To translate the Leuven Knowledge Questionnaire for Congenital Heart Disease into Brazilian Portuguese and to validate its psychometric properties with parents and family caregivers of children with congenital heart disease.
MethodThis was a six-step methodological study, including the translation, synthesis, back-translation, evaluation of the version translated by the committee of experts, pre-testing, and validation, for which two pilot tests were used including the think-aloud protocol. The content validity index and the frequency of socioeconomic data were calculated in a statistical programming environment.
ResultsIn content validation, the instrument showed good applicability among experts, with average content validity index of 0.8–1, while kappa agreement analysis was between 0.76 to 1; both results were considered adequate for validation.
ConclusionsThe results suggest reliability among the evaluators, indicating the instrument’s accuracy and the possibility of using it to assess the knowledge of parents and family caregivers about congenital heart disease.
Congenital heart disease (CHD) is a group of defects in cardiac formation and/or large blood vessels with significant incidence, prevalence, morbidity, and mortality. Recent data show that cardiac malformations are among the leading causes of death in early childhood; CHD is the most frequent and has the highest mortality, representing 40% of malformations.1
In Brazil, CHD has a prevalence of six per 1000 children, and in the last 15 years this rate has increased to nine out of 1000 children. Recent data show that children with severe heart disease will not reach 18 years of age. These diseases are among the leading causes of death worldwide, reaching 30%.2
The aim of this study was thus to translate the Knowledge Congenital Heart Disease Questionnaire (LKQCHD) into Brazilian Portuguese and to validate its psychometric properties in a population of parents and family caregivers of children with CHD.
BackgroundPatients and parents/family caregivers of children with CHD need to be aware of the care required and signs and symptoms of the condition in order to intervene and recognize when conditions are getting worse. The Leuven (LKQCHD) was developed to verify the knowledge of patients with heart disease.3,4
Based on these experiences, the questionnaire was slightly adapted for use with parents of children with CHD.5
Given this context, there is a need to prepare for action in this scenario, and educational strategies for parents or caregivers to gain more knowledge of CHD are necessary. Parents and family caregivers of children with CHD must understand the state of health of their child in order to provide excellent care. As these children have signs and symptoms of the pathology, and their condition frequently worsens, rapid interventions are necessary to avoid hospitalization and even death.6
According to various authors, parents and family caregivers of children with CHD and other cardiac pathologies present significant knowledge gaps that may affect the care of these children, possibly due to a lack of understanding or remembering important information.7–10 It is noteworthy that the knowledge of the patient and family about their heart defects and relevant health practices can improve health outcomes.10
In terms of complications, the relationship between congenital heart defects and child’s teething increases risks for the development of infectious endocarditis.
These children may develop endocarditis on exposure to bacteremia, and good oral hygiene with appropriate brushing and supervision by caregivers and parents will reduce the frequency and magnitude of bacteremia, which plays a crucial role in the development of infectious endocarditis.9
The lack of mastery of certain knowledge by parents and family caregivers of cardiopaths may show a failure in communication and information between parents, family, and professionals, especially at this stage of life. It is import to question whether this communication failure is related to lack of knowledge about certain topics.10,11
One of the studies found that parents were not well informed about medication, side effects and interaction with other drugs or foods, endocarditis, and the possibility of their child taking part in competitive sports.7
In the outpatient care practice of pediatric patients with heart disease, it is evident that factors such as parents’ knowledge of the diagnosis, supervision of physical activity, food, and other types of protection are important for the favorable outcome of care for the pediatric patient in question. In this sense, studies that focus on this aspect are of fundamental importance.
No instrument in Portuguese validated in Brazil that could measure the knowledge of parents and family caregivers about heart disease was found. The LKQCHD is an instrument that was developed to comprehensively measure the level of knowledge in patients and families with CHD.8,9
Materials and methodsThis is a methodological study of the translation, cross-cultural adaptation, and content validation of the LKQCHD which was carried out from March 2017 to December 2018 and approved by the research Ethics Committee, under No. 1207652.
