To determine decision limits for total cholesterol, LDL-cholesterol, non-HDL cholesterol, HDL-cholesterol, and triglycerides in healthy children and adolescents from Cuiabá, Brazil.
MethodsThis was a cross-sectional study of 1866 healthy children and adolescents randomly selected from daycare centers and public schools in Cuiabá. The desirable levels of serum lipids were defined using the classic criteria, i.e., total cholesterol, LDL-cholesterol, non-HDL cholesterol, and triglycerides levels below the P75 percentile, and HDL-c above the P10 percentile.
ResultsFor CT, P75 was: 160mg/dL for the age range of 1 to <3 years, 170mg/dL for ≥3 to <9 years, and 176mg/dL for ≥9 to <13 years. For non-HDL cholesterol, it was 122mg/dL for the age range of 1 to <13 years. For LDL-c, it was 104mg/dL at the age range of 1 to <9 years and 106mg/dL from ≥9 to <13 years. For TG, it was 127mg/dL from 1 to <2 years; 98mg/dL from ≥2 to <6 years; and 92mg/dL from ≥6 to <13 years. As for HDL-cholesterol, P10 was 24mg/dL, 28mg/dL, 32mg/dL, and 36mg/dL, for the age ranges of 1 to <2 years, ≥2 to <3 years, ≥3 to <4 years, and ≥4 to <13 years, respectively.
ConclusionThe decision limits for the serum lipid levels defined in this study differed from those observed in the current Brazilian and North-American guidelines, especially because it differentiates between the age ranges. Using these decision limits in clinical practice will certainly contribute to improve the diagnostic accuracy for dyslipidemia in this population group.
Determinar limites de decisão (LD) para o colesterol total (CT), LDL-colesterol (LDL-c), colesterol não-HDL (c-NHDL), HDL-colesterol (HDL-c) e triglicérides (TG) em crianças e adolescentes saudáveis de Cuiabá.
MétodoEstudo transversal envolvendo 1.866 crianças e adolescentes saudáveis de creches e escolas municipais públicas de Cuiabá, aleatoriamente selecionadas. Os LD desejáveis dos lipídeos séricos foram definidos pelos critérios clássicos, isto é, níveis de CT, LDL-c, c-NHDL, TG abaixo do percentil 75, e de HDL-c acima do percentil 10.
ResultadosOs P75 para CT foram: 160mg/dL para a faixa etária de 1 a < 3 anos, 170mg/dL para ≥ 3 a < 9 anos e 176mg/dL para ≥ 9 a < 13 anos. Para o c-NHDL, de 122mg/dL na faixa etária de 1 a <13 anos. LDL-c: 104mg/dL na faixa etária de 1 a < 9 anos e 106mg/dL de ≥ 9 a < 13 anos. TG: 127mg/dL entre 1 a < 2 anos; 98mg/dL de ≥ 2 a < 6 anos; e 92mg/dL de ≥ 6 a < 13 anos. Quanto ao HDL-c, o P10, foi de 24mg/dL, 28mg/dL, 32mg/dL e 36mg/dL, para as faixas etárias de 1 a < 2 anos, ≥ 2 a < 3 anos, ≥ 3 a < 4 anos e ≥ 4 a < 13 anos, respectivamente.
ConclusãoOs LD dos níveis séricos de lipídeos definidos neste estudo diferem daqueles apresentados nas diretrizes brasileiras e americanas atuais, especialmente por fazer a diferenciação entre as idades. Utilizar tais LD em nossa prática clínica certamente contribuirá para melhorar a acurácia do diagnóstico de dislipidemia nesse grupo populacional.
