To translate and validate the Brazilian Portuguese version of the Transition Readiness Assessment Questionnaire in a population of adolescents and young adults with chronic rheumatologic disorders. This questionnaire evaluates the patient's readiness for making the transition from the pediatric health service to adult care.
MethodsThe four-phase methodology for the translation and validation of generic questionnaires was followed, including translation, back-translation, pilot testing and clinical validation of the final tool. The confirmatory factor analysis was used for clinical validation and the Cronbach's alpha coefficient was used to assess the overall internal consistency of the final tool.
ResultsA total of 150 patients with a mean age of 17.0 years (SD=2.2 years, range 14–21 years) were enrolled for the final tool validation. Of those, 71 patients had juvenile systemic lupus erythematosus (47.3%), 64 had juvenile idiopathic arthritis (42.7%), and 15 had juvenile dermatomyositis (10%). During the confirmatory factor analysis, the dimension “Talking with providers” consisting of two questions, was considered as not fitting the translated questionnaire due to a very high ceiling effect and was therefore excluded. All other translated items favorably contributed to the overall consistency of the final tool; removing that dimension did not result in a substantial increase in Cronbach's alpha, which was 0.776.
ConclusionsThe Brazilian Portuguese version of the Transition Readiness Assessment Questionnaire was validated in a population of transitional patients with chronic rheumatologic disorders, after one dimension from the original questionnaire was excluded. It is a non-specific disease questionnaire; thus, it can be used to evaluate the transition readiness of Brazilian patients with other chronic diseases.
Traduzir para o português brasileiro e validar o Questionário de Avaliação do Preparo para a Transição em uma população de adolescentes e adultos jovens com doenças reumáticas crônicas. Este questionário avalia o preparo do paciente para realizar a transição do serviço de saúde pediátrico para a assistência ao adulto.
MétodosSeguimos a metodologia de quatro etapas para a tradução e validação de questionários genéricos que inclui tradução, retrotradução, teste piloto e validação clínica do instrumento final. Utilizamos Análise Fatorial Confirmatória e Coeficiente Alfa de Cronbach para testar a validade do instrumento e sua consistência interna.
ResultadosResponderam ao questionário traduzido e adaptado 150 pacientes. A média de idade foi de 17,0 anos (DP = 2,2 anos, variação 14-21 anos). Tinham o diagnóstico de lúpus eritematoso sistêmico juvenil 71 pacientes (47,3%), 64 (42,7%) artrite idiopática juvenil e 15 (10%) dermatomiosite juvenil. Durante a análise fatorial confirmatória, a dimensão “Falando com a Equipe Médica” contendo duas questões teve que ser removida devido à presença de expressivo efeito teto. Todas as outras questões restantes contribuíram favoravelmente para aumentar a consistência interna do questionário, obteve-se um Coeficiente Alfa de Cronbach de 0,776.
ConclusõesO Questionário de Avaliação do Preparo para a Transição na sua versão em português brasileiro pode ser validado em uma população de pacientes com doenças reumáticas crônicas em transição, com a exclusão de uma dimensão do questionário original. Por ser um questionário não específico para doenças reumáticas, poderá ser utilizado para avaliar o preparo para a transição de outros pacientes brasileiros com doenças crônicas.