Authorization was initially requested from the author, Phillip Moons, PhD, to carry out the translation of the instrument. After authorization, the project was sent to the Research Ethics Committee for consideration and approval.
The instrument consists of 24 questions grouped into four dimensions: Disease and Treatment, Prevention of Complications, Physical Activity, and Sexuality and Heredity. There are various response formats, such as Yes, No, and I don’t know, and some answers include signs and symptoms, types of pathologies, a drawing for the recognition of the type of heart disease, and answers about the general knowledge of the pathology. The answers were categorized as 1 (correct), 2 (wrong), or 3 (incomplete) according to the author’s orientation.
Inclusion criteria were parents (mother and father), grandparents, and brothers or sisters (family caregivers) of children of both sexes aged 1–12 who had CHD, and who had been treated for one year in a reference hospital for CHD in Porto Alegre, Rio Grande do Sul, Brazil. Parents and family caregivers accompanying the children provided written informed consent and responded to the LKQCHD. The LKQCHD translation and cross-cultural adaptation process was based on international guidelines and consisted of six stages: 1 — initial translation; 2 — synthesis of translations; 3 — back translation; 4 — evaluation by the committee of experts; 5 — pre-test; 6 — content validation.12
Process of the translation, transcultural adaptation, and validation of the LKQCHD instrument into Brazilian PortugueseStage 1: TranslationIn the first step of the translation process, the original English version of the LKQCHD was independently translated into Brazilian Portuguese by two translators; these versions were dubbed T1 and T2.
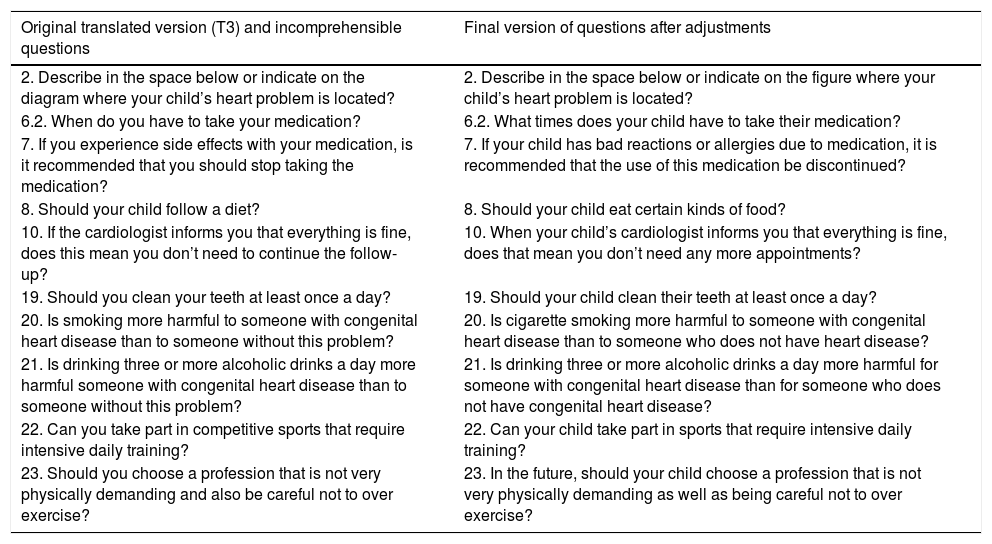
Stage 2: SynthesisIn this stage, the synthesis of the translations took place at a consensus meeting between two translators and two nursing researchers working with children with CHD, and this was called T3 in Table 1.