Coronary disease, secondary to atherosclerosis, is the main cause of current mortality.1,2 Although it is a significant cause of death in adults, atherosclerotic manifestations are rarely observed in children. The lifestyle that causes this disease usually begins in childhood, with the ingestion of caloric foods and a sedentary lifestyle, which leads to obesity, an important risk factor.3–5 According to the Family Budget Survey,6 one in three Brazilian children aged 5–9 years is overweight. Among adolescents, this picture is also of concern.6
Another risk factor is dyslipidemia, whose prevalence in pediatrics is described as high in Brazil,7,8 especially in the ERICA9 (Study of Cardiovascular Risks in Adolescents), as also shown in international publications.2,10,11 Studies indicate that 50% of children with dyslipidemia will present the disease during adulthood.12
In Brazil, the values that define serum lipid levels for children and adolescents have been proposed based on studies performed in the North American pediatric population for almost three decades2,13 and are considered in the I Guideline for Atherosclerosis Prevention in Childhood and Adolescence (I Diretriz sobre Prevenção da Aterosclerose na Infância e na Adolescência [IDPAIA]),3 in the I Brazilian Guideline for Familial Hypercholesterolemia (I Diretriz Brasileira de Hipercolesterolemia Familiar [IDBHF]),14 and, recently, in the update of the Brazilian Guideline for Dyslipidemia and Atherosclerosis prevention (Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose [ADBDPA]).15
In the IDPAIA,3 serum lipids were categorized as desirable, borderline and increased decision limits (DL), considering the P50, P75, and P90 percentiles, respectively. In other guidelines,14,15 including the North American,2 the same classification is equivalent to P75, between P75 and P95, and above P95.
In the ADBDPA,15 the values are described considering the presence or absence of fasting. For serum lipids, except for triglycerides (TG), the values are the same regardless of fasting, and there is only a reference to the desirable DL.
In all guidelines, lipid values were defined in a single age range, except for TG, which are presented in two age groups in the IDBHF14 and the ADBDPA15: 0–9 years and 10–19 years.
Serum lipid measurement is part of the pediatric assessment routine.3,14,16 Adequate interpretation of levels of total cholesterol (TC), high-density lipoprotein (HDL-c), non-high-density lipoprotein (NHDL-c), low-density lipoprotein (LDL-c), and triglycerides (TG) is crucial for the diagnosis of dyslipidemia and for adequate decision-making.
The reports issued by the laboratories show the reference intervals (RIs) next to the result, for test interpretation. Usually, RIs are obtained from the test package inserts, literature data, or the RI validation from other laboratories; ideally, however, laboratories should establish their own RIs.17,18
Establishing the laboratory's own RIs is an arduous task, but it is the best method, as it reflects the characteristics of the population to which the tests will be applied daily.17–19 As 70% of medical decisions are based on laboratory results,20 it is important to have adequate RIs.
Considering that, in Brazil, DL for serum lipids in pediatrics were established based on data from North American children,3,14,15 disregarding genetic, environmental, cultural, and demographic differences, the present study aimed to establish DL for lipids in healthy children and adolescents in the city of Cuiabá.
MethodsThe study was carried out in Cuiabá, capital city of the state of Mato Grosso. The ethnic composition of the population resulted from intense race-mixing at two different moments: the first between populations from the state of São Paulo, Native Brazilians and blacks, during the colonial period; and the second, from 1970 onwards, when there was intense migration originating mainly from the South and Southeast regions of Brazil. The municipality is divided into four large regions, which include 83 municipal schools and 48 municipal public daycare centers, in which 38,934 children were enrolled in 2012.21
Children and adolescents aged 1 year up to 12 years, 11 months, and 29 days, enrolled in municipal daycare centers or schools of Cuiabá were eligible for the study. The number of classrooms in schools was similar between the regions (approximately 13 classrooms), with approximately 30 students per room. The daycare centers had only one class and the number of children did not exceed 15. Due to this homogeneity, a cluster sampling procedure was used, with five schools and five daycare centers being chosen per region by drawing lots. Since not all daycare centers had a nursery, all five daycare centers that had a nursery were added to the clusters that had been drawn. Thus, a total of 20 schools and 25 daycare centers were selected.
The sample calculation was based on the Clinical and Laboratory Standards Institute (CLSI) document, which recommends that at least 120 reference individuals per age group are necessary to define the RI.17 Therefore, the drawing of five students/classroom was initially proposed to meet the goal. The resulting number was 1420 students. However, to correct the effect of the cluster sample design and to compensate for losses (absence, refusal), this number was increased by 40%, which resulted in a sample of 1988 students. This sample represented 1.5% of the population of the municipality in the study's age group.22
Children and adolescents without any known underlying disease, with no clinical signs or symptoms, and no health complaints at the time of collection were included in the study. Participants did not use any type of medication on a regular basis, except for prophylactic ferrous sulfate.
