this triple-masked controlled trial aimed to assess the effects of vitamin D supplementation on insulin resistance and cardiometabolic risk factors in obese children and adolescents.
Methodsthe study comprised 50 participants, aged 10 to16 years, who were randomly assigned into two groups of equal number. In this 12-week trial, one group received oral vitamin D (300,000 IU) and the other group received placebo. Cardiometabolic risk factors, insulin resistance, and a continuous value of metabolic syndrome (cMetS) were determined. Statistical analysis was conducted after adjustment for covariate interactions.
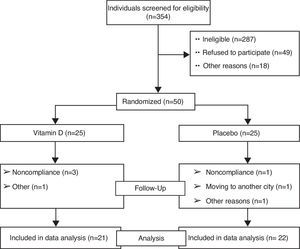
Resultsoverall, 21 patients in the vitamin D group and 22 in the placebo group completed the trial. No significant difference was observed in the baseline characteristics of the two groups. After the trial, in the vitamin D group, serum insulin and triglyceride concentrations, as well as HOM -IR and C-MetS decreased significantly, both when compared with the baseline and with the placebo group. No significant difference was observed when comparing total cholesterol, LDL-C, HDL-C, fasting blood glucose, and blood pressure.
Conclusionthe present findings support the favorable effects of vitamin D supplementation on reducing insulin resistance and cardiometabolic risk factors in obese children.
este ensaio clínico triplo-cego controlado visa investigar os efeitos da suplementação de vitamina D sobre a resistência à insulina e os fatores de risco cardiometabólico em crianças e adolescentes obesos.
Métodoso estudo contou com 50 participantes com idade entre 10 e 16 anos, aleatoriamente divididos em dois grupos de igual número de participantes. Neste ensaio clínico de 12 semanas, um grupo recebeu vitamina D via oral (300000 IU) e o outro grupo recebeu placebo. Foram determinados fatores de risco cardiometabólico, resistência à insulina e valor contínuo da síndrome metabólica (cMetS). A análise estatística foi conduzida após o ajuste das interações covariáveis.
Resultadosno todo, 21 pacientes no grupo vitamina D e 22 no grupo placebo concluíram o ensaio clínico. Nenhuma diferença significativa foi encontrada nas características de base dos dois grupos estudados. Após o ensaio clinico, no grupo vitamina D, as concentrações séricas de insulina e triglicerídeos, bem como HOMA-RI e cMetS caíram significativamente em comparação ao início do estudo; e também em comparação ao grupo placebo. Nenhuma diferença significativa foi vista ao comparar o colesterol total, LDL-C, HDL-C, glicemia de jejum e pressão sanguínea.
Conclusãonossas conclusões indicam efeitos favoráveis da suplementação de vitamina D sobre a redução da resistência à insulina e de fatores de risco cardiometabólico em crianças obesas.
Most chronic non-communicable diseases and their risk factors begin early in life. Therefore, in recent years, much attention has been focused on the primary prevention of diseases from childhood. The long-term effects of childhood obesity, such as cardiometabolic risk factors including metabolic syndrome (MetS) are of special concern.1
In this context, prevention and early control of risk factors are of crucial importance. MetS is an underlying cause of most chronic diseases, and insulin resistance is suggested to have an underlying role in the development of MetS. Such disorders are not limited to adults and to industrialized countries, rather, they are becoming as an important health problem for children and adolescents in several countries.2 The authors’ previous national studies reported a high prevalence of MetS and cardiometabolic risk factors in Iranian children and adolescents.3,4 In recent years, significant relationships have been documented between vitamin D deficiency and various non-communicable diseases, notably cardiovascular diseases and diabetes, as well as with their predisposing factors, such as MetS and insulin resistance. Vitamin D has an important role in glucose and insulin metabolism.5 It affects pancreatic islet cells through its receptors and may increase insulin secretion. Vitamin D deficiency leads to elevated PTH levels, and in turn to decreased insulin sensitivity. Moreover, vitamin D has anti-inflammatory and immune modulating effects, and might lead to a decrease in insulin resistance and an increase in insulin secretion by modulating the immune system.6 Low serum levels of vitamin D are suggested to be associated with insulin resistance and cardiometabolic risk factors even in young age. Thus, different doses of vitamin D supplementation are proposed for prevention of these risk factors in healthy children and adolescents.7 However, whether vitamin D supplementation would improve insulin sensitivity and metabolic risk factors in the pediatric age group is controversial. The current study aimed to investigate the effects of oral vitamin D supplementation on insulin resistance and cardiometabolic risk factors in obese children and adolescents.