Synthesis of the translations (T3) and the final version of the questions that underwent alteration.
| Original translated version (T3) and incomprehensible questions | Final version of questions after adjustments |
|---|---|
| 2. Describe in the space below or indicate on the diagram where your child’s heart problem is located? | 2. Describe in the space below or indicate on the figure where your child’s heart problem is located? |
| 6.2. When do you have to take your medication? | 6.2. What times does your child have to take their medication? |
| 7. If you experience side effects with your medication, is it recommended that you should stop taking the medication? | 7. If your child has bad reactions or allergies due to medication, it is recommended that the use of this medication be discontinued? |
| 8. Should your child follow a diet? | 8. Should your child eat certain kinds of food? |
| 10. If the cardiologist informs you that everything is fine, does this mean you don’t need to continue the follow-up? | 10. When your child’s cardiologist informs you that everything is fine, does that mean you don’t need any more appointments? |
| 19. Should you clean your teeth at least once a day? | 19. Should your child clean their teeth at least once a day? |
| 20. Is smoking more harmful to someone with congenital heart disease than to someone without this problem? | 20. Is cigarette smoking more harmful to someone with congenital heart disease than to someone who does not have heart disease? |
| 21. Is drinking three or more alcoholic drinks a day more harmful someone with congenital heart disease than to someone without this problem? | 21. Is drinking three or more alcoholic drinks a day more harmful for someone with congenital heart disease than for someone who does not have congenital heart disease? |
| 22. Can you take part in competitive sports that require intensive daily training? | 22. Can your child take part in sports that require intensive daily training? |
| 23. Should you choose a profession that is not very physically demanding and also be careful not to over exercise? | 23. In the future, should your child choose a profession that is not very physically demanding as well as being careful not to over exercise? |
The researchers suggested changes in the instrument format, given that some questions in the instrument were now incomprehensible for the Brazilian reality: specifically, questions 2, 6.2, 7, 8, 10, 19, 20, 21, 22, and 23; the table below shows the changes made in the structure of the questions that were incomprehensible to the research participants.
Stage 3: Retranslation or back translationIn this stage, the T3 version was back-translated from Portuguese into English by two other independent translators who did not know the original instrument. Each back translator produced a new version, called BT1 and BT2, and this stage aimed to assess whether the content of the synthesis version was similar to the original instrument in Table 1.
Stage 4: Evaluation by the committee of expertsAfter the back translation, a committee of six experts, comprising professional nurses with experience in the subject under study, including five university teachers with MAs, and one university professor with a PhD. This committee met with the purpose of producing the so-called pre-final version of the instrument, which also included the analysis of semantic and conceptual equivalences of the version translated into Brazilian Portuguese in Table 2.
Final version of the Leuven Knowledge Questionnaire for Congenital Heart Disease instrument translated into Brazilian Portuguese.
| Leuven Knowledge Questionnaire for Congenital Heart Disease |
|---|
| Doença e Tratamento |
| 1. Qual o nome da doença cardíaca do seu filho(a)? |
| 2. Descreva no espaço abaixo ou indique na figura onde se localiza o problema cardíaco do seu filho(a) |
| 3. De quanto em quanto tempo seu filho(a) tem que ir a consulta com o médico ou pediatra para acompanhamento do problema cardíaco? |
| 4. Para que serve o acompanhamento do seu filho(a) com médicos e pediatra? |
| 5. Como foi tratado até agora o problema cardíaco do seu filho(a)? |
| 6. Seu filho foi orientado que certos alimentos e certas medicações podem alterar o efeito da sua medicação? |
| 6.1. Qual a dose que seu filho tem que tomar e qual via (comprimido, injeção na veia ou musculo)? |
| 6.2 Quais os horários que seu filho(a) tem que tomar o medicamento? |
| 6.3 Você sabe qual o efeito ou a função desta medicação? |
| 6.4 Você sabe quais as reações ruins que pode ocorrer devido ao uso desta medicação? |
| 7. Se seu filho(a) tiver reações ruins ou alergias devido a medicação é indicado que o uso desta medicação seja interrompido? |
| 8. Seu filho deve seguir uma alimentação? |
| 8.1 Se responder sim por favor indicar o tipo de alimentação? |
| 9. Assinale todos os sintomas se caso a doença cardíaca do seu filho(a) piore e que você deva procurar um médico(cardiologista) com urgência. |
