Necrotizing enterocolitis (NEC) is characterized by a rich infiltration of macrophages in the intestines, which is derived from monocytes in the blood. The authors aimed to explore the changing trend of absolute monocyte counts (AMC) over time in NEC infants and to verify whether the reduction of AMC correlates with the severity of NEC and whether it can be used to identify infants who need surgery.
MethodThe authors collected the clinical data of 66 control and 222 NEC infants. The NEC infants were divided into medical NEC (M-NEC) and surgical NEC (S-NEC). The counting of monocyte and their percentage change were compared at the time of birth, before NEC (baseline), the onset of NEC and after NEC (recovery). In addition, the same comparison was made among stages 1, 2 and 3 of Bell's staging, respectively.
ResultsThe authors found that the AMC in NEC infants decreased sharply at the onset. Further comparison was made between 172 cases of M-NEC and 50 cases of S-NEC. It was discovered that the AMC reduced more in S-NEC infants at onset, but it increased more at recovery. In addition, the authors found that among stage 1,2 and 3, stage 3 had the lowest AMC and the largest percentage decrease at the onset.
ConclusionThe AMC decreases sharply in NEC infants at onset, and the degree of decline is associated with the severity of NEC. AMC is expected to be a marker of NEC and provide a reference for clinicians in the diagnosis and treatment of NEC.
Necrotizing enterocolitis (NEC) is a serious pediatric gastrointestinal pathology, which is the leading factor of death from gastrointestinal disease in premature infants.1,2Most NEC cases occur in premature infants, especially in very low-birth-weight (VLBW) infants. The incidence of NEC reaches up to 13% in infants with a birth weight ≤ 2500 g or born at ≤ 33 weeks of gestation.3 The mortality rate of infants with NEC is between 20% and 30%, and the infants who need surgery have the highest mortality rate.2 NEC has a variety of clinical manifestations, including enteral intolerance with mild abdominal distension, or other non-specific manifestations that are common in neonatal intensive care unit (NICU). But in severe cases, it can be life-threatening, such as peritonitis, intestinal obstruction, intestinal perforation, and other serious systemic symptoms involving hypotension, shock, multiple organ dysfunction, and so on.
At present, Bell's staging4 is the most widely used classification system for NEC diagnosis, but heavily depends on clinicians and cannot be diagnosed early, resulting in frequent interruption of enteral feeding, which may increase the risk of NEC.2,3,5 Therefore, there is an urgent need for molecular markers for early diagnosis of NEC. Molecular markers that have been proposed include C-reactive protein, tumor necrosis factor, interleukin-6 (IL-6), IL-8, intestinal fatty acid-binding protein, liver fatty acid-binding protein, fecal calprotectin, trefoil factor 3 and Claudin-3.3,5 However, the diagnostic efficiency is low and some of them are not common in clinical work.
Different from adults, the intestinal injury of neonates with NEC is rich of macrophage infiltration,6-9 which has also been verified in the animal model.10 Intestinal macrophages which are derived from monocytes cannot proliferate, and their maintenance depends on the continuous recruitment and differentiation of blood monocytes.11 When intestinal inflammation occurs, blood monocytes, as the only source of intestinal monocyte-derived macrophages, need to migrate extensively to the gut.12,13 Meanwhile, in infants with NEC, the absolute monocyte counts (AMC) decreased in varying degrees,11,14,15 also associated with mortality in NEC.16 The activation of monocytes participates in the development of NEC,17,18 and blocking NF-κB activation in Ly6c+ monocytes alleviates NEC.11 However, the current studies did not discuss whether there is a difference in AMC between surgical NEC (S-NEC) and medical NEC (M-NEC).
Therefore, the authors hypothesized that after the occurrence of NEC, a large number of blood monocytes migrate to the gut and differentiate into macrophages, which leads to a severe inflammatory reaction. At the same time, the AMC decreases sharply, which may be associated with the severity of NEC. In order to verify this hypothesis, the authors compared AMC in the peripheral blood of NEC patients admitted to our center from July 2014 to June 2021 with matched controls excluding severe infectious and gastrointestinal diseases. The AMC differences between M-NEC and S-NEC and between different stages of NEC were further compared to verify whether AMC can be used as one of the effective predictors of the severity of NEC and the need for surgical treatment.