The transition between pediatric and adult healthcare is a matter of growing concern. Advances in medical treatments have improved the survival rates of patients with chronic and complex diseases.1,2 An unsuccessful transition can result in high rates of loss to follow-up, poor treatment adherence, and poor outcomes, ranging from disease reactivation to death.3 Therefore, establishing a good quality transition program should be among the priorities of any healthcare team that provides pediatric care, including physicians, nurses, and other professionals.4,5
Recommendations on when to begin introducing the concept of transition and when to effectively transfer patients vary widely, but it is generally accepted that this transition should ideally occur between 15 and 21 years of age.5
Although many aspects that influence the transition outcome are related to the intrinsic problems of each healthcare system, such as lack of an integrated medical communication system and lack of adequate infrastructure, other aspects are related to the patients’ self-preparation to undergo the transition. Emotional bonding with the healthcare team, financial difficulties, and difficulty in making decisions about issues pertaining to the individuals themselves appear to be particularly prominent among young individuals with chronic diseases during the transition phase.6–9 As a result, reliable and standardized tools that assess transition readiness need to be available to ensure a continuous, planned transition process that promotes self-care capacity, by maximizing the well-being and functionality of these patients in adult life.5
The American Academy of Pediatrics recently recommended that the transition should be individualized based on the patient's readiness and that transition readiness analysis tools should be adapted to each patient's specific situation. However, regardless of the chosen tool, it should contain specific components that provide a minimum of accuracy to assess the patients’ ability to undergo the transition at a given time.5
A systematic review of the literature on the psychometric properties of assessment tools for transitional readiness in adolescents with chronic diseases has shown that most cannot be well evaluated, and that only the Transition Readiness Assessment Questionnaire (TRAQ) has proven reliability in its key measurement components.10 TRAQ is a self-administered questionnaire that was developed and modified over a two-year period and had its validity, reliability, and accuracy established in several studies. It contains 20 questions, which measure a patient's readiness to transition from pediatric care to adult care. Although comprehensive, TRAQ is short and easy to administer and, unlike other transitional questionnaires, it is “disease-neutral,” which facilitates data comparison across different patient populations.11,12
Since the transition problem is universal, it is important that TRAQ be culturally adapted and made available in other languages for use in local and multicenter studies. TRAQ is currently available only in English and Spanish, including an Argentinian Spanish version.11–14 To date, no validated transition readiness tool is available in Brazil.
The objective of this study was to translate TRAQ into Brazilian Portuguese and to validate its psychometric properties in a population of young individuals of transition age with chronic rheumatologic disease.
MethodsThe TRAQ questionnaire contains 20 questions grouped into five dimensions: Managing Medications, Appointment Keeping, Tracking Health Issues, Talking with Providers, and Managing Daily Activities. It was specifically designed to evaluate adolescents and young adults with chronic illnesses and special health care needs.11,12 It contains simple and short sentences that were created to be comprehensible to young individuals and quantify their level of readiness to make the transition. These phrases were tested to fit the definitions of the transition stages proposed by Prochaska and DiClemente.15
Each scale ranges from 1 to 5, scoring the level of patient's readiness for the transition. The mean score of the items indicated in the questions results in a scale also ranging from 1 to 5.
The study was approved by the local Ethics Committee, and the author of the questionnaire (Gregory Sawicki) granted authorization for TRAQ to be translated into Brazilian Portuguese.
All study participants signed the free and informed consent and/or assent form. The four-step methodology used for the translation and validation of generic questionnaires developed in other languages was followed, as described below.16–19
Step 1: TranslationThe original English version of TRAQ was independently translated into Brazilian Portuguese by two of the authors (C.G.A., C.A.L.), followed by a joint discussion to combine the two independent versions into a single version. At this stage, the researchers’ efforts were devoted to achieving linguistic and conceptual equivalence with the original English version. Ultimately, there were few differences and no changes were required regarding the cultural aspect of the language, since the questions involved common aspects of patients’ daily lives.
Step 2: Back-translationThe initial Brazilian Portuguese version, resulting from the combination of the two independent versions by the two authors, was back-translated into English by a certified translator. In a second discussion, the same authors reviewed and included the necessary changes, resulting in the translated questionnaire.
Step 3: Pilot testing and cognitive interviewThe cross-cultural adaptation of the questionnaire was performed during this stage to achieve semantic equivalence (equivalence between words), idiomatic equivalence (equivalent expressions), and experimental equivalence (words and situations appropriate to the Brazilian cultural context). The translated TRAQ was administered by one of the authors (C.G.A) to 16 patients with rheumatologic diseases, on the day of the medical consultation in a favorable environment (waiting room/office). Two patients of each age between 14 and 21 years were selected to have their responses evaluated and identify possible comprehension difficulties resulting from the translation. Sample calculation was not performed at this stage of the study. Some difficulties were observed in the questionnaire development, since certain questions were misunderstood by some patients. These questions (9, 10, and 15) of the original TRAQ asked about “health insurance coverage,” “how to deal with a potential loss of current health insurance coverage,” and “whether there is financial aid for work or study.” As a result, the text “are you a member of a private or public health system” was added to question 10, but questions 9 and 15 remained unchanged. Based on this stage of the process, last version was edited into a final version, which was used in the next phase of the study.