| 10. Quando o cardiologista do seu filho(a)lhe informa que está tudo bem, isso significa que você não precisa de mais nenhuma consulta? |
| Prevenção de complicações |
| 11. Você sabe o que é endocardite? |
| 12. Indique qual o principal sinal de endocardite? |
| 13. Seu filho(a) só pode ter endocardite uma vez na vida? |
| 14. Estão indicados abaixo alguns fatores que podem levar a endocardite. Você acha que esses fatores contribuem para o aparecimento da endocardite? |
| 15. Sempre que seu filho(a) tiver febre ele deve tomar imediatamente antibiótico mesmo sem consulta médica? |
| 16. Você deve levar seu filho(a) ao dentista pelo menos uma vez ao ano. Para realizar uma avaliação? |
| 17. Seu filho deve tomar antibiótico antes de cada visita ao dentista? |
| 18. Quando seu filho apresentar sangramento nas gengivas você deve comunicar o médico dele? |
| 19. O seu filho deve escovar os dentes pelo menos uma vez ao dia? |
| 20. O uso do cigarro é mais prejudicial para alguém com doença cardíaca congênita do que para outra pessoa que não possuí a doença cardíaca? |
| 21. Consumir bebida alcoólica 3 ou mais vezes por dia é mais prejudicial para quem tem uma doença cardíaca congênita do que para outra pessoa que não possui a doença cardíaca congênita? |
| Atividade Física |
| 22. Seu filho(a) pode realizar esportes que exijam um treino todos os dias com muita intensidade? |
| 23. Seu filho(a)deve escolher para o futuro uma profissão que não exija muito fisicamente? |
| Sexualidade e Hereditariedade |
| 24. Qual a chance de seu filho(a) ter filhos com a mesma doença dele (cardiopatia congênita)? |
A pre-test of the final version of the instrument was performed, consolidating the evaluation stage of the committee, in order to assess the comprehension and clarity of the items. It consisted of 20 subjects from the target population who completed the questionnaire and who were interviewed to investigate each item of the questionnaire and the choice of answers. The final version of the instrument was obtained after analysis of the pre-test results and presented in Table 2, as follows:
Stage 6: ValidationPilot Test 1Before being applied on a daily basis, any new instrument must be tested, i.e., pilot tested, in order to make sure it is operational and ready for use in a real-life study or intervention.11 The think-aloud protocol is closely related to interviewing when it comes to collecting data. The researcher applies this technique simultaneously while respondents are conducting the assessment, asking questions about the perceived clarity and understandability of each instruction and item, and recording the respondents’ perceptions and reactions.13–15 In Pilot Study 1, five family members were informed informally through the researcher, so as to present them with the study objectives and to request their participation in the pilot study.
It should be emphasized that the self-report instrument to which they responded did not evaluate them; on the contrary, they evaluated the Brazilian Portuguese version of the self-report form. These family members were brought together, and while responding to the LKQCHD, the think-aloud protocol was applied. The aim was to listen to the opinion of respondents about this version of the self-report form and thus ensure that this Portuguese version of the LKQCHD was clear and accurate. After each instruction and item were read aloud by one of the researchers, respondents were instructed to comment on the clarity and comprehensibility of the formulation in Portuguese. Participants identified certain aspects of the questionnaire structure, which were then corrected. During the application of the think-aloud technique, in Question 1 the lack of awareness of family members in relation to CHD was observed, but the changes caused by the pathology could be seen in the illustration. In Question 11, on endocarditis, most participants were unaware of the terminology of the disease but most could describe its symptoms, demonstrating that they know of its existence, but not from its scientific terminology. At the end of the application of the think-aloud protocol, it was found that the instrument is suitable for application in the population under study. The final translated version was approved by the author of the LKQCHD questionnaire.
Pilot Test 2The cross-cultural adaptation of the questionnaire performed during this step to achieve semantic equivalence (word equivalence), idiomatic equivalence (equivalent expressions), and experimental equivalence (words and situations appropriate to the Brazilian cultural context). The translated LKQCHD was applied by one of the researchers to 171 relatives of patients with congenital cardiovascular disease on the day of medical consultation in a favorable environment (waiting room/office). The estimated time to complete the questionnaire was ten minutes.16
ResultsIn Stage 1, the discrepancies most found in the translations were related to words or terms with similar meanings in Brazil, such as cleaning and brushing teeth, diagram and figure, palpitation and tachycardia, and diet and food.