MethodsThis triple-masked controlled trial was conducted in 2012 in Isfahan, Iran, and was approved by the Research Council and the Ethics Committee of the Isfahan University of Medical Sciences. The trial was registered with the code IRCT201110271434N5 in the Iranian Registry of Clinical Trials, which is a primary registry in the World Health Organization (WHO) Registry Network. This trial was conducted in accordance with the principles of the Helsinki Declaration. An informed consent was obtained from parents and oral assent from participants. Considering an α error of 0.05 and a β error of 20%, and also considering the effect of vitamin D supplementation on insulin sensitivity in a previous trial among obese individuals,8 the sample size was calculated as 20 in the intervention group, and 20 in the placebo group. Due to possible attrition during the trial, the sample size was increased to 25 in each group.
ParticipantsThe study was conducted among children and adolescents referred to the pediatrics clinics affiliated to Isfahan University of Medical Sciences. Eligibility criteria were age between 10 years and 16 years, body mass index equal to or greater than three Z-scores,9 and presence of MetS.10,11 Exclusion criteria were any medication or supplementation use and any chronic disease. Obese children and adolescents were invited to participate, and if after receiving their laboratory data, they fulfilled the criteria of MetS, they were recruited to the trial. Sampling continued until reaching the necessary number of participants for the trial. The demographic variables were determined through a validated questionnaire.12
Physical examination and laboratory testsAnthropometric indexes, as well as systolic (SBP) and diastolic (DBP) blood pressure were measured by trained nurses according to standard protocols, using calibrated instruments. Fasting venous blood sample was examined for fasting plasma glucose (FPG) and lipid profile by autoanalyzer with standard kits (Pars Azmoun - Tehran, Iran). Serum concentration of 25-hydroxy vitamin D (25(OH)D) was analyzed using the chemiluminescent immunnoassay (CLIA) method (25 OH VitD CLIA kit, Diasorin - Stillwater, MN,United States); the kit's expected range is 4 to 150 ng/mL. The lowest reportable value was 4.0 ng/mL, which is based on an inter-assay precision that approximates 20% CV (functional sensitivity).
Plasma insulin was measured by radioimmunoassay (RIA) (LINCO Research Inc), which is 100% specific for human insulin with less than 0.2% cross-reactivity with human proinsulin and no cross reactivity with c-peptide or insulin-like growth factor. Insulin resistance (IR) was calculated on the basis of the homeostasis model assessment of IR (HOMA-IR), using the following formula:
[HOMA-IR=(fasting insulin (mU/L) x fasting glucose (mmol/L)/22.5].