Three workstations were set up in each institution: in the first station, the questionnaire was filled out and the informed consent form was signed. Anthropometric measurements were obtained in the second workstation,23 and blood was collected in the third workstation. The questionnaire contained questions about ethnicity, which was self-reported or characterized by the respondent.24
Weight was measured using a digital scale (Bioland EB 9015, SP, Brazil) and the length/height was measured using an anthropometric ruler for patients aged up to 2 years, whereas older children and adolescents were measured in the standing position using an anthropometer.23 Subsequently, these results were analyzed using age Z-scores by body mass index.25 For the age range of 1–2 years, weight/length Z-scores were used.25 After obtaining the Z-score results, nutritional status was classified as normal weight; risk of overweight (up to 5 years); overweight; obesity; severe obesity (5 years and older); underweight; and severe underweight (WHO Anthro and WHO Anthro Plus, version 3.2.2, WHO Headquarters, Geneva, Switzerland).
Since the aim of the study was to establish DLs for clinical practice, it was decided to include children and adolescents from all nutritional categories, provided they did not show any evidence of comorbidity. Thus, nutritional deviations were not considered as an exclusion criterion. In Brazil, 33% of children aged 5–9 years are overweight or obese.5
A total of 8mL of blood was collected in a single tube. For children younger than 2 years, a 3-h fast was recommended; for those aged 2–5 years, a 6-h fast; and for those older than 5 years and adolescents, a 12- to 14-h fast.
TC and TG were measured using the colorimetric method and HDL-c was measured using the homogeneous colorimetric method. NHDL-c and LDL-c were estimated by calculations: NHDL-c was calculated by subtracting the HDL-c from the TC, whereas LDL-c was calculated using the Friedewald formula. The analyses were carried out on the Cobas 6000 equipment (Roche®, CA, USA), at the Cedilab Laboratory of the DASA network in Cuiabá.
The statistical analysis of each serum lipid was performed to establish the DLs. Initially, variance homogeneity in the 12 age groups was analyzed using Bartlett's test. Then, the analysis of variance (ANOVA) test was used for homogeneous variances. For non-homogeneous variances, the Kruskal–Wallis test was applied. Subsequently, the Bonferroni post hoc test was used, which adjusted the level of significance for multiple comparisons in 2 by 2 groups, which allowed grouping the age ranges.
Once grouped into age ranges for each serum lipid, Bartlett's test was repeated, and the ANOVA or Kruskal–Wallis tests were applied. While keeping the age groups, outliers were excluded by calculating the mean plus or minus three standard deviations.26 After outlier exclusion, the distribution was carried out in percentiles, considering, as the classical criterion, the distribution of values up to P75 as desirable.2,14 Values ranging from P75 to P95 were considered as borderline and values ≥P95, as elevated. These percentiles were used for the analysis of serum lipids, except HDL-c, for which P10 was used as the lower limit, with P50 corresponding to the desirable level.2 The level of significance was set at 5% (alpha=0.05). These analyses were performed using the MINITAB, version 15 (MINITAB Inc., United Kingdom) and SPSS, version 16 (SPSS for Windows, Version 16.0., Chicago, USA).
Study participation occurred only after meeting the inclusion criteria and signing of the informed consent. The study was approved by the Research Ethics Committees of Hospital Universitário Julio Muller (947/2010) and Faculdade de Medicina da USP (318/2011).
ResultsThe sociodemographic and nutritional characteristics of the 1866 children and adolescents who participated in this study are shown in Table 1.
Sociodemographic and nutritional characterization of 1866 healthy children and adolescents participating in the study, Cuiabá, MT, Brazil. 2012.
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Female | 947 | 50.8 |
| Male | 919 | 49.2 |
| Age range (years) | ||
| 1 | 181 | 9.7 |
| 2 | 248 | 13.3 |
| 3 | 280 | 15.0 |
| 4 | 162 | 8.7 |
| 5 | 168 | 9.0 |
| 6 | 177 | 9.5 |
| 7 | 152 | 8.1 |
| 8 | 150 | 8.0 |
| 9 | 112 | 6.0 |
| 10 | 114 | 6.1 |
| 11 | 65 | 3.5 |
| 12 | 57 | 3.1 |
| Ethnicity (n=1854) | ||
| Mixed-race | 1202 | 64.4 |
| White | 366 | 19.6 |
| Black | 233 | 12.5 |
| Asian | 53 | 2.8 |
| Nutritional status (n=1864) | ||
| Normal weight | 1368 | 73.4 |
| Overweight | 176 | 9.4 |
| Risk of overweight | 117 | 6.3 |
| Obesity | 92 | 4.9 |
| Underweight | 50 | 2.7 |
| Severe obesity | 38 | 2.1 |
| Severe underweight | 23 | 1.2 |
Obs, variation in n due to lack of information for the variable.