In Stage 2, the synthesis of the translations, all the changes occurring in the previous stage were studied, and the professionals opted for most common terms in Brazilian Portuguese. In this stage there was a need for correction of the verb tenses.
In Stage 3, the back-translated versions BT1 and BT2 were the same in eighteen questions (77%), and all translation differences were considered as synonyms. In Stage 4, the committee of experts made cross-cultural adaptations to produce a pre-test version. At this stage of the study, no sample calculation was performed. Based on this stage of the process, the version was corrected, and this resulted in the final version used in the next phase of the study.
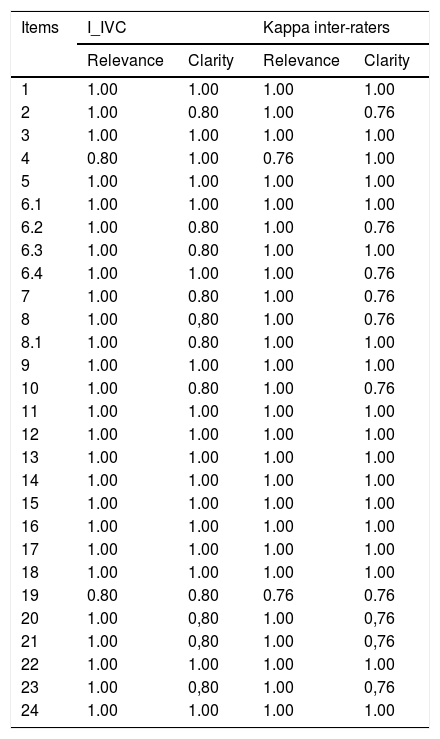
Also in Stage 4, the content validity index (CVI) calculated for each evaluator is described in Table 3, where it can be seen that the lowest CVI was 0.80 in relation to the clarity of the questions, corresponding to 80% agreement in the answers of the evaluators, which indicates that the instrument’s content validity was demonstrated in the results by the kappa coefficient. The kappa agreement test was used to verify the relevance and clarity among the evaluators, considering cutoff values above 0.6. Analyzing the results, it was found that all items presented values above 0.6, and Questions 2, 6.2, 7.8, 10, 19, 20, 21, and 23 presented 0.76 either in clarity or relevance in Table 4.
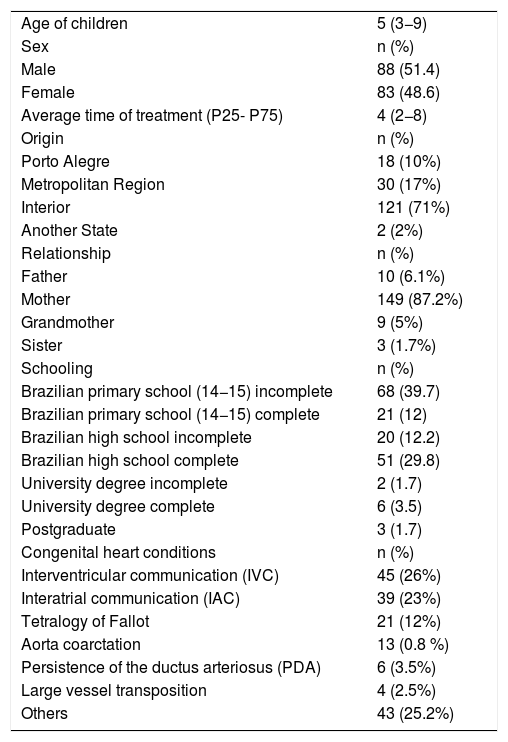
Demographic data on children and families (n = 171). Porto Alegre, Rio Grande do Sul, Brazil, 2019.