Step 4: Clinical validation – validity and reliability testThis phase consisted of a field study performed after a sample calculation, in which the questionnaire was administered consecutively to a larger number of transitional youngsters with chronic rheumatologic diseases treated at the pediatric rheumatology outpatient clinic. As they are the most prevalent diseases in pediatric rheumatology, only patients with juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, and juvenile dermatomyositis were included in the study. Patients with cognitive deficits that might hinder the understanding of the proposed questions were excluded. The interviews were carried out by a lay interviewer with no medical background and no previous information about the disease or the patient's condition. The interviewer was instructed to intervene as little as possible and the missing data should not be inserted. Moreover, data on socioeconomic factors, gender, age, disease status and follow-up time in the service, ethnicity, place of residence, educational level of the patients and their parents, and current occupation were collected in order to apply the Brazilian Economic Classification Criteria of the Brazilian Association of Research Companies (Critério de Classificação Econômica Brasil da Associação Brasileira de Empresas de Pesquisa [ABEP]) for structural poverty assessment.
Statistical analysisConfirmatory factorial analysis (CFA) was used to evaluate the validity of the Brazilian Portuguese version of the TRAQ. To perform the validation, sample size was calculated using PSS 2008 (Power Analysis and Sample Size System, NCSS, UT, USA) statistical software, so that for a correlation of 0.50 (for items that comprise the same subscale), its estimate does not differ by more than 0.10 with a 95% confidence. According to Hair et al., the ratio between the number of individuals and the number of variables should exceed five to one, with at least 100 cases being recommended to ensure more robust results.20
The model adequacy was verified through indexes such as root mean square error of approximation (RMSEA), confirmatory fit index (CFI), Tucker-Lewis index (TLI), and normalized chi-squared (X2/d.f.). The overall internal consistency of the final questionnaire was analyzed by Cronbach's alpha coefficient, a statistical test that evaluates how a set of items measures a single latent construct. This same index was used to validate the original English version of the TRAQ and is a reliability (or consistency) coefficient, depending on the number of items and mean correlations between items, typically ranging from 0 to 1. The closer to 1, the greater the consistency between items on a scale or subscale. A value ≥0.7 indicates high reliability; 0.5 to <0.7, moderate reliability; >0.2 to <0.5, fair reliability; and ≤0.2, low reliability. Student's t-test was used for the comparison of means between two groups. In the statistical analysis, a significance level of 5% was adopted for all tests and the analyses were performed using a software (Stata Statistical Software: Release 12. College Station, TX, USA).
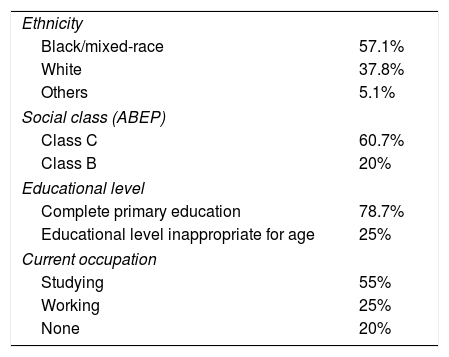
ResultsPatientsIn total, 150 patients with a mean age of 17.0 years (SD=2.2 years, range 14–21 years) answered the questionnaire. The sample consisted of 71 patients with juvenile systemic lupus erythematosus (JSLE; 47.3%), 64 with juvenile idiopathic arthritis (JIA; 42.7%), and 15 with juvenile dermatomyositis (JDM; 10%). The mean follow-up time was 7.7 years, and 75% of the patients in the study were females. Of the population that answered the questionnaire, 42% had clinical disease activity. Six patients were excluded due to cognitive impairment and two patients refused to participate (Table 1).