Definition of cardiometabolic risk factors and MetSCardiometabolic risk factors were defined according to the latest cut-off points provided by the National Heart, Lung, and Blood Institute for the pediatric age group.13 As there is no universal definition of MetS in the pediatric age group, a continuous value of MetS (cMetS) was used, as recommended by the American Diabetes Association and the European Association for the Study of Diabetes for children and adolescents.9 The cMetS score was derived by first standardizing the residuals for waist circumference (WC), high density lipoprotein-cholesterol (HDL-C), triglycerides (TG), fasting blood glucose (FBG), and mean arterial blood pressure (MAP) by regressing them based on age and gender to account for age- and gender-related differences. MAP was calculated using the following equation: MAP=[(SBP-DBP)/3]+DBP. Since the standardized HDL-C is inversely related to the MetS risk, it was multiplied by -1. The cMetS score was calculated as the sum of the standardized residuals (Z-scores) for the individual variables. A higher cMetS score indicates a less favorable metabolic profile. The cMetS score has been previously validated by the authors in Iranian children and adolescents.11
Medication and placeboThe Zahravi Pharmaceutical company, which manufactures soft gel capsules containing 50,000 IU of vitamin D3, collaborated with the trial in preparing placebo. They were identical in appearance with the vitamin D capsules, and both were tasteless and odorless.
Study interventionThe trial statistician generated a randomization list using Stata, version 9 (College Station - TX, United States). Participants were randomly assigned to two groups of equal number. All trial staff, participants, and the statistician were masked to treatment allocation throughout the study.
One group received 300,000 IU (one capsule per week) of vitamin D3,14 and the other group received placebo. Both groups received similar recommendations for healthy eating and reduction of sedentary activities.
Compliance with consumption of medication or placebo was assessed by weekly phone follow-up and monthly visits in the clinic. Twelve weeks after randomization, all baseline clinical and laboratory examinations were repeated in both groups. The entire program was offered free of charge.
Statistical analysisData analyses were performed using the Statistical Package for Social Sciences (SPSS), version 20.0 (SPSS Inc. - Chicago, IL, United States). The normality of the distribution of variables was confirmed by the Kolmogorov–Smirnov test. The intention to treat principle was used throughout the analysis. Student's t-test was used to compare the mean of quantitative variables before and after the intervention. The comparison of pre-and-post-intervention within each group was calculated by paired t-test. Analysis of covariance were used to adjust results for the dependent variables measured before and after the trial for treatment covariate interactions. All values were reported as mean±SD. ptime, pgroup and ptime×group were calculated for all variables.
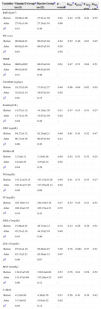
ResultsThe study flow chart shows the screening, randomization, and follow-up of the participants (Fig. 1). Overall, 21 patients in the vitamin D group and 22 in the placebo group completed the trial. No significant difference was observed in the baseline characteristics of the two groups studied. The intra-group and inter-group differences in variables before and after the trial are presented in Table 1.