| Age of children | 5 (3−9) |
| Sex | n (%) |
| Male | 88 (51.4) |
| Female | 83 (48.6) |
| Average time of treatment (P25- P75) | 4 (2−8) |
| Origin | n (%) |
| Porto Alegre | 18 (10%) |
| Metropolitan Region | 30 (17%) |
| Interior | 121 (71%) |
| Another State | 2 (2%) |
| Relationship | n (%) |
| Father | 10 (6.1%) |
| Mother | 149 (87.2%) |
| Grandmother | 9 (5%) |
| Sister | 3 (1.7%) |
| Schooling | n (%) |
| Brazilian primary school (14−15) incomplete | 68 (39.7) |
| Brazilian primary school (14−15) complete | 21 (12) |
| Brazilian high school incomplete | 20 (12.2) |
| Brazilian high school complete | 51 (29.8) |
| University degree incomplete | 2 (1.7) |
| University degree complete | 6 (3.5) |
| Postgraduate | 3 (1.7) |
| Congenital heart conditions | n (%) |
| Interventricular communication (IVC) | 45 (26%) |
| Interatrial communication (IAC) | 39 (23%) |
| Tetralogy of Fallot | 21 (12%) |
| Aorta coarctation | 13 (0.8 %) |
| Persistence of the ductus arteriosus (PDA) | 6 (3.5%) |
| Large vessel transposition | 4 (2.5%) |
| Others | 43 (25.2%) |
Kappa concordance coefficients for the Leuven evaluators. Porto Alegre, Rio Grande do Sul, Brazil, 2019.
| Items | I_IVC | Kappa inter-raters | ||
|---|---|---|---|---|
| Relevance | Clarity | Relevance | Clarity | |
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.00 | 0.80 | 1.00 | 0.76 |
| 3 | 1.00 | 1.00 | 1.00 | 1.00 |
| 4 | 0.80 | 1.00 | 0.76 | 1.00 |
| 5 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6.1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6.2 | 1.00 | 0.80 | 1.00 | 0.76 |
| 6.3 | 1.00 | 0.80 | 1.00 | 1.00 |
| 6.4 | 1.00 | 1.00 | 1.00 | 0.76 |
| 7 | 1.00 | 0.80 | 1.00 | 0.76 |
| 8 | 1.00 | 0,80 | 1.00 | 0.76 |
| 8.1 | 1.00 | 0.80 | 1.00 | 1.00 |
| 9 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10 | 1.00 | 0.80 | 1.00 | 0.76 |
| 11 | 1.00 | 1.00 | 1.00 | 1.00 |
| 12 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13 | 1.00 | 1.00 | 1.00 | 1.00 |
| 14 | 1.00 | 1.00 | 1.00 | 1.00 |
| 15 | 1.00 | 1.00 | 1.00 | 1.00 |
| 16 | 1.00 | 1.00 | 1.00 | 1.00 |
| 17 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18 | 1.00 | 1.00 | 1.00 | 1.00 |
| 19 | 0.80 | 0.80 | 0.76 | 0.76 |
| 20 | 1.00 | 0,80 | 1.00 | 0,76 |
| 21 | 1.00 | 0,80 | 1.00 | 0,76 |
| 22 | 1.00 | 1.00 | 1.00 | 1.00 |
| 23 | 1.00 | 0,80 | 1.00 | 0,76 |
| 24 | 1.00 | 1.00 | 1.00 | 1.00 |
The final process of validation of the content of the questionnaire was carried out with 171 family members, whose children’s average age was 5 years. The majority were boys (51.4%), with an average treatment time of four years. The majority (71%) came from the hinterland of the state of Rio Grande do Sul, with the mother as the main caregiver (87.2%), and the majority of caregivers had completed Brazilian primary schooling (up to 14−15 years old). The most common pathology was intraventricular communication (IVC), as described in Table 3.
DiscussionIn the process of translation and transcultural adaptation of the LKQCHD,1 the translated version, called the Questionnaire on the knowledge of family members of children with CHDs, showed semantic, cultural, and conceptual equivalence, after the expert’s evaluation.
During the process of translation and adaptation of the questionnaire, grammatical adjustments were necessary in order to adapt it to the Brazilian context.
