The aim of this study is to translate and validate the Nutritional Pathway for Infants with Congenital Heart Disease before Surgery British nutritional protocol into Brazilian Portuguese and test its clinical feasibility for specialized clinicians in Brazilian hospitals.
MethodThe translation and validation process followed strict methodological standards over the following steps: 1) initial translation; 2) synthesis; 3) back-translation; 4) expert committee content validation, and pre-test clinical feasibility with 30 health professionals. Data were collected through the Research Electronic Data Capture software data system, and then extracted and analyzed through statistical analysis software.
ResultsThe culturally adapted version was considered equivalent to the original. In the first round, 82% agreement was achieved, and after consensus, there was 100% agreement among the experts. Regarding the ease of use of the protocol in clinical practice, the instrument obtained a minimum agreement rate of 93.4% and a 0.92 content validity index.
ConclusionsThe results indicate that the instrument adapted to Brazilian Portuguese has high content validity, and high reliability among the experts, suggesting a high level of accuracy of the instrument and cultural adaptation for Brazilian culture and medical systems. It was easily understandable by health professionals, as well as simple to apply in clinical practice. The Nutritional protocol for preoperative infants with congenital heart disease can reproduce the outcomes found in the pilot of this instrument carried out in the United Kingdom, which may promote better pre-surgical nutritional status for infants with congenital heart disease in Brazil.
There is a wide variability of nutritional care practices for pre-surgical infants with congenital heart disease (CHD) in Brazil. This reflects the absence of scientific protocols and public health guidelines, which contributes to inadequate energy intake and malnourished preoperative profiles in this vulnerable population.1 It has been reported that malnourished pre-surgical infants with CHD are associated with higher post-surgery complications and mortality.2,3
Researchers from different countries have been formulating interventions with the objective of improving the nutritional status of infants with CHD.4 They differ in aspects such as the type of CHD,5 feeding route,6 breastfeeding recommendations,7 and macronutrients requirements.8 It has been shown that rehabilitation of malnourished children in the perioperative period using hypercaloric formulas is associated with better anthropometric parameters, shorter intensive care unit (ICU) stay, less mechanical ventilation time, and reduced use of antibiotics.8,9
Adequate nutrition and nutritional monitoring by a multidisciplinary team are essential to promote the physical growth and neurodevelopment of children with CHD, favoring recovery and clinical progress.10 Hence, the need for interventions from a multidisciplinary team, specialized in CHD, is evident to broaden access to care from early diagnosis (in-utero) to the post-surgical period.11
Despite the evidence of the benefits of providing a high-calorie diet to children with CHD,12,13 formulas of this type are not readily available for infants in the Brazilian healthcare system. To have access to high-calorie formulas for example, a request through the court system is required, which is not always approved, and is a lengthy process which prevents the intervention from occurring at the appropriate time for best postoperative and developmental outcomes.14
One of the great challenges of the Brazilian healthcare system is the lack of comprehensive care for children with CHD, among which is the lack of pre-surgical and follow-up nutritional protocols.15 To our knowledge, there are no validated nutritional instruments for the Brazilian population. The need to implement national guidelines focused on nutritional assistance for infants with CHD is dire, especially considering our nations’ mortality rates (neonatal mortality in Brazil accounts for 60 to 70% of all childhood deaths, with CHD accounting for 10% of all childhood deaths).15
A nutritional protocol was developed in the UK aiming to provide structured nutritional support, through a multi-disciplinary team, for infants with CHD prior to cardiovascular surgery.16 According to the pilot data of this protocol, weight maintenance, improved growth, and reduced ICU stay were observed post-intervention in 44 infants.17 The objective of this study is to carry out the translation, cross-cultural adaptation, content validation, and the clinical feasibility, through a clinical expert panel consensus, of the Nutritional Pathway for Infants with Congenital Heart Disease before Surgery (NPICHDBS). The Brazilian version of this protocol, called Protocolo Nutricional para Lactentes com Cardiopatia Congenita Pre-operatório (PRNCCP), aims to contribute to future clinical practice, national medical guidelines, and policy change.
MethodThis is a methodological study of the translation, cross-cultural adaptation, and content validation of the NPICHDBS.16 This study was approved by the ethics committees of two reference hospitals in Rio Grande do Sul, both among the largest pediatric cardiology centers in Brazil. Data were collected between November 2019 and June 2021.