Demographic data (n=142).
| Ethnicity | |
| Black/mixed-race | 57.1% |
| White | 37.8% |
| Others | 5.1% |
| Social class (ABEP) | |
| Class C | 60.7% |
| Class B | 20% |
| Educational level | |
| Complete primary education | 78.7% |
| Educational level inappropriate for age | 25% |
| Current occupation | |
| Studying | 55% |
| Working | 25% |
| None | 20% |
ABEP, Associação Brasileira de Empresas de Pesquisa.
In general, the tool was well understood and was completed in a short time by the study participants. No major difficulties were observed and most of the patients were able to read and answer the questionnaire. However, 42 (28%) of the 150 questionnaires were incomplete. The main difficulties found were in questions 9, 10, and 15, difficulties that had already been identified during the pilot-testing phase in the questionnaire translation process. The answers to these questions showed the highest rate of incompleteness: question 9 (15/150), question 10 (17/150), and question 15 (11/150). When analyzing the general TRAQ scores based on age and gender, a statistically significant difference was observed between patients aged 18 years and older when compared with younger patients (3.91, SD=0.61 versus 3.46, SD=0.68; p<0.001), but these differences were not observed for gender (3.67, SD=0.67 for females versus 3.59, SD=0.73 for males; p=0.526).
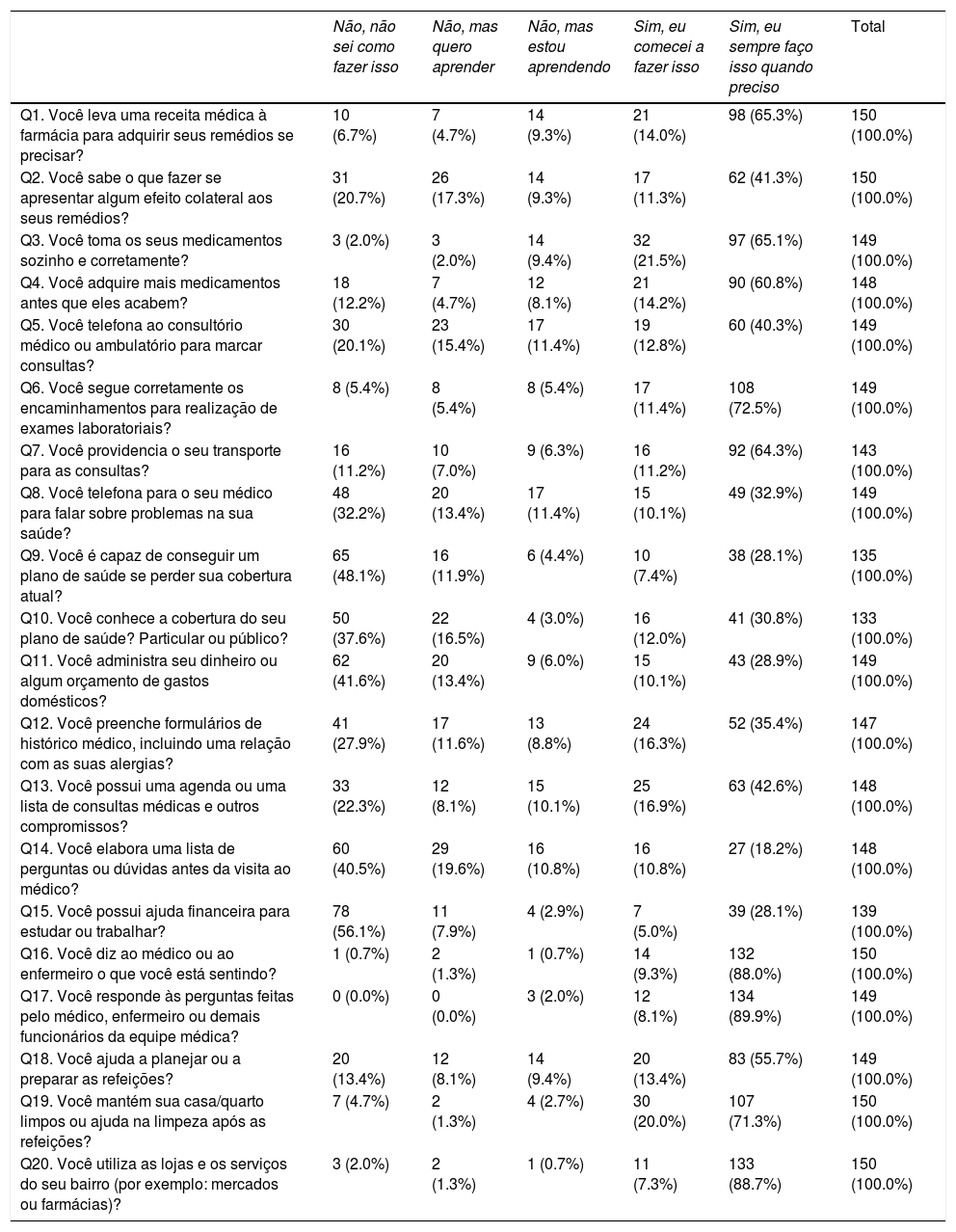
Table 2 shows the distribution of responses for each TRAQ item.
Distribution of the answers obtained with the translated version of the Transition Readiness Assessment Questionnaire.
| Não, não sei como fazer isso | Não, mas quero aprender | Não, mas estou aprendendo | Sim, eu comecei a fazer isso | Sim, eu sempre faço isso quando preciso | Total | |
|---|---|---|---|---|---|---|
| Q1. Você leva uma receita médica à farmácia para adquirir seus remédios se precisar? | 10 (6.7%) | 7 (4.7%) | 14 (9.3%) | 21 (14.0%) | 98 (65.3%) | 150 (100.0%) |
| Q2. Você sabe o que fazer se apresentar algum efeito colateral aos seus remédios? | 31 (20.7%) | 26 (17.3%) | 14 (9.3%) | 17 (11.3%) | 62 (41.3%) | 150 (100.0%) |