The LKQCHD was developed in 2001 by a Belgian nurse, Philip Moons, PhD, and was initially applied to adult patients with CHD and covered four domains that evaluate the disease and its treatment, prevention of complications, physical activities, and reproductive issues. In Taiwan, there are two versions: the first was developed in 2001 and covered four areas: (a) the disease and its treatment; (b) prevention of complications; (c) physical activities; (d) reproductive issues.
Later, in 2009, a new version of the instrument was elaborated, retaining the previous structure but dividing the fourth domain into two: sexuality and heredity, and contraception and pregnancy planning, the latter domain being applicable to female patients only. According to the author, although this questionnaire was designed to be answered by patients, it can be adapted for use with parents of children with CHD.11–13 The studies that translated and adapted the LKQCHD used a methodology similar to that presented in the research, differing only in the composition of the specialists who were part of the committee of experts. Despite cultural and methodological differences between evaluators, the LKQCHD proved to be a reliable and valid instrument for use by caregiving parents and family members.
The Portuguese version, adapted for use with the Brazilian population, presented an excellent inter-rater evaluation related to clarity and relevance, with all ratings above 0.6. After the translation and back translation of the LKQCHD, few questions required cultural adaptation, and no items were omitted. However, it does not provide detailed information on the structure of agreement and disagreement. Beaton et al.16 mention that for the estimation of inter-rater reliability, the kappa method is the most appropriate.17 Questions 7, 20, and 21 presented lower values reliability; the kappa method is the most appropriate evaluation related to clarity and relevance, with all ratings above 0.6 in Table 4.
Knowledge about the disease is believed to improve self-care and help control risk factors. As cases of children with CHD increase, it is critical that caregiving parents or family members are prepared to provide appropriate care to this population. A study on the knowledge about the care provided to children with CHD states that having information about these conditions is fundamental in order to improve quality of life and reduce the number of hospitalizations.
Patients, parents, and family caregivers are expected to have appropriate knowledge of heart disease in order to foster responsibility and commitment to care.6,7 Studies that verify the level of knowledge of patients, parents, and relatives of individuals with CHD have presented important outcomes, showing that there is a need to improve educational strategies for this population.18–24 However, some instruments focus only on specific aspects of CHD, such as diagnosis and location of the disease, endocarditis, and physical activity issues. Thus, the validation and cross-cultural adaptation of the LKQCHD for Brazil is necessary due to the increasing number of patients with CHD.
Hence, the results suggest the reliability of the inter-evaluators, indicating the accuracy of the instrument and the possibility of using it to assess the knowledge of parents and family caregivers about CHD. It is important to highlight that this questionnaire is not filled in by family members and should instead be applied by researchers, as it is an instrument that, despite being translated and cross-culturally adapted to Brazilian Portuguese, has items that are difficult to understand for less educated family members, and this was perceived during its application in Pilot Tests 1 and 2. In summary, this study demonstrates that tools such as this are necessary for the improvement of educational strategies focused on the population under study. A recent study conducted with mothers of children with CHD showed positive results regarding knowledge after educational interventions.20
Relevance to clinical practiceIt is believed that the guidance directed to the gaps presented in the application of this questionnaire will have a direct impact on the health of children with CHD, reducing health complications and even hospitalizations, improving the quality of life of children and their families, since there are reports that interventions focused on education reduce stress for family members and improve the quality of life and emotional state of individuals.21
Limitations of the studyA particular limitation of the study is that the instrument has gone through the process of adaptation and validation in only a few countries, making it difficult to discuss and compare the results in a wider range of locations.
ConclusionsIn conclusion, the Brazilian version of the LKQCHD, applied in only a few countries, making it difficult to discuss and compare the results in a wider scenario, proving to be valid and reliable for assessing the knowledge of parents and family caregivers about the child’s CHD. The application of this instrument may help in the elaboration of educational strategies and contribute to the improvement of the patients and family members’ quality of life, as well as improving the care practices of cardiology and nursing.
During its validation, there were some gaps in the knowledge of this population; after its publication, a study evaluating knowledge will be performed, using these results to develop a playful educational booklet for family members.
Conflicts of interestThe authors declare no conflicts of interest.