statistically significant as a protective factor in the regression model. This statistically significant fact may be possibly explained by the higher frequency of RDS in AGA infants (76.8%) when compared to SGA infants (23.2%).
As SGA infants are discharged with greater growth restriction, it gives rise to a false interpretation that RDS reduces the chance of EUGR. The male gender also emerged as a protective factor for EUGR in the univariate analysis for the weight variable; however, this variable did not remain statistically significant and was not included in the regression model. Kurtoglu et al. also demonstrated that male infants, when compared to females, were heavier for the same corrected gestational age at hospital discharge.29
The low power of the study for part of the variables, mainly for HC, was a limitation of the present study. However, the frequency of EUGR for HC was low, indicating that this variable suffered less influence from clinical variables. Another issue was the lack of nutritional information in the database, which would have allowed for greater detailing of growth restriction causes. Prospective studies that include these variables are necessary.
In conclusion, EUGR remains a frequent and universal problem in preterm infants with very low birth weight in neonatal intensive care units, and, therefore, long-term studies are required to determine better methods to feed and care for these infants, especially those born SGA.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Lima PA, de Carvalho M, da Costa AC, Moreira ME. Variables associated with extra uterine growth restriction in very low birth weight infants. J Pediatr (Rio J). 2014;90:22–27.