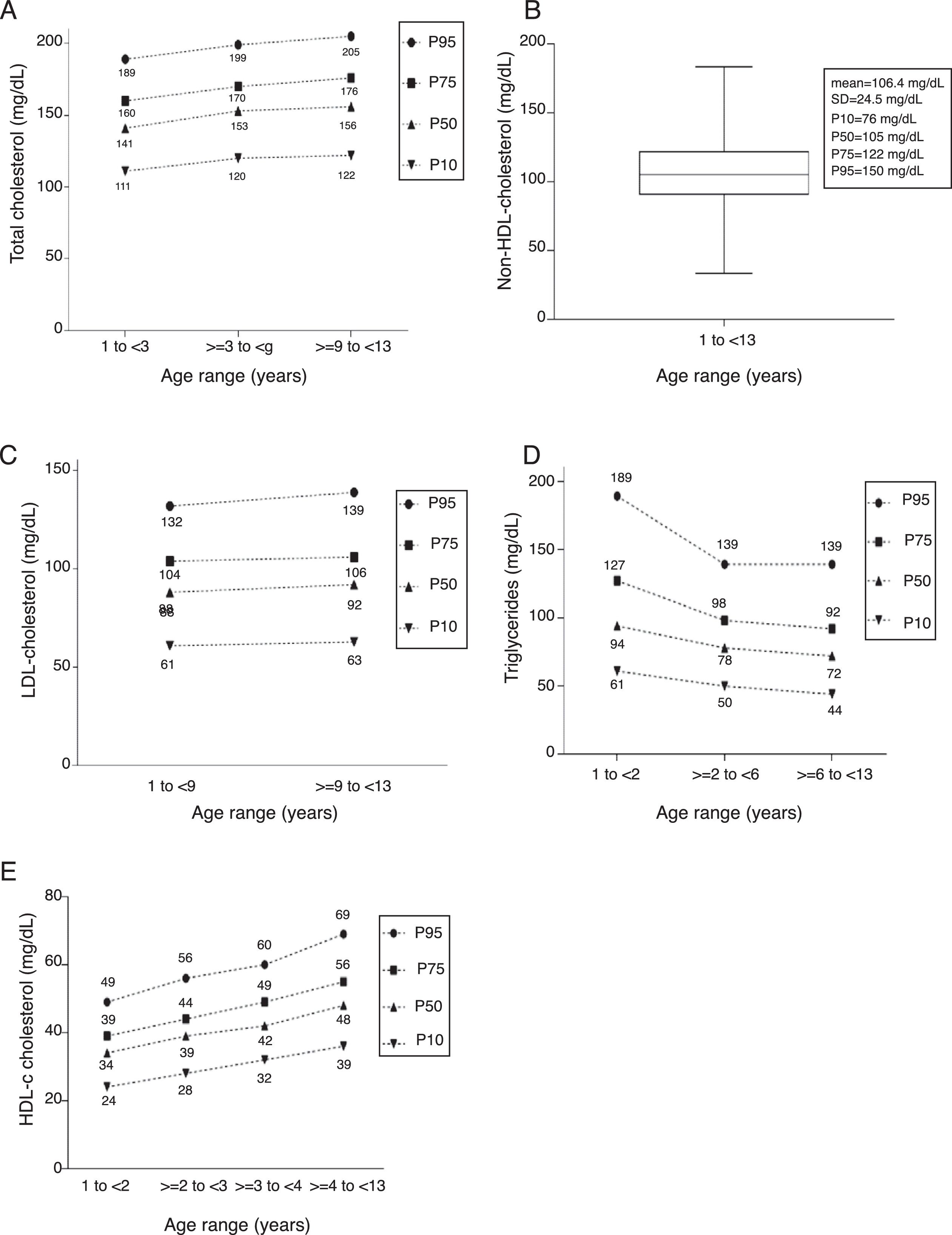
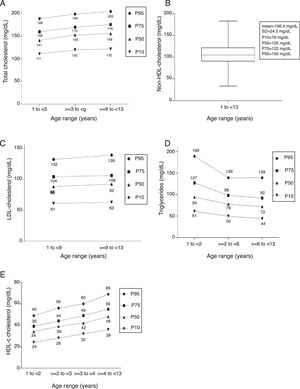
For the TC analysis, 1858 children and adolescents were divided into three age groups: 1 to <3 years, ≥3 to <9 years, and ≥9 to <13 years. The percentile distribution of TC levels is shown in Fig. 1A.