MethodsPatient enrollmentThis study was approved by the Ethics Boards of Shanghai Children's Hospital. Parents of the newborns understood the purpose of the study and provided informed consent. All the methods were performed in accordance with relevant guidelines and regulations, including the Declaration of Helsinki for human studies by the World Medical Association. The authors retrospectively collected medical records and banked peripheral blood samples from preterm patients diagnosed with NEC in Shanghai Children's Hospital from July 2014 to June 2021, including those who underwent medical and surgical treatment. Patients with one of the following conditions were excluded: full-term infants, intestinal stenosis confirmed by surgery or imaging, refusing surgery or treatment, or incomplete medical records. After the diagnosis of NEC, NEC infants who received medical treatment only were defined as M-NEC, and those who had indications for surgery and eventually underwent surgery were defined as S-NEC. Clinicians comprehensively judge the stage of NEC according to the clinical manifestations, imaging features and laboratory indicators of the infants. If NEC recurred during hospitalization, the first data was used. Patients in the control group matched the NEC infants for gestational age and birth weight, without severe inflammation or associated gastrointestinal anomalies and other diseases that affect AMC such as severe viral infection and hematological malignancy.
Patient demographics and data collectionDemographic characteristics included gestational age, birth weight, gender, delivery mode (vaginal delivery or cesarean section), Apgar score, outcome and length of stay. Other information including patent ductus arteriosus (PDA), patent foramen ovale (PFO), atrial septal defect (ASD) ventricular septal defect (VSD), sepsis and transfusion (before the occurrence of NEC) also has been collected. The AMC at the time of birth, before NEC (baseline), the onset of NEC, and after NEC (recovery) were separately collected (Supplementary Fig. 1). The baseline was defined as the last available peripheral blood AMC before NEC onset. Recovery was defined as the first AMC after restarting feeding. The percentage change of AMC was calculated by subtracting the baseline AMC from the onset AMC and dividing by the baseline AMC. Control AMC was matched and collected according to the number of days from birth at each time point in the NEC group.
Statistical analysisStatistical analysis was performed using SPSS software version 23 (IBM SPSS Statistics, Armonk, NY) and graphed with GraphPad Prism version 7 (GraphPad Software, San Diego, CA). Quantitative variables were described by median (interquartile ranges, IQRs) and visualized as box-and-whisker plots, and compared by Mann-Whitney U. Qualitative variables were described by frequency (percentage), and compared by Chi-square Tests. All statistical tests were two-sided, and P<0.05 was considered a significant difference.
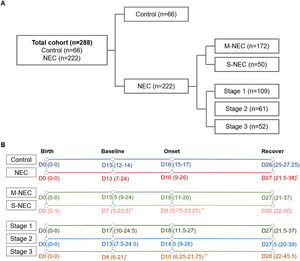
ResultsA total of 308 infants with NEC treated at Shanghai Children's Hospital from July 2014 to June 2021 were included in the study. The authors excluded 13 full-term infants, 34 cases of intestinal stenosis, 12 cases of refusing surgery or treatment, and 27 cases of incomplete clinical records. Finally, a total of 222 premature infants with NEC were enrolled, including 172 cases of M-NEC and 50 cases of S-NEC. And 66 infants matched for birth weight and gestational age of NEC infants were enrolled as control (Figure 1A). Compared with the control group (length of hospital stay: median 31, IQR 26–44.25 days; mortality: 0%), NEC infants had a longer length of hospital stay (median 47.5, IQR 33–67.25 days, p<0.001) and higher mortality (8.1%, p = 0.036). Other demographic characteristics, including gestational age, birth weight, gender, mode of delivery, Apgar scores, congenital heart diseases, sepsis, and transfusions were not significantly different (Table 1).
Patient demographics and clinical characteristics.
The authors collected the AMC of the NEC infants at each time point, including birth, baseline, onset, and recovery. Control AMC was matched and collected according to the number of days from birth at each time point in the NEC group. For birth AMC, the most rapid blood was collected from a patient 1 hour after birth, and the slowest sample was collected 17 h after birth in control infants. In NEC infants, the most rapid blood was collected 0.5 h after birth, and the slowest sample was collected 63.5 h after birth. Among all NEC cases, AMC was available for 173 cases at birth, 182 cases at baseline, 201 cases at onset, and 201 cases at recovery. The recovery days were later in the NEC cases (median 27, IQR 21.25–38 days) than in the controls (median 26, IQR 25–27.25 days, p = 0.044). Within the NEC group, baseline and onset were earlier in S-NEC (baseline: median 7, IQR 5–23.5 days; onset: median 9, IQR 5.75–23.25 days) than in M-NEC (Baseline: median 15.5, IQR 9–24 days, p = 0.008; onset: median 18, IQR 11–26 days, p = 0.001). Similarly, infants with stage 3 (baseline: 8, 6–21; onset: 10, 6.25–21.75) had earlier baseline and onset than infants with stage 1 (baseline: median 17, IQR 10–24.5 days, p = 0.017; onset: median 18, IQR 11.5–27days, p = 0.007) (Figure 1B). The medians and IQRs of the time lag for each group were shown in Supplementary Table 1 and Supplementary Table 2.
