This study aimed to identify the predictors and threshold of failure in neonatal acute respiratory distress syndrome.
MethodsNewborns with severe acute respiratory distress syndrome aged 0–28 days and gestational age ≥36 weeks were included in the study if their cases were managed with non-extra corporal membrane oxygenation treatments. Patients were divided into two groups according to whether they died before discharge. Predictors of non-extra corporal membrane oxygenation treatment failure were sought, and the threshold of predictors was calculated.
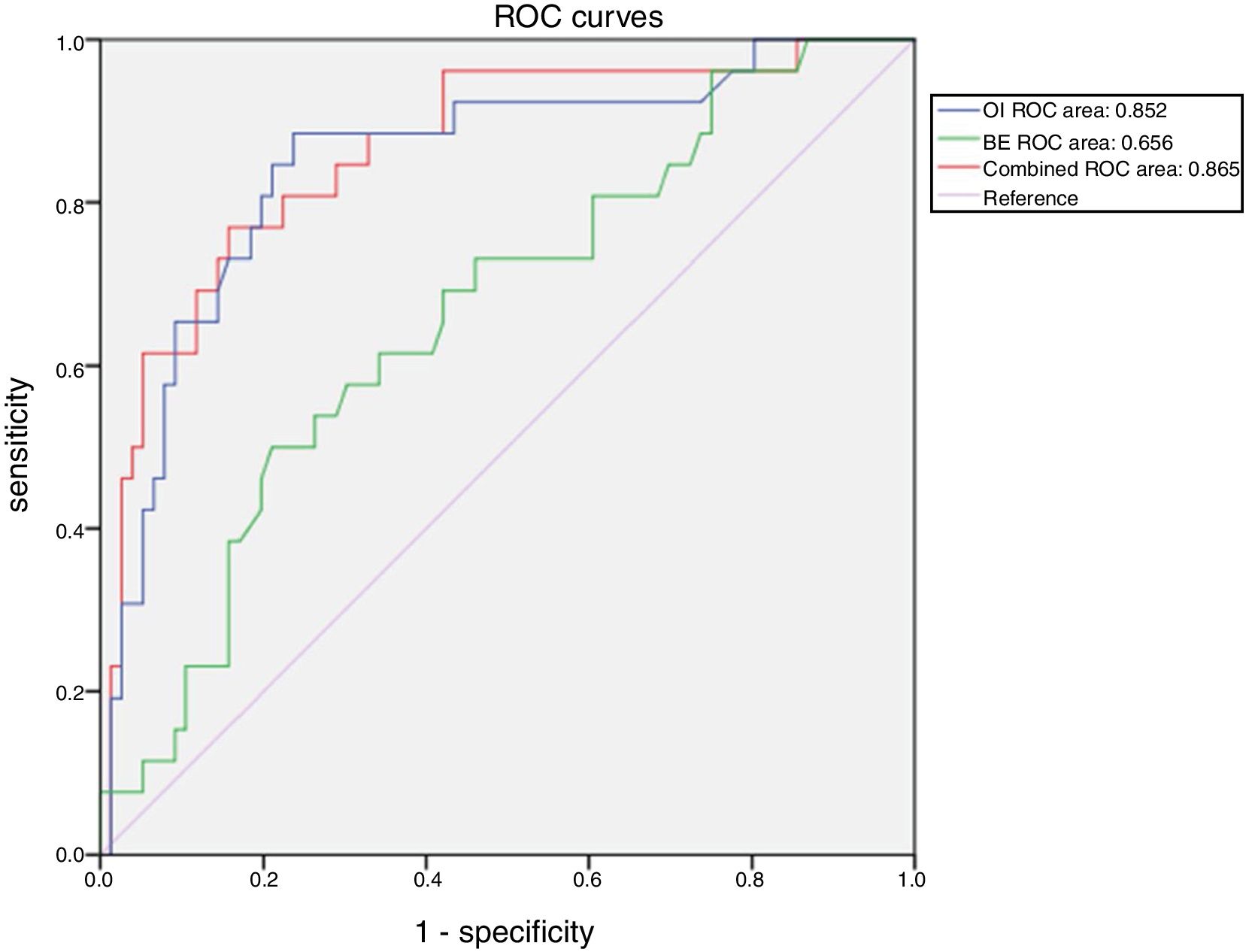
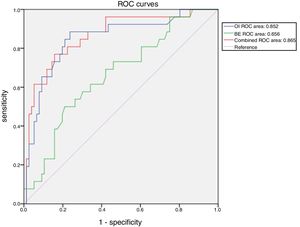
ResultsA total of 103 patients were included in the study. A total of 77 (74.8%) survived hospitalization and were discharged, whereas 26 (25.2%) died. Receiver operating characteristic analysis of oxygen index, pH, base excess, and combinations of these indicators demonstrated the advantage of the combination of oxygen index and base excess over the others variables regarding their predictive ability. The area under the curve for the combination of oxygen index and base excess was 0.865. When the cut-off values of oxygen index and base excess were 30.0 and −7.4, respectively, the sensitivity and specificity for predicting death were 77.0% and 84.0%, respectively. The model with base excess added a net reclassification improvement of 0.090 to the model without base excess.