The NHDL-c was analyzed in 1854 individuals in a single age group, from 1 to <13 years, whose parameters are shown in Fig. 1B. The values corresponding to P10, P50, P75, and P95 were 76mg/dL, 105mg/dL, 122mg/dL, and 150mg/dL, respectively. Chart representation of this variable was not possible.
As for LDL-c, 1856 children and adolescents were studied and stratified into the age groups from 1 to <9 years and ≥9 to <13 years (Fig. 1C).
For the 1839 children and adolescents analyzed for TG, it was possible to identify three age groups of significant variation: 1 to <2 years, ≥2 to <6 years, and ≥6 to <13 years. The percentile distribution is shown in Fig. 1D.
Four age groups were defined for the analysis of HDL-c in 1848 individuals: 1 to <2 years, ≥2 to <3 years, ≥3 to <4 years, and ≥4 to <13 years. The percentile distribution is shown in Fig. 1E.
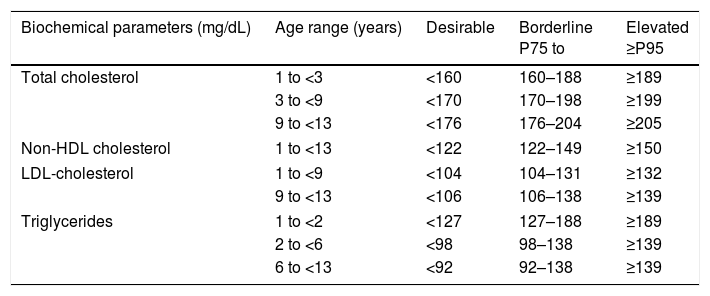
The DLs found in this population for serum lipids are shown in Table 2. The comparison of the desirable DLs of the present study with those described in the different guidelines is shown in Table 3.
Desirable, borderline, and altered concentrations of TC, NHDL-c, LDL-c, TG, and HDL-c for healthy children and adolescents in the municipality of Cuiabá, Mato Grosso, Brazil.
| Biochemical parameters (mg/dL) | Age range (years) | Desirable | Borderline P75 to | Elevated ≥P95 |
|---|---|---|---|---|
| Total cholesterol | 1 to <3 | <160 | 160–188 | ≥189 |
| 3 to <9 | <170 | 170–198 | ≥199 | |
| 9 to <13 | <176 | 176–204 | ≥205 | |
| Non-HDL cholesterol | 1 to <13 | <122 | 122–149 | ≥150 |
| LDL-cholesterol | 1 to <9 | <104 | 104–131 | ≥132 |
| 9 to <13 | <106 | 106–138 | ≥139 | |
| Triglycerides | 1 to <2 | <127 | 127–188 | ≥189 |
| 2 to <6 | <98 | 98–138 | ≥139 | |
| 6 to <13 | <92 | 92–138 | ≥139 | |
| Biochemical parameters (mg/dL) | Age range (years) | Desirable >P50 | Borderline P10 to P50 | Low P10 |
|---|---|---|---|---|
| HDL-cholesterol | 1 to <2 | >34 | 25–34 | ≤24 |
| ≥2 to <3 | >39 | 29–39 | ≤28 | |
| ≥3 to <4 | >42 | 33–42 | ≤32 | |
| ≥4 to <13 | >48 | 37–48 | ≤36 |
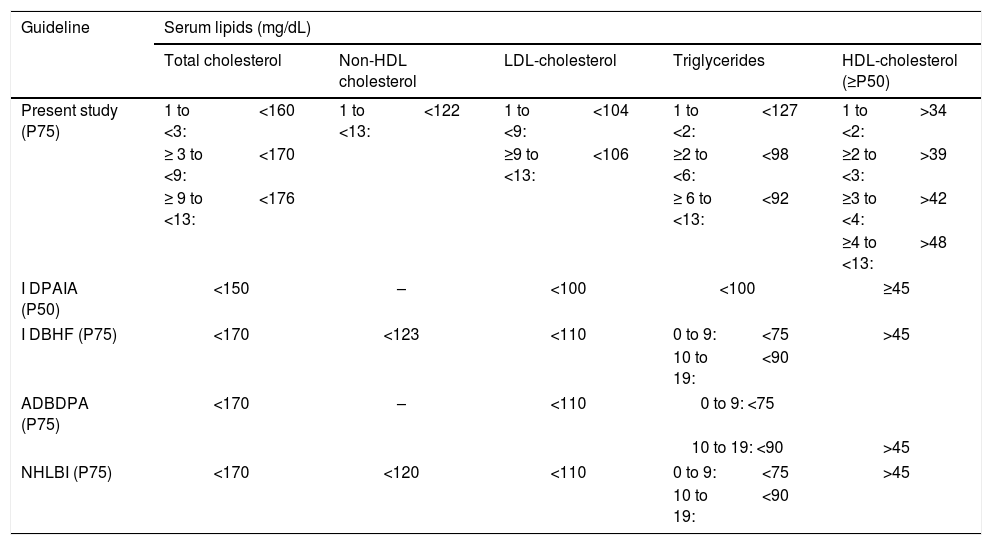
Comparison of the desirable decision limits for CT, HDL-c, NHDL-c, LDL-c, and TG in children and adolescents from the Cuiabá study with the pediatric guidelines.