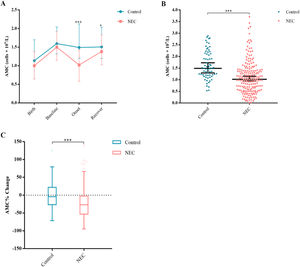
A drop in AMC differentiates NEC from controlsFirst, the authors compared AMC between NEC infants and control infants at each point of time and found that the AMC of NEC infants (median 1.02 × 109/L, IQR 0.58–1.43) was significantly lower than those of the controls at onset (1.49 × 109/L, 1.1975–2.125, p<0.001) (Figure 2A, B). The AMC of the infants with NEC (1.38 × 109/L, 1.025–1.83) gradually recovered, but was still lower than that of the controls (1.505 × 109/L, 1.1925–2.035, p = 0.042) at recovery (Figure 2A). There was no significant difference between the two groups in AMC levels at birth and at baseline. The authors further calculated the percentage change of AMC and found that the decrease of AMC percentage in NEC infants (−26.96%, −53.15 to −2.65) was much greater than that in the controls (−4.26%, −26.87 to 22.03, p<0.001) at onset (Figure 2C).
Changes in AMC for controls and NEC infants. Median AMCs are plotted at birth, baseline, onset and recover (A). Scatter dot plot for AMCs at onset (B). Tukey boxplots for percent changes in monocytes (C) at onset compared with baseline. Bars represent 25 and 75 percentiles (A) or 1.5× the interquartile ranges (IQR) (C). *Significance: *p < 0.05, **p < 0.01, ***p < 0.001. ° Indicates outliers and x indicates extreme outliers (>1.5 × IQR).
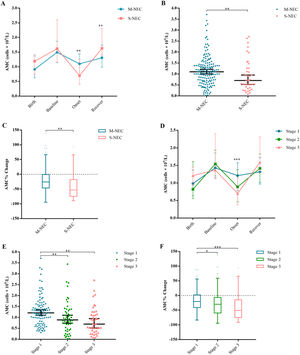
Next, the authors divided NEC infants into S-NEC and M-NEC according to whether they received surgery during the treatment, and compared the AMC level and the percentage change of AMC respectively. At the onset, the AMC of S-NEC infants (0.7 × 109/L, 0.4–1.26) was significantly lower than that of M-NEC infants (1.1 × 109/L, 0.69–1.44, p = 0.005) (Figure 3A, B). Furthermore, the AMC of S-NEC infants (−53.18%, −74.98 to 17.70, p = 0.005) decreased by a greater percentage at onset (Figure 3C). On the contrary, at recovery, the AMC of S-NEC infants (1.63 × 109/L, 1.405–2.31) was higher than that of M-NEC infants (1.32 × 109/L, 0.985–1.74, p = 0.003) (Figure 3A).
Changes in AMC for NEC infants. Median AMCs are plotted at birth, baseline, onset and recover for S-NEC and M-NEC (A) or for different NEC stages (D). Scatter dot plot for AMCs at onset for S-NEC and M-NEC (B) or for different NEC stages (E). Tukey boxplots for percent changes in monocytes for S-NEC and M-NEC (C) or for different NEC stages (F) at onset compared with baseline. Bars represent 25 and 75 percentiles (A, D) or 1.5× the interquartile ranges (IQR) (C, F). *Significance: *p < 0.05, **p < 0.01, ***p < 0.001. ◦ Indicates outliers and x indicates extreme outliers (>1.5 × IQR).
Clinicians classified NEC infants into stages 1, 2 and 3 according to Bell's staging. AMC and its percentage change were compared among different stages. The authors found that AMC in both stage 2 (0.89 × 109/L, 0.4625–1.3775, p = 0.007) and stage 3 (0.7 × 109/L, 0.38–1.1425, p<0.001) were significantly lower than those in stage 1 (1.21 × 109/L, 0.78–1.575) at onset (Figure 3D, E), and the decrease of AMC percentage in stage 2 (−29.70%, −59.46 to −6.73, p = 0.010) and stage 3 (−50.77%, −75.32 to −14.74, p<0.001) was also greater than that in stage 1 (−20.26%, −40.68 to 2.24) (Figure 3F). Although there was no significant difference in AMC among different Bell's stages at recovery, the median of AMC followed a stepwise progression from stage 1 to stage 3 (Figure 3D).
DiscussionAs a serious gastrointestinal disease in children, NEC is one of the important factors leading to the death from gastrointestinal disease in premature infants.1,2 However, at present, the diagnosis depends more on the clinical experience of the clinician, unless the infant has obvious surgical indications, the decision to operate sometimes is at the discretion of pediatric surgeons. The authors hope that more objective indicators can be put forward to provide a reference for clinicians’ diagnosis and treatment. The authors compared the AMC and its percentage change at different time points between the control group and NEC group, M-NEC and S-NEC, and different NEC stages. It was found that the AMC decreased significantly in NEC infants at onset, and it could reflect the severity of NEC and related to whether the infant needed surgery or not.