| Q3. Você toma os seus medicamentos sozinho e corretamente? | 3 (2.0%) | 3 (2.0%) | 14 (9.4%) | 32 (21.5%) | 97 (65.1%) | 149 (100.0%) |
| Q4. Você adquire mais medicamentos antes que eles acabem? | 18 (12.2%) | 7 (4.7%) | 12 (8.1%) | 21 (14.2%) | 90 (60.8%) | 148 (100.0%) |
| Q5. Você telefona ao consultório médico ou ambulatório para marcar consultas? | 30 (20.1%) | 23 (15.4%) | 17 (11.4%) | 19 (12.8%) | 60 (40.3%) | 149 (100.0%) |
| Q6. Você segue corretamente os encaminhamentos para realização de exames laboratoriais? | 8 (5.4%) | 8 (5.4%) | 8 (5.4%) | 17 (11.4%) | 108 (72.5%) | 149 (100.0%) |
| Q7. Você providencia o seu transporte para as consultas? | 16 (11.2%) | 10 (7.0%) | 9 (6.3%) | 16 (11.2%) | 92 (64.3%) | 143 (100.0%) |
| Q8. Você telefona para o seu médico para falar sobre problemas na sua saúde? | 48 (32.2%) | 20 (13.4%) | 17 (11.4%) | 15 (10.1%) | 49 (32.9%) | 149 (100.0%) |
| Q9. Você é capaz de conseguir um plano de saúde se perder sua cobertura atual? | 65 (48.1%) | 16 (11.9%) | 6 (4.4%) | 10 (7.4%) | 38 (28.1%) | 135 (100.0%) |
| Q10. Você conhece a cobertura do seu plano de saúde? Particular ou público? | 50 (37.6%) | 22 (16.5%) | 4 (3.0%) | 16 (12.0%) | 41 (30.8%) | 133 (100.0%) |
| Q11. Você administra seu dinheiro ou algum orçamento de gastos domésticos? | 62 (41.6%) | 20 (13.4%) | 9 (6.0%) | 15 (10.1%) | 43 (28.9%) | 149 (100.0%) |
| Q12. Você preenche formulários de histórico médico, incluindo uma relação com as suas alergias? | 41 (27.9%) | 17 (11.6%) | 13 (8.8%) | 24 (16.3%) | 52 (35.4%) | 147 (100.0%) |
| Q13. Você possui uma agenda ou uma lista de consultas médicas e outros compromissos? | 33 (22.3%) | 12 (8.1%) | 15 (10.1%) | 25 (16.9%) | 63 (42.6%) | 148 (100.0%) |
| Q14. Você elabora uma lista de perguntas ou dúvidas antes da visita ao médico? | 60 (40.5%) | 29 (19.6%) | 16 (10.8%) | 16 (10.8%) | 27 (18.2%) | 148 (100.0%) |
| Q15. Você possui ajuda financeira para estudar ou trabalhar? | 78 (56.1%) | 11 (7.9%) | 4 (2.9%) | 7 (5.0%) | 39 (28.1%) | 139 (100.0%) |
| Q16. Você diz ao médico ou ao enfermeiro o que você está sentindo? | 1 (0.7%) | 2 (1.3%) | 1 (0.7%) | 14 (9.3%) | 132 (88.0%) | 150 (100.0%) |