| Guideline | Serum lipids (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total cholesterol | Non-HDL cholesterol | LDL-cholesterol | Triglycerides | HDL-cholesterol (≥P50) | ||||||
| Present study (P75) | 1 to <3: | <160 | 1 to <13: | <122 | 1 to <9: | <104 | 1 to <2: | <127 | 1 to <2: | >34 |
| ≥ 3 to <9: | <170 | ≥9 to <13: | <106 | ≥2 to <6: | <98 | ≥2 to <3: | >39 | |||
| ≥ 9 to <13: | <176 | ≥ 6 to <13: | <92 | ≥3 to <4: | >42 | |||||
| ≥4 to <13: | >48 | |||||||||
| I DPAIA (P50) | <150 | – | <100 | <100 | ≥45 | |||||
| I DBHF (P75) | <170 | <123 | <110 | 0 to 9: | <75 | >45 | ||||
| 10 to 19: | <90 | |||||||||
| ADBDPA (P75) | <170 | – | <110 | 0 to 9: <75 | ||||||
| 10 to 19: <90 | >45 | |||||||||
| NHLBI (P75) | <170 | <120 | <110 | 0 to 9: | <75 | >45 | ||||
| 10 to 19: | <90 | |||||||||
I DPAIA, I Diretriz de Prevenção da Aterosclerose na Infância e na Adolescência, 2005; I DBHF, I Diretriz Brasileira de Hipercolesterolemia Familiar, 2012; ADBDPA, Atualização da Diretriz Brasileira de Dislipidemia e Prevenção da Aterosclerose, 2017; NHLBI, National Heart, Lung and Blood Institute, 2012.
When comparing test results of the Brazilian population with DLs obtained from other populations, erroneous interpretations may occur. The diagnosis of dyslipidemia can be made more accurately using DLs obtained from the local population.17
The results of this study demonstrated a difference in serum lipid levels between the age groups, which is contrary to the recommendations of the guidelines that show DL for a single pediatric age group, except for TG, which is presented in different levels for the age groups of 0–9 years and 10–19 years.14,15 Only the desirable DLs of serum lipids will be discussed.
Regarding TC, the DLs of the present study were higher than those proposed by the IDPAIA.3 In the age group of 1–2 years, they were lower, but identical in the age range of 3–8 years, when compared with the other guidelines.2,14,15 In the 9–12 years age range, the values of the present study were higher than those shown in the guidelines.2,14,15 Elevated CT levels have been correlated as an important risk factor for atherosclerotic cardiovascular disease.3
As for NHDL-c, a powerful predictor of atherosclerosis, the DL obtained in this study was lower to that suggested in the IDBH14 and higher than that proposed by the NHLBI.2 The other guidelines3,15 do not address this parameter.