The inclusion criteria for the process of Translation and Cross-Cultural Adaptation (TCA) expert committee included: bilingual health professionals, and PhD in language sciences or health fields with experience in pediatric CHD. Experts agreed to participate by signing an electronic informed consent (e-IC) and filling out the self-administered forms through a REDCap platform link18 sent by e-mail or messaging application. During the content validation and clinical feasibility stage, the inclusion criteria were health professionals with expertise in CHD from the fields of nutrition, pediatric cardiology, Speech-Language Therapy (SLT), and nursing.
The lead author of the original UK protocol authorized the translation and validation of the Brazilian protocol and became a collaborator and co-author of this study. The protocol contains 5 steps: I) nutritional risk assessment according to the type of heart disease (those with more than one type of congenital lesions or those with greater severity being at greater risk); II) monitoring of the infant growth through weight, length, and head circumference measurements; III) assessment of feeding skills; IV) assessment of food intake, whether adequate or not for weight gain; and V) determination of which nutritional care plan is appropriate according to all previous assessments, from plans A, B, or C (Appendix B).
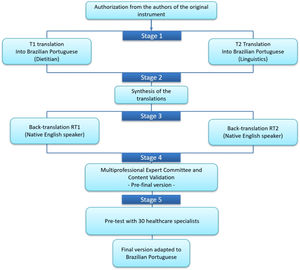
Translation, cross-cultural adaptation, content validation and the clinical feasibility processThe TCA process followed strict rules, and was based on the methodological model by Beaton et al. (2000), as shown in Figure 1, following 5 steps: (1) Initial Translation; (2) Synthesis; (3) Back Translation; (4) Expert Committee (EC); and (5) Pre-test with CHD Specialist Clinicians.19
Translation and Cross-Cultural Adaptation of the NPICHDBS.
Based on Beaton et al., 2000.19
The NPICHDBS was translated into Brazilian-Portuguese by two independent translators who were native speakers of the Brazilian-Portuguese language. The first translator (T1) is a Portuguese-English bilingual dietitian, specialized in CHD, with a Master's in Medical Sciences, and a PhD in nutrition and genetics, while the second translator (T2) was a bilingual PhD scholar in Language Sciences, specialized in Portuguese-English/English-Portuguese translation, who had no prior knowledge of the study's topic.
Step 2: SynthesisAfter discussing the two translations, T1, T2, and a licensed pediatric dietitian (author VPC), obtained the new version called T1.2 in a virtual meeting. As recommended in the literature,19 there must be a consensus between the two translated versions, and any discrepancies were eliminated, producing a single agreed-upon version by all three parties. Then, this version was submitted for back-translation.
Step 3: Back translationThis step was performed by two other professional translators (neither of them, a health professional). They were native speakers of English and did not receive the original document, in order to avoid information bias, as recommended in the literature. The back translations (BTs) produced were named BT1 and BT2.19
Step 4: Evaluation by the expert committee (EC)In this stage, the objective was to evaluate the T1.2 version produced, verifying equivalences of meaning, language, culturally appropriate practices, dietary habits and concepts between the NPICHDBS and the PRNCCP. A committee of 7 bilingual Portuguese-English experts included clinicians from pediatric cardiology, nutrition, SLT, pediatric neurodevelopment (all health experts in CHD, with BSc, MD, or PhD degrees), and a language studies professor. Of those, four experts had a PhD degree, one had an MD degree, and one expert had a postdoctoral degree. All committee members independently reviewed version T1.2, comparing each item side-by-side with the original British version, on a RedCap survey. They responded with a Likert scale to the level of their agreement with the content and translation of each item: 1) Totally Agree, 2) Partially Agree; 3) Indifferent (Neutral), 4) Disagree, and 5) Totally Disagree. Experts were instructed to provide suggestions or modifications, when scoring items as Neutral, Disagree, or Totally Disagree, and those items were reviewed and discussed by the EC in an online meeting.
Consensus was analyzed quantitatively prior to the meeting. Content validity index analyses were done to measure statistical agreement between experts. At the online meeting, the translated items that experts did not unanimously agree on were discussed (Suplementary material Appendix A). The discrepancies in their responses were discussed, and any disagreements were resolved and consolidated into the preliminary version of the protocol to be tested by CHD clinicians, as recommended in the literature, in stage 5 (Figuer 1).
Content validityFor content validation, the committee analyzed whether the nutritional and health concepts were properly represented, that is, whether there are semantic, idiomatic, conceptual, and experimental equivalences between the original version and the translated version.19
For cultural reasons, the authorsclassified items marked as Neutral as discordant, since Latin Americans tend to prefer taking impartial or neutral positions, over negative or divergent ones.20
Content validity was assessed in two ways: item-level content validity index (I-CVI) and the average content validity index of the instrument (Average CVI), see analysis section.