Characteristics of participants at baseline and after the trial.
| Variables | Vitamin D Groupa (n=21) | Placebo Groupb (n=22) | p overallc | ptimed | pgroupe | p time × groupf | ptime × ageg |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | |||||||
| Before | 28.08±1.06 | 27.81±1.04 | 0.61 | 0.42 | 0.56 | 0.41 | 0.55 |
| After | 27.91±1.04 | 27.24±1.01 | 0.68 | ||||
| ph | 0.51 | 0.48 | - | ||||
| WC (cm) | |||||||
| Before | 90.08±6.01 | 90.03±5.04 | 0.64 | 0.52 | 0.46 | 0.61 | 0.45 |
| After | 89.02±5.04 | 89.07±5.01 | 0.58 | ||||
| ph | 0.61 | 0.58 | - | ||||
| WHtR | |||||||
| Before | 06/01±0/02 | 06.03±0.04 | 0.61 | 0.47 | 0.51 | 0.64 | 0.51 |
| After | 06.01±0.01 | 06.02±0.05 | |||||
| ph | 0.51 | 0.48 | - | ||||
| 25(OH)D (ng/mL) | |||||||
| Before | 18.27±2.04 | 17.91±2.27 | 0.48 | 0.06 | 0.04 | 0.02 | 0.03 |
| After | 32.01±2.14 | 19.07±2.01 | 0.02 | ||||
| ph | 0.01 | 0.15 | - | ||||
| Insulin(μU/L) | |||||||
| Before | 14.27±1.32 | 14.19±1.20 | 0.31 | 0.37 | 0.31 | 0.51 | 0.27 |
| After | 13.71±1.58 | 14.07±1.04 | 0.02 | ||||
| ph | 0.04 | 0.28 | - | ||||
| FBG (mg/dL) | |||||||
| Before | 94.27±5.32 | 92.20±6.21 | 0.48 | 0.61 | 0.41 | 0.52 | 0.47 |
| After | 90.71±4.58 | 90.07±5.64 | 0.21 | ||||
| ph | 0.06 | 0.28 | - | ||||
| HOMA-IR | |||||||
| Before | 3.21±0.11 | 3.15±0.26 | 0.48 | 0.42 | 0.51 | 0.32 | 0.25 |
| After | 2.81±0.25 | 3.07±0.14 | 0.02 | ||||
| ph | 0.04 | 0.28 | - | ||||
| TG(mg/dL) | |||||||
| Before | 141.21±24.15 | 143.15±23.26 | 0.48 | 0.41 | 0.31 | 0.35 | 0.27 |
| After | 102.81±27.20 | 137.07±25.14 | 0.02 | ||||
| ph | 0.04 | 0.08 | - | ||||
| TC(mg/dL) | |||||||
| Before | 161.50±3.21 | 164.18±5.18 | 0.27 | 0.32 | 0.25 | 0.21 | 0.51 |
| After | 160.18±2.35 | 162.15±4.21 | 0.35 | ||||
| P8 | 0.54 | 0.41 | - | ||||
| HDL-C(mg/dL) | |||||||
| Before | 47.06±4.01 | 48.72±4.12 | 0.34 | 0.12 | 0.26 | 0.38 | 0.51 |
| After | 45.21±3.18 | 44.35±2.19 | 0.48 | ||||
| ph | 0.45 | 0.46 | - | ||||
| LDL-C(mg/dL) | |||||||
| Before | 97.01±4.19 | 95.68±2.87 | 0.58 | 0.76 | 0.861 | 0.57 | 0.51 |
| After | 93.17±5.21 | 92.56±4.12 | 0.67 | ||||
| ph | 0.08 | 0.07 | - | ||||
| MAP (mmHg) | |||||||
| Before | 134.01±5.89 | 136.61±6.08 | 0.53 | 0.76 | 0.81 | 0.58 | 0.52 |
| After | 131.47±4.69 | 135.26±4.52 | 0.07 | ||||
| ph | 0.06 | 0.12 | - | ||||
| C-MetS | |||||||
| Before | 4.21±0.89 | 4.36±0.78 | 0.51 | 0.26 | 0.41 | 0.38 | 0.42 |
| After | 3.17±0.61 | 4.01±0.52 | 0.02 | ||||
| ph | 0.04 | 0.12 | - | ||||
BMI, body mass index; C-MetS, continuous metabolic syndrome; FBG, fasting blood glucose; HDL-C, high density lipoprotein-cholesterol; HOMA-IR, homeostasis model of assessment - insulin resistance; LDL-C, low density lipoprotein-cholesterol; MAP, mean arterial blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WHtR, waist to height ratio; 25(OH)D, 25-hydroxyvitamin D.
All values are presented as mean ± SD.
At baseline, the mean concentrations of serum 25(OH)D were not significantly different between the groups studied; after the trial, the group receiving vitamin D had significant intra-group (p=0.01) and inter-group (p=0.02) increases. This was confirmatory evidence of the compliance of participants in taking vitamin D capsules.