AMC is correlated with gestational age and days of birth. The mean value for AMC increased linearly between 22 and 42 weeks of gestation and showed a trend of first increase and then reduction within one month after birth.19 Studies have shown that the normal reference range of monocytes on the day of birth of full-term infants is 0.3–3.3 × 109/L.19 Monocytosis is associated with lower mean birth weight and gestational age, leukocytosis, multiple transfusions, albumin transfusions, theophylline therapy20 and intrauterine infections such as candidiasis and syphilis.21,22 Interestingly, the decline of monocytes may be a unique feature of NEC.
At present, there is no specific normal reference range of AMC for newborns with different gestational ages. Premature birth is a relatively clear pathogenic factor of NEC, and most NEC cases occur in premature infants,1,2 so the authors focus on the changes of AMC in preterm NEC. To be more comparable, the authors matched the gestational age and birthdays of the control group and the NEC group to eliminate their influence on the AMC level as much as possible. In order to reflect the change process of AMC more comprehensively, the authors selected four-time points, including birth, baseline, onset, and recovery. The authors found that at birth and baseline, there was no difference in AMC between the NEC group and the control group. At the onset, the AMC of NEC infants reduced sharply, which is consistent with the conclusion of Remon et al.15 This phenomenon may be due to the large number of monocytes from peripheral blood being recruited to the intestinal lamina propria, and also echoes the pathology of NEC which is rich in macrophages.6-9 After the recovery of NEC, the AMC was also restored, but it was still lower than that of the control group with the same birth days.
The authors also made a comparison between S-NEC and M-NEC. At the onset, the AMC of S-NEC was lower than that of M-NEC, and its percentage decreased more dramatically. This finding suggests that NEC infants with lower AMC and a larger percentage of AMC decline may need more and closer attention from clinicians and are more likely to need surgery. The authors also found that after recovery, AMC in S-NEC infants was higher than that in M-NEC infants, possibly because lower AMC at onset stimulated the marrow more, causing the marrow to produce more monocytes to replenish the peripheral blood. Next, the authors also found that the more severe the NEC stage, the lower AMC and the greater the reduction of AMC at the onset. In the present study, there were statistical differences between stage 1 and stage 2, stage 1 and stage 3, but there was no statistical difference between stage 2 and stage 3, which was different from the results of Desiraju et al.23 and Tajalli et al.14 At recovery, AMC was highest in stage 3, followed by stage 2, and lowest in stage 1, although there was no statistical difference.
The authors speculate that after the occurrence of NEC, a large number of monocytes in peripheral blood are recruited to the intestinal lamina propria and transformed into macrophages, resulting in a reduction in the number of monocytes in peripheral blood. In newborn mice, embryo-derived macrophages with proliferative activity account for the main part of intestinal macrophages. At 2–3 weeks of age, monocyte-derived macrophages replace most embryo-derived macrophages.24 However, some studies have shown that intestinal macrophages have no proliferative activity, and their supplements all depend on peripheral blood monocytes.12 It is clear that when inflammation occurs, intestinal macrophages usually originate from the recruitment and differentiation of monocytes in the blood.11 However, the circulating monocyte pool of premature infants is restricted,19 and the marrow lacks a large number of mature monocytes,25 which leads to the lack of timely replenishment of monocytes in peripheral blood. And the more serious the inflammation, the more the number of macrophages infiltrated in the intestinal lamina propria, and the lower the monocytes in the peripheral blood.
As a common laboratory index, AMC has the characteristics of being easy to obtain, low cost and strong consistency. It can provide an objective and specific reference basis for clinicians to diagnose NEC and is expected to become a new marker for the diagnosis of NEC. This will greatly help clinicians to identify NEC infants and indicate the need for surgery. However, this study has some limitations. This study is a retrospective study, some cases lack AMC data at certain time points, which may cause certain bias. And this study is a single-center study, the number of M-NEC infants and stage 1 infants is more. In the future, the authors hope to carry out a prospective, multicenter large-scale study to further investigate whether AMC can become a new marker of NEC. And improve diagnostic efficiency by combining other laboratory indicators and clinical findings.
In conclusion, the authors confirmed the phenomenon of decreased AMC in NEC infants at onset, and the AMC declined even more in those who needed surgery. In addition, the more severe the NEC, the lower AMC. AMC is expected to become a new marker of NEC, and can reflect the severity of NEC, and provide more guidance for clinicians.