Step 5: Pre-testThis stage included the recruitment of 30 hospital-based CHD clinicians, according to Beaton's TCA model,19 with the eligibility criteria: 1-regionally local clinical specialists in the areas of cardio pediatrics, nutrition, SLT, and nursing; 2-with experience in CHD. Among the 30 professionals recruited, there were medical doctors (n = 5), nurses (n = 4), SLTs (n = 6), and dietitians (n = 15). All participants in the research signed the informed consent. After recruitment, participants received by email a link to a Redcap online survey. The clinical specialists answered a demographic questionnaire, and they were asked to read the translated protocol in order to assess their understanding and clarity of the final version of the translated 30-item instrument. All survey data were extracted from Redcap onto an excel spreadsheet. Frequency outputs were generated by the RedCap program.
This was a convenience sample of clinicians who were recruited by recommendation or through hospital directories from two reference medical centers in Southern Brazil. Clinicians had a mean of 11 years of experience. Their mean age was 36 years (Table 1).
Demographic characteristics of the Expert Committee sample (a); and the pretest clinical specialist sample (b).
SD= standard deviation.
An important aspect analyzed in the pre-test is the clinical feasibility of the translated and adapted version of the protocol to clinical practice. A Brazilian 5-question clinical feasibility questionnaire, by Coluci et al.,21 using a Likert scale (ranging from 1= “Totally Disagree” to 5 = “Totally agree”), was administered. Feasibility was measured by the percentage of expert responses of 4 (“partially agree”) and 5 (“Totally agree”) scores (see Table 3).
AnalysisData were initially collected on the REDCap platform, and then transferred and analyzed using the statistical program SPSS, v25.0, where descriptive statistical analyses were made for the characterization of the samples (stages 4 and 5), and clinical feasibility agreement (stage 5).
For stage 4, the content validity index (CVI) was calculated for each item (I-CVI) using the following formula: number of experts who agreed on the equivalence of each item, divided by the total number of experts. The calculation of the CVI of the instrument (Average CVI) was the average of all I-CVIs divided by the total number of items.22
ResultsThe characterization of the clinicians who participated in the EC, stage 4 is shown in Table 1(a), and pretest stage 5 sample characterization is shown in Table 1(b).
In stage 1, the suggested changes were related to: a) exchanging words for synonyms more applicable to the context where the instrument will be used (e.g. pathway was translated to guide and then finally agreed on protocol), b) inclusion of definitive articles (gender inclusive language of both gender forms in Portuguese language, o and a, e.g. o/a bebê); c) To avoid cacophony and remove a developmentally inaccurate term (The sentence “how does the infant eat and drink?” or “Como o bebê come e bebe?” in Portuguese, where the word bebe seemed inappropriate for an infant who is not independently drinking yet, was changed to “how does the infant feed?” or “Como o/a bebê se alimenta?”, which includes both how the infant eats and drinks).
In stage 2, the modifications suggested above were evaluated, where the clinicians selected terms and expressions according to professional practice and with the correction of the most appropriate verb tenses (e.g., verb "depend on" is conjugated in the future for the present indicative tense: “As necessidades nutricionais de bebês com cardiopatia congênita dependem do tipo de lesão cardíaca”).
In stage 3, the BT1 and BT2 versions were observed to be very similar, and when differences were found in the BTs, these were synonymous terms (e.g., pre-surgery and before surgery). In stage 4, the EC requested the modification of the writing of the name of some CHDs to improve understanding in Brazilian-Portuguese (e.g., Patent Ductus Arteriosus: “Ducto Arterioso Patente” was changed to Patent Arterial Chanel: “Canal Arterial Patente”). Conceptual adaptations were also made so that the words matched the originally proposed terms (e.g., flat or falling growth curve: “Curva plana ou em queda” was changed to flat or descending growth curve: “Curva de crescimento plana ou descendente”). Finally, cultural adaptations according to Brazilian socioeconomic and dietary habits were made (e.g., peanut butter, rarely found in Brazilian supermarkets, was replaced by vegetable oil). The justifications for cultural and language adaptations by the expert committee is shown in Appendix A.
In stage 4, after the first evaluation by the EC, a new round of evaluation was carried out, with the objective of confirming the content validity of the items with a CVI less than or equal to 0.86. These items were discussed by the EC and adaptations were made to rectify any disagreements. The new evaluation reached a CVI of 1 in each item of the instrument. Thus, the CVI of the instrument (CVI/total Average) as a whole was 1, demonstrating a content validity considered by the literature as excellent22 (Table 2).
CVI of the items and the instrument in the first and second EC evaluation.
CVI/Ave: average content validity index of the instrument.