| Q17. Você responde às perguntas feitas pelo médico, enfermeiro ou demais funcionários da equipe médica? | 0 (0.0%) | 0 (0.0%) | 3 (2.0%) | 12 (8.1%) | 134 (89.9%) | 149 (100.0%) |
| Q18. Você ajuda a planejar ou a preparar as refeições? | 20 (13.4%) | 12 (8.1%) | 14 (9.4%) | 20 (13.4%) | 83 (55.7%) | 149 (100.0%) |
| Q19. Você mantém sua casa/quarto limpos ou ajuda na limpeza após as refeições? | 7 (4.7%) | 2 (1.3%) | 4 (2.7%) | 30 (20.0%) | 107 (71.3%) | 150 (100.0%) |
| Q20. Você utiliza as lojas e os serviços do seu bairro (por exemplo: mercados ou farmácias)? | 3 (2.0%) | 2 (1.3%) | 1 (0.7%) | 11 (7.3%) | 133 (88.7%) | 150 (100.0%) |
The floor effect and ceiling effect were considered present when more than 15% of participants’ responses showed extreme values. A floor effect was observed in ten items and ceiling effect in all 20 items of the Brazilian Portuguese version of the TRAQ. Questions 16, 17, and 20 presented the most pronounced ceiling effect, with over 88% of the responses concentrated on the “Yes, I always do this when I need to” option.
Questionnaire validityTo determine the adequacy of the scale, including the verification of the original five dimensions, a confirmatory factorial analysis of the maximum likelihood method was performed using the maximum likelihood estimation (MLE). The initial five-dimensional analysis did not demonstrate convergence due to the dimension “Talking with Providers.” The latter consisted of two individual questions (questions 16 and 17), and a very significant ceiling effect was observed for both questions (89% of patients answered “Yes, I always do this when I need to”). As a result, this scale dimension was removed in the subsequent analyses. There was no need to remove question 20, despite pronounced floor and ceiling effects.
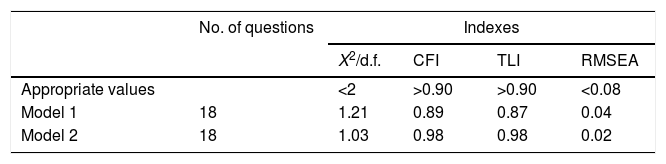
Questionnaire adequacyWhen applying the CFA to demonstrate the construct adequacy, the CFI and TLI indexes were statistically insufficient for the translated questionnaire as a whole. However, by adding covariance between items 9 and 10, as suggested by the change indexes in the statistical test, these adequacy indicators increased and reached acceptable levels (Table 3).