For LDL-c, the DLs found were lower than those proposed by all the guidelines,2,14,15 except the IDBPAIA.3 For a long time, this parameter, together with TC, was deemed very important for cardiovascular risk evaluation.
As for TG, this parameter is approached in a single age group (2–19 years) in IDPAIA,3 whereas in the other guidelines, they are shown from 0 to 9 years and from 10 to 19 years.2,14,15 The present study proposes the division into three age groups. The DLs were higher than those shown in the IDBHF14 and the NHLBI2 in all age groups. In subjects aged ≥2 to <6 years and ≥6 to <13 years, the values were compatible with those proposed in the ADBDPA15 without fasting. When compared with the IDPAIA,3 the values obtained here were lower in the age groups from ≥2 to <6 years and from ≥6 to <13 years. The higher TG levels found in this study in children aged <2 years are certainly due to the shorter fasting time.
Because of the improved accuracy of laboratory techniques, lipemia has ceased to interfere with many laboratory tests. Moreover, the postprandial state predominates most of the time; thus, the measurement of fasting serum lipids would not reflect the daily mean of lipids in blood and would not indicate the real impact on cardiovascular risk assessment.27 Currently, the flexibilization of fasting is recommended, due to the need to assess patients’ cardiac risk in their usual diet status.28
As for HDL-c, it has been observed that its levels tend to increase over the years. The evaluation of this analyte deserves special attention, since the value of medical interest is the lower limit. The IDBHF14 adopted P5, whereas the NHLBI2 has adopted P10. The other Brazilian guidelines have proposed only the desirable values (P50).3,15
HDL-c values, considering the P10, were 24mg/dL, 28mg/dL, 32mg/dL, and 36mg/dL for the four defined age groups, respectively. When considering the P5, the values obtained were 21mg/dL, 25mg/dL, 29mg/dL, and 32mg/dL. In both situations, the lower limit values found in this study were lower than those proposed by the IDBHF14 and the NHLBI.2
Considering the desirable DL, in the age range of <4 years, the values found herein were lower than those suggested by the guidelines.2,3,14,15 For those aged ≥4 years, it was higher than that recommended by all the guidelines.2,3,14,15
The serum lipid levels recommended by the Brazilian guidelines were observed in North American children and adolescents, with different genetic profiles and eating habits, with the aggravation of having been performed 30 years ago, when eating habits were better.2,3,13–15
Even though the information of the present study originated from a regional population, the social and demographic characteristics of this population make them very close to those of the rest of the Brazilian population. The current Cuiabá population resulted from an intense migratory movement. The population of Cuiabá has increased from 100,860 in 1970 to 590,118 in the year 2017.22 Therefore, although regional, the assessed population is potentially more similar to the Brazilian population than those used for the creation of the national guidelines.
The present study has limitations, such as the selection bias, as individuals from private schools were not included in the sample. Another limitation refers to the shorter duration of fasting for younger children, which could overestimate TG values. However, as previously mentioned, fasting is not currently required to assess the lipid profile.27,28 Finally, there is the concern about the exclusion of outliers. Considering the frequency of outlier exclusion of less than 4% in the sample,29 there is little chance they have interfered with the obtained limits, as shown in other studies.26
The values that allow the diagnosis of dyslipidemias must be reliable. Ideally, each country should establish its own RIs and DLs.17 The values of the parameters evaluated in the present study may represent the DLs for the Brazilian pediatric population, contributing to improve the diagnostic accuracy in this population group.
FundingAll exams were carried out at Diagnósticos da América, DASA, in Cuiabá and in Alphavile, São Paulo, at no cost to the researchers. The author received a grant from CAPES.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank their esteemed professor Cristina Jacob, for her true teachings and invaluable contributions.
Please cite this article as: Slhessarenko N, Fontes CJ, Slhessarenko ME, Azevedo RS, Andriolo A. Proposition of decision limits for serum lipids in Brazilian children aged one to 13 years. J Pediatr (Rio J). 2019;95:173–79.
Institution or service where the study was carried out for indexing at the Index Medicus/MEDLINE: Universidade Federal de Mato Grosso, Universidade de São Paulo (USP), São Paulo, SP, Brazil.