After the trial, in the vitamin D group, serum TG concentration decreased significantly compared with the baseline (p=0.04); and also compared with the placebo group (p=0.02). A significant decrease was observed in serum insulin levels and HOMA-IR in the vitamin D group at the end of the study in comparison with the baseline (p=0.04). At the end of the study, the serum insulin levels and HOMA-IR had a significant difference between the groups (p=0.02 and p=0.02, respectively), showing improvement in IR in the group receiving vitamin D. Comparison of cMetS at the baseline and after the trial showed significant decrease in the vitamin D group when compared to the placebo group (p=0.04). However, no significant differences were observed when comparing the serum levels of total cholesterol, LDL-C, HDL-C, FBG, and BP at baseline and after the trial both intra-group (p=0.54, p=0.08, p=0.45, p=0.06, and p=0.06, respectively) and inter-group (p=0.41, p=0.07, p=0.46, p=0.28, and p=0.07).
DiscussionTo the best of the authors’ knowledge, the present study is one of the first of its kind in the pediatric age group, and revealed favorable effects of oral vitamin D3 (300,000 IU) on insulin resistance, MetS, and TG in obese children and adolescents. There is increasing evidence that vitamin D deficiency is associated with risk factors of non-communicable diseases, including components of the MetS and other cardiometabolic risk factors even in children and adolescents.15–17 Insulin resistance is considered as one of the main underlying causes of MetS. Some studies have demonstrated an inverse relationship between vitamin D levels and insulin resistance.18,19 Inflammatory cytokine production is considered to be one of the mechanisms of the effect of vitamin D on insulin resistance; inflammatory cytokines are associated with both obesity and insulin resistance.20 There is further evidence of a relationship between vitamin D metabolism and diabetes mellitus. Vitamin D is involved in insulin secretion and probably on its function, hypophysis regulation and glucose homeostasis, which eventually can lead to the development of MetS.21 It has been suggested that low serum levels of vitamin D may increase insulin resistance, and in turn the risk of diabetes mellitus type 2 over time.20
Previous trials on the effects of vitamin D on cardiometabolic risk factors and insulin resistance have been performed with adults.19,22–26
The present results confirm a significant relationship between vitamin D deficiency and increased blood pressure, TG, insulin resistance, and MetS. The findings of this study are in agreement with those of other clinical trials conducted among adults; and an inverse relationship has been observed between serum concentration of vitamin D and the risk of MetS and insulin resistance.22–24 Furthermore, several cross-sectional studies presented the same results.19,25,26 For instance, in a study among postmenopausal women, an inverse relationship was documented between serum levels of vitamin D with TG, HDL/TG, and MetS, but the corresponding figures were not significant for LDL-C, HDL-C, and insulin.22
Conversely, other studies conducted among adults did not document a significant association between vitamin D levels and the abovementioned indexes.27,28 Likewise, a cross-sectional study on Turkish high school students did not find a significant correlation between vitamin D levels and insulin resistance or MetS.29 Differences in the age groups studied, severity of weight excess, and cardiometabolic risk factors, as well as the doses of vitamin D supplementation can explain the controversies in the findings of various studies. The dose and interval of vitamin D supplementation for improving the serum vitamin D levels of children remain undetermined.30
Study limitations and strengthsAlthough some significant differences were observed, which indicate that a sufficient number of subjects were studied, a larger sample size with longer period of follow-up perhaps would have obtained more favorable results. The strengths of the present study are its novelty in the pediatric age group and the assessment of independent association of vitamin D with the risk factors studied.
ConclusionVitamin D supplementation was inversely associated with insulin resistance and some cardiometabolic risk factors. Vitamin D supplementation may have beneficial effects on controlling some complications of childhood obesity.
FundingThis trial was conducted as a thesis funded by the Isfahan University of Medical Sciences.
Conflicts of interestThe authors declare no conflicts of interest.
This study was conducted as a thesis funded by Isfahan University of Medical Sciences. The authors would like to thank the participants of the study and their families.
Please cite this article as: Kelishadi R, Salek S, Salek M, Hashemipour M, Movahedian M. Effects of vitamin D supplementation on insulin resistance and cardiometabolic risk factors in children with metabolic syndrome: a triple-masked controlled trial. J Pediatr (Rio J). 2014;90:28–34.