Indices of adequacy of the initial and final modelsa – factorial confirmatory analysis.
| No. of questions | Indexes | ||||
|---|---|---|---|---|---|
| X2/d.f. | CFI | TLI | RMSEA | ||
| Appropriate values | <2 | >0.90 | >0.90 | <0.08 | |
| Model 1 | 18 | 1.21 | 0.89 | 0.87 | 0.04 |
| Model 2 | 18 | 1.03 | 0.98 | 0.98 | 0.02 |
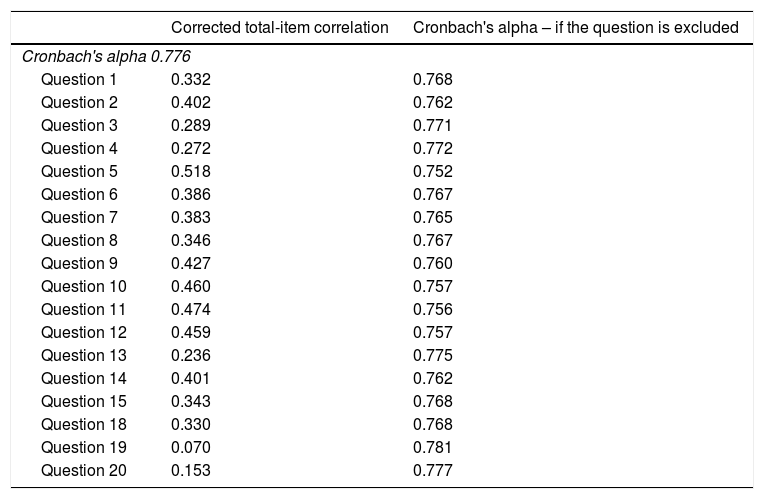
The internal consistency values (Cronbach's alpha) for the remaining 18 questions and their respective alpha values, if the question were excluded, are shown in Table 4. A globally acceptable internal consistency (Cronbach's alpha=0.776) was observed. Additionally, all items favorably contributed to the internal consistency, and the removal of one question did not result in a substantial increase in the Cronbach's Alpha value. In turn, internal consistency in the remaining four dimensions was moderate, with a Cronbach alpha index of 0.56 for “Managing Medications,” 0.59 for “Managing Daily Activities,” 0.60 for “Tracking Health Issues,” and 0.67 for “Appointment Keeping”.
Internal consistency: Cronbach's alpha and related measures.
| Corrected total-item correlation | Cronbach's alpha – if the question is excluded | |
|---|---|---|
| Cronbach's alpha 0.776 | ||
| Question 1 | 0.332 | 0.768 |
| Question 2 | 0.402 | 0.762 |
| Question 3 | 0.289 | 0.771 |
| Question 4 | 0.272 | 0.772 |
| Question 5 | 0.518 | 0.752 |
| Question 6 | 0.386 | 0.767 |
| Question 7 | 0.383 | 0.765 |
| Question 8 | 0.346 | 0.767 |
| Question 9 | 0.427 | 0.760 |
| Question 10 | 0.460 | 0.757 |
| Question 11 | 0.474 | 0.756 |
| Question 12 | 0.459 | 0.757 |
| Question 13 | 0.236 | 0.775 |
| Question 14 | 0.401 | 0.762 |
| Question 15 | 0.343 | 0.768 |
| Question 18 | 0.330 | 0.768 |
| Question 19 | 0.070 | 0.781 |
| Question 20 | 0.153 | 0.777 |
n=108.