ConclusionThe combination of oxygen index and base excess can be used as a predictor of outcomes in neonates receiving non-extra corporal membrane oxygenation treatment for acute respiratory distress syndrome. In neonates with acute respiratory distress syndrome, if oxygen index >30 and base excess <−7.4, non-extra corporal membrane oxygenation therapy is likely to lead to death.
Acute respiratory distress syndrome (ARDS) is a life-threatening disease in critically ill patients.1,2 The American and European Consensus Conference (AECC) established specific clinical criteria for adult ARDS and acute lung injury (ALI) in 1994, including acute and sudden onset of severe respiratory distress, bilateral infiltrates on chest radiography, absence of carcinogenic pulmonary edema, and severe hypoxemia.3 In 2012, the “Berlin definition” was published to provide better separation of prognosis and treatment selection. Adult ARDS was divided into three categories based on the values of partial pressure of arterial oxygen (PaO2), fraction of inspired oxygen (FiO2), positive end-expiratory pressure (PEEP), and continuous positive airway pressure (CPAP): mild ARDS (200mm Hg<PaO2/FiO2≤300mm Hg with PEEP or CPAP≥5cm H2O), moderate (100mm Hg<PaO2/FiO2≤200mm Hg with PEEP≥5cm H2O), and severe ARDS (PaO2/FiO2≤100mm Hg with PEEP≥5cm H2O).4 Based on the Berlin definition of adult ARDS, in the Pediatric Acute Lung Injury Consensus Conference (PALICC) definition of pediatric ARDS, oxygen index (OI) is considered to be a primary indicator of stratification of respiratory disease severity in mechanically ventilated patients.5
Although representing a relatively small percentage of the total number of pediatric intensive care unit (ICU) admissions, patients with ARDS are considered to be the most challenging patients.6 Epidemiological surveys show that, from 1992 to 2013, pediatric ARDS mortality has ranged from 10% to 50%.7 At present, the most commonly used ARDS treatment in pediatrics mainly includes mechanical ventilation, nitric oxide, and pulmonary surfactant.8 With advances in the understanding of the pathophysiology of respiratory failure, other advanced rescue therapies, such as extra corporal membrane oxygenation (ECMO), are increasingly being adopted.9 As a primary indicator of the severity of ARDS, OI>40 is often considered to be associated with high mortality and an indicator of ECMO used for infants or children.10 Newborns (infants within 28 days of birth) are a special type of pediatric inpatient. Compared with other patients, newborns have greater metabolic requirements and fewer cardiopulmonary reserves, which may require lower thresholds for intervention. This study analyzed the predictive value of OI and other indicators for non-ECMO treatment failure in neonatal ARDS. The study aimed to identify early predictors and provide a basis for the use of advanced treatments such as ECMO.
MethodsStudy designThis was a retrospective, multi-center study, conducted from July 2013 to July 2018. This study was registered at ClinicalTrials.gov (identifier: NCT03607760). The patients were selected from the neonatal (NICU) or pediatric ICU (PICU) of five tertiary hospitals (Jilin University First Hospital, Bayi Children's Hospital affiliated with the Chinese People's Liberation Army General Hospital, Children's Hospital of Chongqing Medical University, Zhengzhou University Children's Hospital, and Henan Provincial People's Hospital), and data was collected from electronic medical records. The inclusion criteria were: (1) newborns with severe ARDS11; (2) aged 0 days to 28 days; (3) gestational age ≥36 weeks; (4) submitted to non-ECMO treatment. Survivors were defined as patients who were cured and discharged from the hospital, while the group deaths included those who died before discharge. Patients with severe multiple organ failure, brain death, active intracranial bleeding, fatal chromosomal abnormalities, mechanical ventilation time >14d, or FiO2 ≥70% over seven to ten days were excluded from the study. This study was approved by the ethics committee of the Army General Hospital (No. 2018-54).
TreatmentAll patients were treated with non-ECMO therapy, including respiratory support, pulmonary surfactant (PS) replacement, nutritional support, and fluid management. The ventilation mode mainly included high-frequency oscillation ventilation (HFOV), NO, and control mode ventilation (CMV). Three hospitals in the present study were able to provide ECMO treatment, and the main reason for the patients not using ECMO was family refusal.
Data collectionThe recorded variables included patient demographics (age, sex, mode of delivery, and birth weight), OI, PH, PaCO2, base excess (BE), and lactate (Lac) values. The number of OIs obtained per patient varied with the frequency of arterial blood gas collection as dictated by clinical course, and the maximum OI value after 12h of birth was calculated for each newborn. PH, PaO2, PaCO2, BE and Lac values were obtained at the same time as the maximum OI.
Statistical analysisThe statistical analysis was performed using IBM SPSS 23.0 statistical software and R, version 3.6.1. Normally distributed data were presented as means and standard deviations, non-normally distributed data were presented as medians and quartiles, and categorical variables were described as numbers and frequencies. Comparisons of continuous data employed the t test and non-parametric Wilcoxon rank-sum test. The χ2 test was adopted for comparisons between two groups of categorical variables. Receiver operating characteristic analysis was used to assess the predictive ability of different models for death. The net reclassification index (NRI) was also calculated to compare the predictive ability of different models. A p value <0.05 (two-tailed) was considered to be statistically significant.
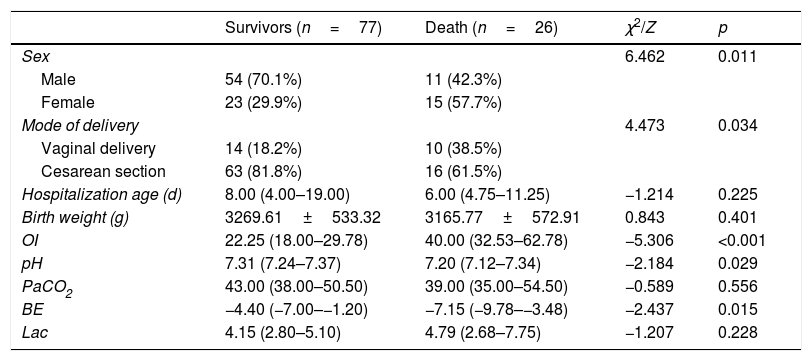
ResultsDemographics and biochemical indicatorsA total of 116 newborns met the inclusion criteria: one newborn had active intracranial bleeding, one had brain death, six had active intracranial bleeding, one had fatal chromosomal abnormalities, and four had mechanical ventilation time >14 days or FiO2 ≥70% over seven to ten days. A total of 103 children were included in the study; 77 (74.8%) survived hospitalization and were discharged, whereas 26 (25.2%) died. Significant differences were observed in sex, mode of delivery, OI, PH, and BE between the survival and non-survival groups (p<0.05). The demographics and biochemical indicators of the subjects are shown in Table 1.
Demographics and biochemical indicators.
| Survivors (n=77) | Death (n=26) | χ2/Z | p | |
|---|---|---|---|---|
| Sex | 6.462 | 0.011 | ||
| Male | 54 (70.1%) | 11 (42.3%) | ||
| Female | 23 (29.9%) | 15 (57.7%) | ||
| Mode of delivery | 4.473 | 0.034 | ||
| Vaginal delivery | 14 (18.2%) | 10 (38.5%) | ||
| Cesarean section | 63 (81.8%) | 16 (61.5%) | ||
| Hospitalization age (d) | 8.00 (4.00–19.00) | 6.00 (4.75–11.25) | −1.214 | 0.225 |
| Birth weight (g) | 3269.61±533.32 | 3165.77±572.91 | 0.843 | 0.401 |
| OI | 22.25 (18.00–29.78) | 40.00 (32.53–62.78) | −5.306 | <0.001 |
| pH | 7.31 (7.24–7.37) | 7.20 (7.12–7.34) | −2.184 | 0.029 |
| PaCO2 | 43.00 (38.00–50.50) | 39.00 (35.00–54.50) | −0.589 | 0.556 |
| BE | −4.40 (−7.00–−1.20) | −7.15 (−9.78–−3.48) | −2.437 | 0.015 |
| Lac | 4.15 (2.80–5.10) | 4.79 (2.68–7.75) | −1.207 | 0.228 |
OI, oxygen index; PaO2, partial pressure of arterial oxygen; BE, base excess; LAC, lactate.
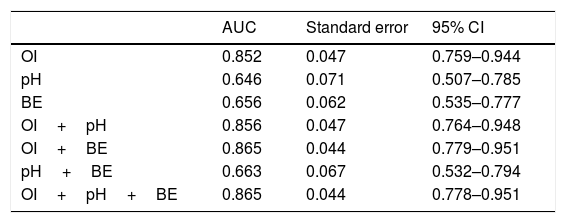
Receiver operating characteristic analysis of OI, pH, BE, and a combination of these indicators demonstrated the advantage of the combination of OI and BE over the others regarding discriminatory ability. The area under the curve for the combination of OI and BE was 0.865 (Table 2).
Prediction of death outcome with OI, pH and BE.
| AUC | Standard error | 95% CI | |
|---|---|---|---|
| OI | 0.852 | 0.047 | 0.759–0.944 |
| pH | 0.646 | 0.071 | 0.507–0.785 |
| BE | 0.656 | 0.062 | 0.535–0.777 |
| OI+pH | 0.856 | 0.047 | 0.764–0.948 |
| OI+BE | 0.865 | 0.044 | 0.779–0.951 |
| pH+BE | 0.663 | 0.067 | 0.532–0.794 |
| OI+pH+BE | 0.865 | 0.044 | 0.778–0.951 |
OI, oxygen index; BE, base excess; AUC, area under the curve.
When the cut-off values of OI and BE were 30.0 and −7.4, respectively, the sensitivity and specificity of OI and BE combined prediction of death were 77.0% and 84.0%, respectively (Fig. 1).
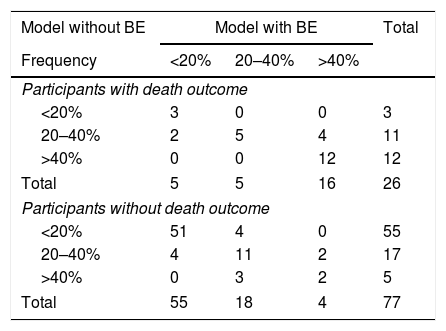
Reclassifications for subjects with and without events are summarized in Table 3. For 26 subjects who died, classification improved using the model with BE, and for four it became worse; the net gain in reclassification proportion for subjects who did not experience an event was not significant, as only one patient became better. The model with BE added an NRI of 0.090 to the model without BE.
Reclassifications for subjects with and without events.
| Model without BE | Model with BE | Total | ||
|---|---|---|---|---|
| Frequency | <20% | 20–40% | >40% | |
| Participants with death outcome | ||||
| <20% | 3 | 0 | 0 | 3 |
| 20–40% | 2 | 5 | 4 | 11 |
| >40% | 0 | 0 | 12 | 12 |
| Total | 5 | 5 | 16 | 26 |
| Participants without death outcome | ||||
| <20% | 51 | 4 | 0 | 55 |
| 20–40% | 4 | 11 | 2 | 17 |
| >40% | 0 | 3 | 2 | 5 |
| Total | 55 | 18 | 4 | 77 |
BE, base excess.
Although non-ECMO respiratory support therapy is effective for ARDS, some patients with severe respiratory failure have a relatively high mortality rate. Máca et al.12 reported that the mortality rate of ARDS patients in the ICU since 2010 was 38%. In the present study, the mortality rate of ARDS was 25.2%. Because this study only included newborns, the mortality rate was lower than that of the entire population. Moreover, since ELSO guidelines13 suggested that newborns using mechanical ventilation >14 days had a poor effect of ECMO, and the present study hoped to predict the failure of conservative treatment to indicate which patient needed ECMO, patients with high mortality risk were excluded. The overall mortality of NICU at this institution is 2% (similar to that in NICUs throughout China). Therefore, this mortality in subjects with severe ARDS was significantly higher than baseline mortality rates and represents a high-risk group.
OI is considered to be the main indicator of stratification of respiratory disease severity.5 In present study, the area under the curve for mortality prediction of OI was greater than pH and BE, which suggests that OI could be a good prognostic indicator of ARDS results. This is consistent with the results of many studies. Hammond et al.’s findings14 that quantify the predictive capability and identify the threshold value of OI will help guide the discussion about appropriate rescue intervention time. Trachsel et al.15 reported that maximum OI is an independent predictor of mortality.
In addition to the comparison between the different indicators, the present study also analyzed the predictive value of combinations of indicators. The combination of OI and BE and the combination of OI, BE, and PH had the same mortality prediction (area under the curve: 0.865), and it was higher than that of other combinations. The authors believe that the combination of OI and BE is more efficient. Although the combined area under the curve of OI and BE has only a slight advantage over the use of OI alone, the combination of OI and BE presented a better reclassification capability than OI alone. After reclassification, four patients in the death cohort became worse, one patient in the survival cohort became better, and NRI increased by 9%. Base excess (BE) is an indicator of the presence and extent of metabolic acidosis.16,17 Metabolic acidosis is common in critically ill patients and is usually associated with higher morbidity and mortality.18 Therefore, the combination of OI and BE may be a good indicator to predict the outcome of neonatal ARDS with conventional therapy.
In addition, it was observed that a OI cut-off value of 30 and a BE cut-off value of −7.40 had the best predictive performance, with 77% sensitivity and 84% specificity. Historically, an OI of 40 in infants was associated with high mortality and was used to identify candidates for rescue therapies, such as ECMO. In fact, recent data suggest that mortality may increase significantly at lower OI levels.19 A previous study of 26 pediatric stem cell transplant recipients with respiratory failure found that an OI>20 was associated with 94% mortality, while an OI>25 had a 100% mortality rate.20 The present study demonstrated that ARDS neonates with OI>30 and BE<−7.4 are more likely to die during non-ECMO treatment and that other treatments, such as ECMO, should be considered.
In the present study, neonatal mortality of ARDS varied with sex and delivery modes. Female sex and vaginal delivery were risk factors for neonatal ARDS death during conventional treatment. Therefore, when OI>30 and BE<−7.4, ARDS newborns who are female or were vaginally delivered should receive special attention.
The present study has several limitations. Firstly, this study is a retrospective analysis, and some indicators (such as chest X-ray, lung ultrasound and blood culture results) could not be obtained, which resulted in the inability to include more non-invasive indicators in the statistical analysis. Secondly, although this was a multi-center study, the sample size was still limited. Thirdly, sicker subjects may have had more frequent OI measurements over the entire mechanical ventilation period, with less inherent bias, than those whose data was obtained over a fixed time frame.
ConclusionsThis study demonstrated that a combination of OI and BE can be used as a predictor of outcomes in neonates undergoing non-ECMO treatment for ARDS. In neonates with ARDS, if OI>30 and BE<−7.4, non-ECMO therapy is likely to lead to death, and other treatments such as ECMO should be considered.
Ethics approval and consent to participateThis study was approved by the ethics committee of the Army General Hospital (No. 2018-54).
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interestThe authors declare no conflicts of interest.