In stage 5, we obtained some suggestions from specialists, but after consulting the experts, as those suggestions did not significantly change the items’ meaning or clarity, new changes were not included at this stage. Thus, the version evaluated in the pre-test stage 5 was the final version of the instrument (Suplementary material Appendix B). Data related to the instrument's feasibility are presented in Table 3.
Feasibility of the Brazilian version of the British Nutritional Protocol, and Content Validity Index.
CVI/Ave: Average I-CVI across items.
The process of TCA and content validation of the PRNCCP protocol was carried out in meticulously through a methodological procedure widely used in the health field,19 in order to sustain the quality and content equivalence of the original British version. Despite being an extensive and expensive process, it is currently widely used and considered the best way to achieve metric equivalence in instrument translation and validation. The differences in the origin and structure of the two languages required careful translations and adaptations to maintain the quality and validity of the translated instrument.
Among the psychometric properties of instruments used in health research, practicability and content validity are critical. We emphasize that this instrument will be used by health professionals, therefore their validation that the protocol is easy to understand and use, demonstrates an excellent clinical feasibility, and face validity of this instrument. Regarding content validity, the calculation of the CVI, through the consensus of an EC, is a widely used method in the literature, which indicates that the newly validated instrument has good content and essentially criterion validity.22
The cultural adaptations allowed the instrument to be adapted according to the guidelines of the Food Guide for Children under 2 years of the Brazilian Ministry of Health,23 making the protocol more applicable and to improve the external validity of the instrument for the Brazilian population.
Pre-surgical nutritional interventions for infants with CHD can improve nutritional needs, food tolerance, weaning from parenteral nutrition, and reduce the duration of mechanical ventilation, hospitalization, and surgical scarring.24 In addition, better nutritional status can reduce the probability of death.2
Pre- and postoperative multidisciplinary interventions and follow-up are extremely important for the outcome of cardiac surgeries in children with CHD and the reduction of neurodevelopmental sequelae.24 This protocol will help Brazilian pediatric cardiothoracic surgical centers to adopt a standardized pre-surgical nutritional intervention that provides better preparation for infants with CHD, who are highly malnourished and have stunted growth.17 This protocol can also reduce hospital costs, infant mortality and morbidity rates, as well as the burden on our public health system.
Clinical relevanceStage 5 results indicate that the PRNCCP is easily understood and considered as a viable clinical tool by Brazilian health specialists. This study shows that this protocol has clinical relevance and is appropriate to be implemented clinically in Brazil.
It is believed that the use of the PRNCCP, which involves a multi-step prescription of multi-disciplinary (e.g. cardiologist, dietitian, and SLT) specialist monitoring, and hypercaloric formula intake (see Appendix B) can reproduce the outcomes found in the British pilot data.17 However, considering that in Brazil we have a greater nutritional deficit in infants than in the UK,17,25 we predict that the benefits will be even wider in a low-income population, with limited access to nutritional and SLT expert care pre-surgery.26 In addition, we hope that the results can be used for the formulation of Brazilian guidelines of comprehensive nutritional pre-surgical care for children with CHD, for public policies related to access to hypercaloric formulas to low-income high-risk children.
Limitations of the studyThe long and meticulous process of translation and validation that required multiple specialized professionals brought on challenges (e.g. recruitment of local specialized experts, length of time to receive responses from busy clinicians, high costs of professional translators).
Additionally, the COVID-19 pandemic was a limiting factor, which prevented face-to-face data collection in hospitals in the pre-test stage 5. We adapted the recruitment using REDCap database to collect consent forms and send questionnaires electronically, which permitted experts to fill out the surveys at a convenient time.
However, the lack of personal interaction between the researchers and potential participants, may have decreased the likelihood of clinicians responding to online surveys. In addition, Brazilian research ethics legislation bans participation compensation for research subjects, and without incentives, busy clinicians may not have felt inclined to participate.
Thus, the type of online sampling used, due to the pandemic, did not allow us to determine the ratio of participant selection by discipline, which hindered our ability to control the number of professionals from each area.
Another limitation is that no other translation and validation of the original instrument has been done, which limits our ability to compare the results with other translations of this protocol. Finally, future studies should put into practice the PRNCCP in a systematic randomized clinical trial to confirm the clinical effectiveness of this protocol in Brazil.
It can be concluded that the translated and validated protocol version developed in this study was adequate for Brazilian-Portuguese based on four aspects of cross-cultural adaptation (semantic, idiomatic-cultural, conceptual, and experimental). High content, face, and criterion validity were observed through the different methodological and statistical techniques used in this study.