As the survival rates of children with chronic diseases and disabilities increase, adult health care providers must be prepared to provide adequate medical care for this population. A well-structured transition process should be considered a priority to ensure continuity of care and maintenance of an adequate health status. Several studies have demonstrated that an inadequate transition from pediatric to adult care is responsible for high rates of school dropout, loss to follow-up, and unsatisfactory outcomes.1–5 Reliable and standardized tools that assess transition readiness need to be available to ensure a continuous, planned transition process that promotes self-care capacity by maximizing the well-being and functionality of these patients in adult life.5
To date, TRAQ is considered one of the most reliable tools to evaluate transition readiness.10 It is currently available only in the English and Spanish languages, including an Argentinian Spanish version.11–14 Since there are no other transition tools that have been validated in Brazil, the authors aimed to translate TRAQ into Brazilian Portuguese and validate the psychometric properties of this version in a population of adolescents and young adults with chronic rheumatologic diseases undergoing care transition.
The present results showed that, unlike the Argentinian Spanish version, it was not possible to validate the translated version of TRAQ in its original version.13,14 Due to the pronounced ceiling effect observed, an entire dimension (two questions) had to be excluded, as it would have compromised the validity of the entire questionnaire. A high incidence of floor effect (10/20 questions) was observed, as well as a ceiling effect on all 20 questions. However, the psychometric properties of the translated tool showed good overall internal consistency assessed by Cronbach's alpha coefficient, exceeding the recommended limit for group comparison (0.70).
This score was lower when compared with the last published version of TRAQ in English in 201412 for both the total coefficient (0.776 versus 0.94) and the isolated dimensions. The reliability of the score for the dimensions was below the recommended minimum of 0.7, which indicates that the tool should only be used to make comparisons as a whole, but not in different dimensions.
Approximately 75% of Brazilians depend on the public healthcare system, which provides universal and free care, including hospitalization, surgeries, and medications.21 Therefore, issues related to “health insurance coverage” and “how to deal with a potential loss of current health coverage” were not applicable to the present study population. When asked “Do you get financial help with school or work?,” 56% of the study participants attributed the lowest score to it, also showing a probable lack of knowledge of the existence of public policies to help individuals with chronic illness in Brazil. The problems found with these questions may not have been related only to the public healthcare system model, but might reflect the lack of education in health and education as a whole, since almost 25% of this patient population did not have the appropriate educational level for their age. This difference in schooling/age found in this study was the same observed in the last Brazilian school census carried out in 2015, taking into consideration the entire country in the last four years of the basic level of schooling of the Brazilian Education Program.22
As expected, and similar to the cohort of patients in the United States, a statistically significant difference was observed for the responses of patients aged 18 years or older when compared with younger patients, when analyzing the general TRAQ scores. However, unlike that cohort, in which women scored higher on TRAQ, this difference by gender was not observed in Brazilian patients.11,12
The present study has some limitations: the number of incomplete questionnaires (28%) resulted in a great loss for the analysis of the entire questionnaire, demonstrating that perhaps the presented questions were not fully understood by the study population.
Moreover, the questionnaire was validated in a subpopulation of patients with a chronic illness, patients with chronic rheumatologic disease, which may not be representative of the entire population. However, because it is a neutral instrument in relation to the underlying disease, it can be applied to any patient with a chronic disease.
In summary, the present study demonstrates that clinical tools, such as the TRAQ, should be adapted to the healthcare system where it is applied. It can be argued that the exclusion of one dimension may have led to the loss of some degree of standardization for comparison between multinational studies. However, due to the lack of adequate alternatives and until a better tool is developed, the authors propose the use of this Brazilian Portuguese version of TRAQ to evaluate the readiness of patients with chronic diseases to undergo the transition.
FundingThis study was supported by MEC/MCTI/CAPES/CNPQ/FAPS (Brazil) as part of the research incentive program: “PROJETO CIÊNCIA SEM FRONTEIRAS – PESQUISADOR VISITANTE ESPECIAL – PVE 2014” with a scholarship granted to Andreas Reiff.
This study was funded by a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 303752/2015-7), awarded to Maria Teresa Terreri.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Anelli CG, Len CA, Terreri MT, Russo GC, Reiff AO. Translation and validation of the Transition Readiness Assessment Questionnaire (TRAQ). J Pediatr (Rio J). 2019;95:180–7.
Study carried out at Escola Paulista de Medicina, Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil.