To evaluate current practices of tracheostomy in children regarding the ideal timing of tracheostomy placement, complications, indications, mortality, and success in decannulation.
Source of dataThe authors searched PubMed, Embase, Cochrane Library, Google Scholar, and complemented by manual search. The guidelines of PRISMA and MOOSE were applied. The quality of the included studies was evaluated with the Newcastle-Ottawa Scale. Information extracted included patients’ characteristics, outcomes, time to tracheostomy, and associated complications. Odds ratios (ORs) with 95% CIs were computed using the Mantel-Haenszel method.
Synthesis of dataSixty-six articles were included in the qualitative analysis, and 8 were included in the meta-analysis about timing for tracheostomy placement. The risk ratio for “death in hospital outcome” did not show any benefit from performing a tracheostomy before or after 14 days of mechanical ventilation (p = 0.49). The early tracheostomy before 14 days had a great impact on the days of mechanical ventilation (-26 days in mean difference, p < 0.00001). The authors also found a great reduction in hospital length of stay (-31.4 days, p < 0.008). For the days in PICU, the mean reduction was of 14.7 days (p < 0.007).
ConclusionsThe meta-analysis suggests that tracheostomy performed in the first 14 days of ventilation can reduce the time spent on the ventilator, and the length of stay in the hospital, with no effect on mortality. The decision to perform a tracheostomy early or late may be more dependent on the baseline disease than on the time spent on ventilation .
As pediatric intensive care continues to improve and children with complex medical conditions survive longer, tracheostomy has been a frequent and necessary procedure for this complex cohort of patients who depend on medical technology for long-term survival. Besides, there has not been a standardization in the quality of care for the procedure.1,2 Pediatric tracheostomies can be associated with increased hospital usage, complications, and mortality.1–3 Great variations are reported on complication and mortality rates, probably due to inconsistencies in the methods, small sample sizes, and studies limited to single institutions.3 Tracheostomy indications have changed in time: prolonged intubations replaced acute airway obstructions as the main indications.4
In adults, randomized controlled studies have shown moderate-quality evidence from lower mortality rates in the early versus late tracheostomy in mechanically ventilated patients.5 The optimal time to tracheostomy in adults is considered as 7 to 4 days of mechanical ventilation, but there are no similar recommendations in children.6
This review aimed to comprehensively summarize the general state of knowledge of current tracheostomy practices in children, indications, expected complications, success in decannulation, and related mortality. As a secondary objective, to look for evidence about the ideal moment to indicate tracheostomy placement in mechanically ventilated children.
Materials and methodsThe authors conducted a systematic search for studies according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,7 using PubMed, Embase, Google Scholar, and Cochrane Library from inception to January 2021. The only used filter was age (child to 18 years old and > 29 days). The review was registered at PROSPERO (CRD42021247211). The authors devised the study question using the PICO (Population, Intervention, Comparison, Outcome) framework: for pediatric patients on prolonged mechanical ventilation (P) who perform early tracheostomy (I), compared with patients who perform late tracheostomy (C), what are current practices and outcomes (O)? The search strategy included the terms “tracheostomy,” “tracheostomy indications,” “tracheostomy complications,” “decannulation.” The authors supplemented electronic search with a manual review of reference lists from eligible publications and relevant reviews. The complete search strategy is in the Supplemental Digital Content.
Eligibility criteriaThe authors of the present study included studies reporting tracheostomy practices in children. Exclusion criteria were the absence of information about indications, complications, or time for tracheostomy (“wrong outcome”); the inclusion of adults and newborns (“wrong population”); reviews, case and series reports, surveys, and abstracts (“wrong design”); The authors also excluded studies with inconclusive results, serious methodological flaws, or which did not bring any relevant or new information (“not relevant”).
Data collection processTwo investigators (R.T.A and F.R.O.C) independently conducted the initial search, which comprised an initial screening of title and abstracts for eligibility. If the studies were potentially relevant, the full text was read for possible inclusion and stored in Rayyan, a website for systematic reviews.8 In cases of disagreement, the other investigators were consulted to determine eligibility. All four investigators approved the final selection of studies, using the Appraisal of Guidelines for Research and Evaluation (AGREE)-II instrument.9 A final decision by the evaluator was to recommend or do not recommend the study, according to MOOSE reporting guidelines.10
For the extracted categorical data, the authors calculated the odds ratios or risk differences, with a 95% confidence interval (95% CI), using the Mantel-Haenszel method. For continuous variables, means and standard deviations were used, with a calculation of the weighted mean difference. When unavailable in the papers, the means and standard deviations were estimated from the values of medians and interquartile ranges, using the equations of Wan et al.11 The meta-analysis was performed using the Review Manager (RevMan) 5.4 software (The Cochrane Collaboration, Copenhagen, 2020).
Assessment of qualityThe authors used the Newcastle-Ottawa Scale (NOS) to evaluate the quality of the most relevant studies, as all of them were retrospective or observational cohorts. The NOS evaluates three quality parameters (selection, comparability, and outcome) divided across 8 specific items. Each item on the scale is scored from one point, except for comparability, which can score up to two points. The maximum score for each study is 9, with studies having less than 5 points being identified as representing a high risk of bias.12
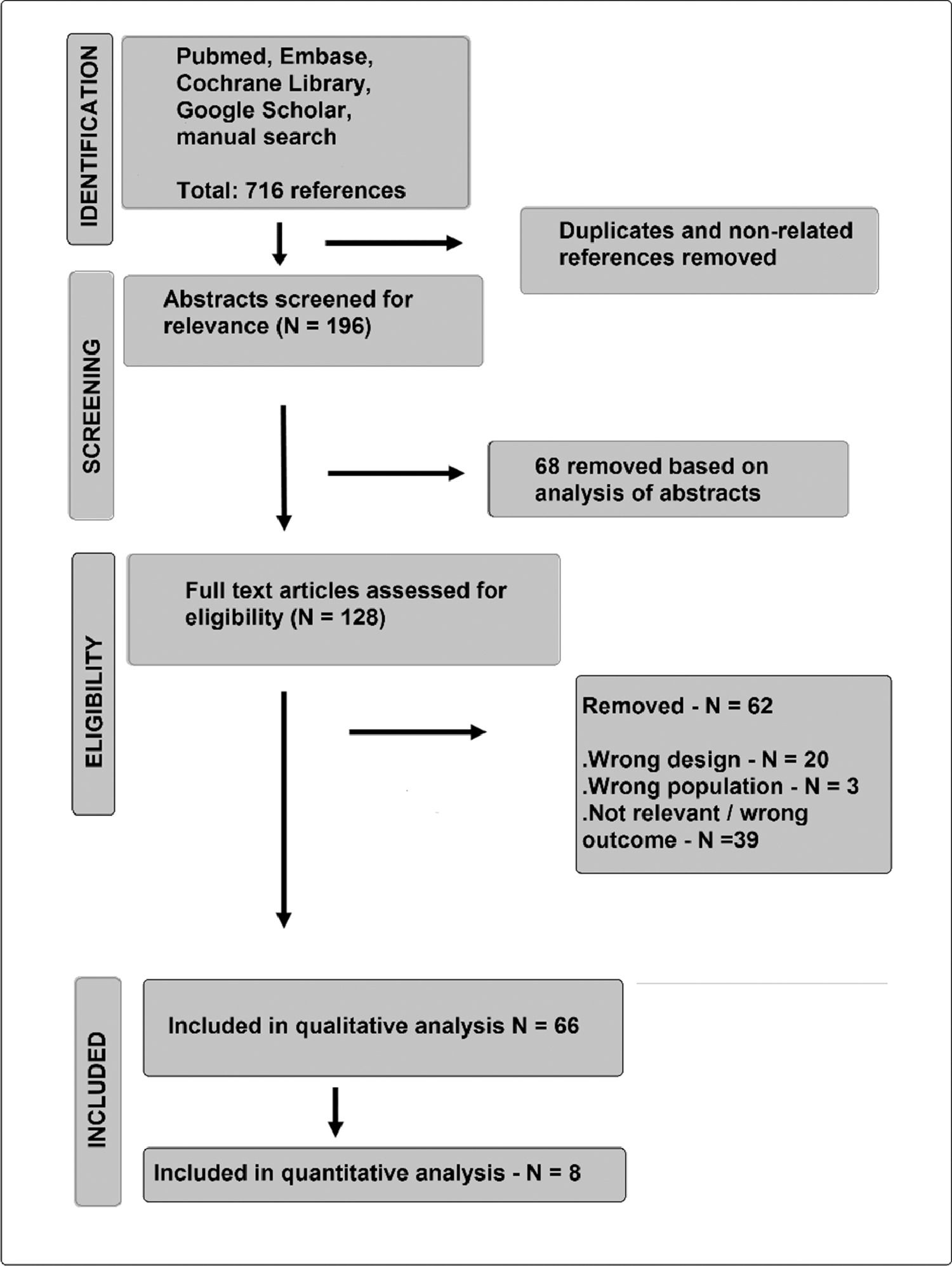
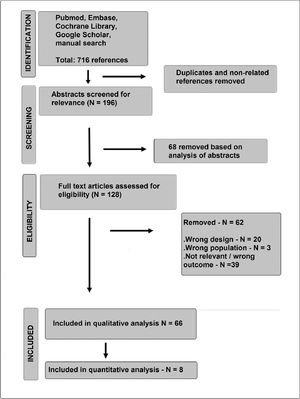
ResultsSearch resultsFrom 128 full-text articles assessed for eligibility, 66 articles were qualitatively analyzed (Supplemental File 1). Eight of them were included in the quantitative evaluation (meta-analysis) of the time for indicating tracheostomy. The iterative process flowchart of selecting articles is illustrated in Fig. 1.
Tracheostomy in pediatric patients – indicationsAn upward trend in the prevalence of tracheostomy, primarily associated with an increase in tracheostomized children receiving invasive ventilation has been observed.13 Prolonged mechanical ventilation was the most frequent condition leading to tracheostomy in various retrospective studies, followed by upper airway obstruction and inadequate airway protection.14–24 The authors summarized the indications listed within these three categories in Table 1.
The indications for a tracheostomy, as listed by authors in three principal categories (Prolonged mechanical ventilation, upper airway obstruction, and inadequate airway protection).
| Principal indications of tracheostomy | Proportions |
|---|---|
| Prolonged mechanical ventilation | 30%,14 27%,15 57%,16 62%,17 26%,18 27%,20 87%22 |
| Obstructions of upper airway | 70%,14 43%,15 25%,16 20%,19 55%,20 23.6%23 |
| |
| Inadequate airway protection | 37%,16 25%20 |
A shift in the indications of pediatric tracheostomy, from upper airway obstruction and infection to an increasing number of procedures performed for prolonged ventilation and chronic diseases, has been reported in various studies since the years 1980.25–31 A reduction in the proportion of PICU patients being tracheostomized was reported in the United Kingdom: The percentage of tracheostomies related to admissions was 2.4 in 2003, being reduced to 0.97 in 2017.30 In a large study from the USA, Edwards et al. reported that the majority of the children with a tracheostomy had complex chronic conditions that presumably led or contributed to their airway compromise or chronic respiratory failure.32 Neurological deficit is the most frequent comorbidity reported, with a prevalence of 33% to 41%.33–36
Complications and outcomesEarly and late complications were reported at rates varying from 5% to 46.9%. Accidental decannulation, stoma infection, bleeding, subcutaneous emphysema, granulation, tracheocutaneous fistula, fistula to the innominate artery, tearing of posterior tracheal wall, subglottic stenosis, pneumothorax, fatal or nearly-fatal tube obstruction, and pneumonia are among the complications cited.14,17,18,22,31,37,38 Deaths directly related to tracheostomy are rarely reported, ranging from 0.6 to 14.4%, due to massive bleeding 21 and cannula obstruction.17,23,39,40 Underlying cardiopulmonary disease and premature birth are factors associated with increased mortality in pediatric tracheostomy patients under 2 years of age.41,42
Decannulation and hospital dischargeMost children with tracheostomy and positive-pressure ventilation at home survive beyond five years.43 Chia et al. report successful decannulation in 39% of these patients at a median of 408 days after tracheostomy.44 In a recent prospective, observational study, Chauhan et al. show an impressive rate of 91% of successful decannulation in a sample of 67 children, but the study excluded congenital syndromes.45 In the series of Mahadevan et al., 75% of patients have been successfully decannulated, on an average of 40 months following tracheostomy, but 6.5% required recannulation.13 Other authors reported rates of successful decannulation varying from 12.6% to 77.8%, after median times from 123 days to 38 months.14,16,19,22,32,37,38
In children requiring surgery for congenital heart disease and a tracheostomy, hospital discharge rates are as low as 50%, in those patients with pulmonary artery shunts, hypoplastic left heart syndrome, and coexisting genetic syndromes.46–49
In neurologically impaired children, Tsuboi et al. reported that rates of successful decannulation within one year and five years were 4% and 17% respectively, compared to 20% and 54% in children without neurological impairment.50 Patients with congenital neurological diseases were also significantly less likely to be decannulated compared with any other indication in the McPherson et al. study.33
For respiratory conditions, when a tracheostomy was indicated for bronchopulmonary dysplasia, successful decannulation could be achieved in 50% of the children.51 Failed decannulation when tracheostomy was indicated for upper airway obstruction was reported to be 20.3%, in the Canning et al. series,52 and in 36.4% in Funamura et al. The latter also reported a high rate of successful decannulation in tracheostomies due to maxillofacial/laryngotracheal trauma.53
Decannulation protocolsIn the protocol described by Bandyopadhyay et al., if the patients were eligible for decannulation and bronchoscopy revealed no airway obstruction, a decannulation challenge was conducted. The ostomy was covered by an occlusive dressing and respiratory parameters were measured awake and asleep during the day and overnight by polysomnography (PSG). Re-cannulation was performed if the study revealed significant airway obstruction (Apnea-Hypopnea Index >10 events/hour), hypoventilation with expired CO2 above 45 torrs for 20% of total sleep time, respiratory distress or prolonged oxygen desaturation. This protocol did not involve the downsizing of a tracheostomy tube or capping trials. The authors report a rate failure of 22.2% at the first attempt. Genetic abnormalities, feeding dysfunction, presence of comorbidities, and decannulation based on the parental expectation of success, rather than medically determining readiness, was associated with a higher chance of failure.54
Beaton et al. also started their protocol with an assessment of the patency of the upper airway through microlaryngoscopy and bronchoscopy. On the first day of the decannulation trial, the tracheostomy tube was downsized to one of a much smaller diameter, as size 3.0. If the child remained stable in the following 24 h with the smaller tube, on day 2 the tracheostomy tube was capped off. During the 24 h when the tube was capped off the child undergone overnight transcutaneous oxygen and carbon dioxide monitoring. If the child continues to be well and overnight monitoring was satisfactory, the tube was removed and the stoma occluded with waterproof adhesive tape. This procedure was successful in 58% of the patients. The commonest causes of failure were suprastomal malacia, adenotonsillar hypertrophy, and chest infection.55
Polysomnography (PSG) has been included in some protocols for decannulation. Parameters of PSG like the apnea-hypopnea index, percentage of total sleep time with oxygen saturation levels less than 90%, and the lowest oxygen saturation levels were predictors of successful decannulation.56–58 Surveillance direct laryngoscopy and bronchoscopy, performed periodically in children with long-term tracheostomies, can diagnose airway lesions that require intervention for airway optimization, such as debridement of granulation tissue, tracheostomy tube exchange, and subglottic dilation.59–61
Discharge to the home of pediatric patients dependent on tracheostomy and/or chronic mechanical ventilation has been proven to be an arduous and time and resources consuming task, and the failure rate is substantial (27%).62
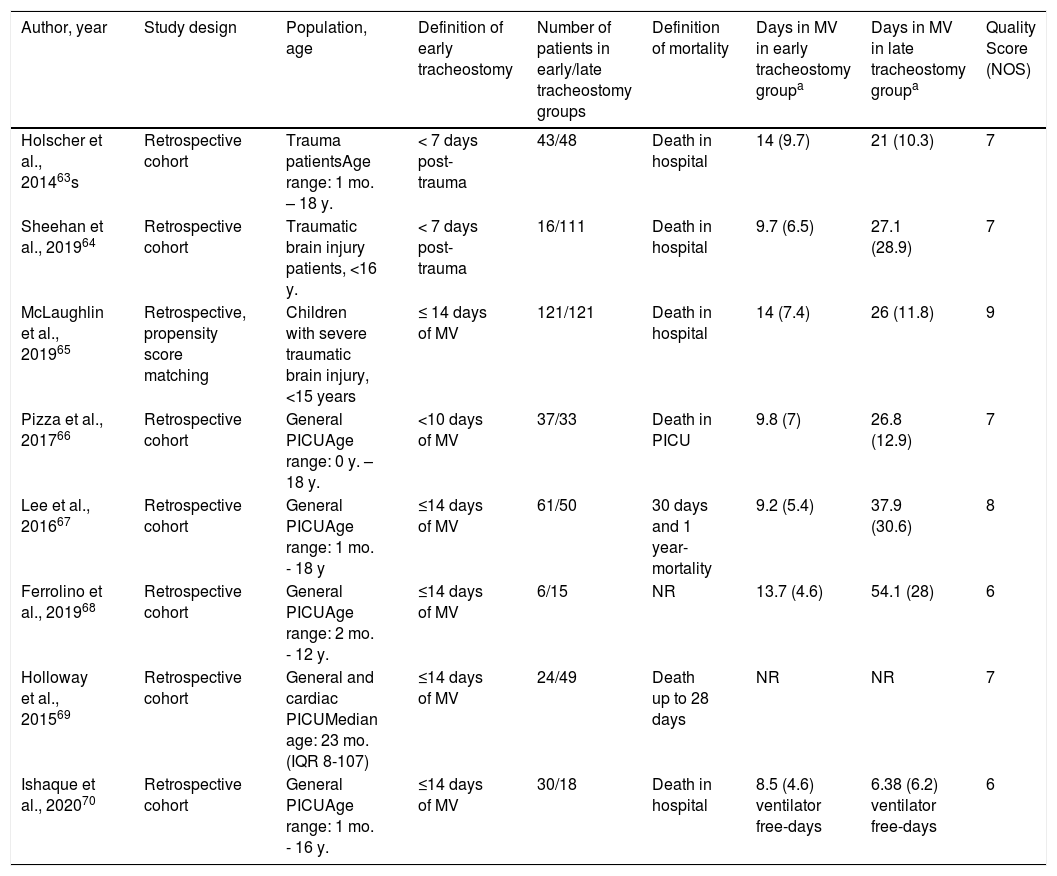
Timing of tracheostomy in pediatric patients on mechanical ventilationThe search did not return any randomized trials. The authors found eight studies63–70 that compared the prognoses of patients undergoing “early” or “late” tracheostomy: in five, “early tracheostomy” was defined as being performed before 14 days of mechanical ventilation, and “late,” after this period. In two studies, “early” was defined as before seven days post-trauma injury, and, in one, as before ten days of ventilation (Table 2). The mean NOS score of these eight studies was 7.3, ranging from 6 (two studies) to 9 (one study).
- A
The effect of early tracheostomy on in-hospital mortality of mechanically ventilated children
Characteristics of the studies isncluded for comparisons between early and late tracheostomy in children.
| Author, year | Study design | Population, age | Definition of early tracheostomy | Number of patients in early/late tracheostomy groups | Definition of mortality | Days in MV in early tracheostomy groupa | Days in MV in late tracheostomy groupa | Quality Score (NOS) |
|---|---|---|---|---|---|---|---|---|
| Holscher et al., 201463s | Retrospective cohort | Trauma patientsAge range: 1 mo. – 18 y. | < 7 days post-trauma | 43/48 | Death in hospital | 14 (9.7) | 21 (10.3) | 7 |
| Sheehan et al., 201964 | Retrospective cohort | Traumatic brain injury patients, <16 y. | < 7 days post-trauma | 16/111 | Death in hospital | 9.7 (6.5) | 27.1 (28.9) | 7 |
| McLaughlin et al., 201965 | Retrospective, propensity score matching | Children with severe traumatic brain injury, <15 years | ≤ 14 days of MV | 121/121 | Death in hospital | 14 (7.4) | 26 (11.8) | 9 |
| Pizza et al., 201766 | Retrospective cohort | General PICUAge range: 0 y. – 18 y. | <10 days of MV | 37/33 | Death in PICU | 9.8 (7) | 26.8 (12.9) | 7 |
| Lee et al., 201667 | Retrospective cohort | General PICUAge range: 1 mo. - 18 y | ≤14 days of MV | 61/50 | 30 days and 1 year-mortality | 9.2 (5.4) | 37.9 (30.6) | 8 |
| Ferrolino et al., 201968 | Retrospective cohort | General PICUAge range: 2 mo. - 12 y. | ≤14 days of MV | 6/15 | NR | 13.7 (4.6) | 54.1 (28) | 6 |
| Holloway et al., 201569 | Retrospective cohort | General and cardiac PICUMedian age: 23 mo. (IQR 8-107) | ≤14 days of MV | 24/49 | Death up to 28 days | NR | NR | 7 |
| Ishaque et al., 202070 | Retrospective cohort | General PICUAge range: 1 mo. - 16 y. | ≤14 days of MV | 30/18 | Death in hospital | 8.5 (4.6) ventilator free-days | 6.38 (6.2) ventilator free-days | 6 |
Days of mechanical ventilation are presented in means (standard deviations), estimated from medians and interquartile ranges using equations by Wan et al.,11 for all, except for Ferrolino et al. and Sheehan et al.
MV, Mechanical ventilation; NR, non-reported; NOS, Newcastle Otawa Score.
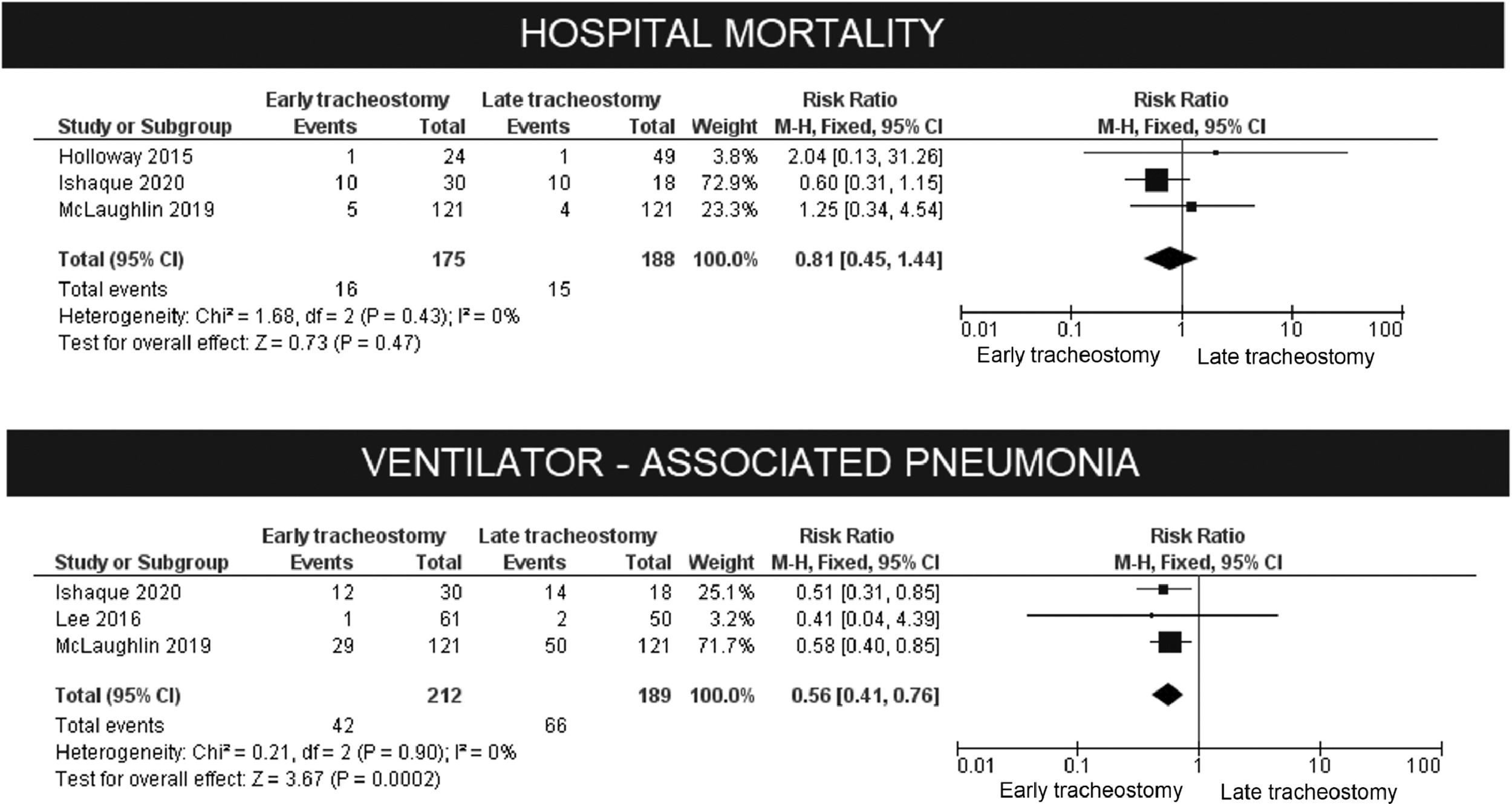
As shown in Table 2, the authors used different times to define mortality. We chose to meta-analyze only the studies that used the outcome "death in a hospital". The risk ratio did not show any benefit from performing a tracheostomy before or after 14 days of mechanical ventilation (p = 0.49), without heterogeneity.
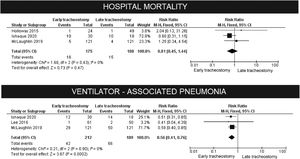
B. The effect of early tracheostomy on the risk of ventilator-associated pneumonia
The authors observed a clear reduction in the risk of ventilator-associated pneumonia, as defined by the authors (RR: 0.56, 95% CI 0.41–0.76, p = 0.0002). These results are shown in Fig. 2.
C. The effect of early tracheostomy on the duration of mechanical ventilation
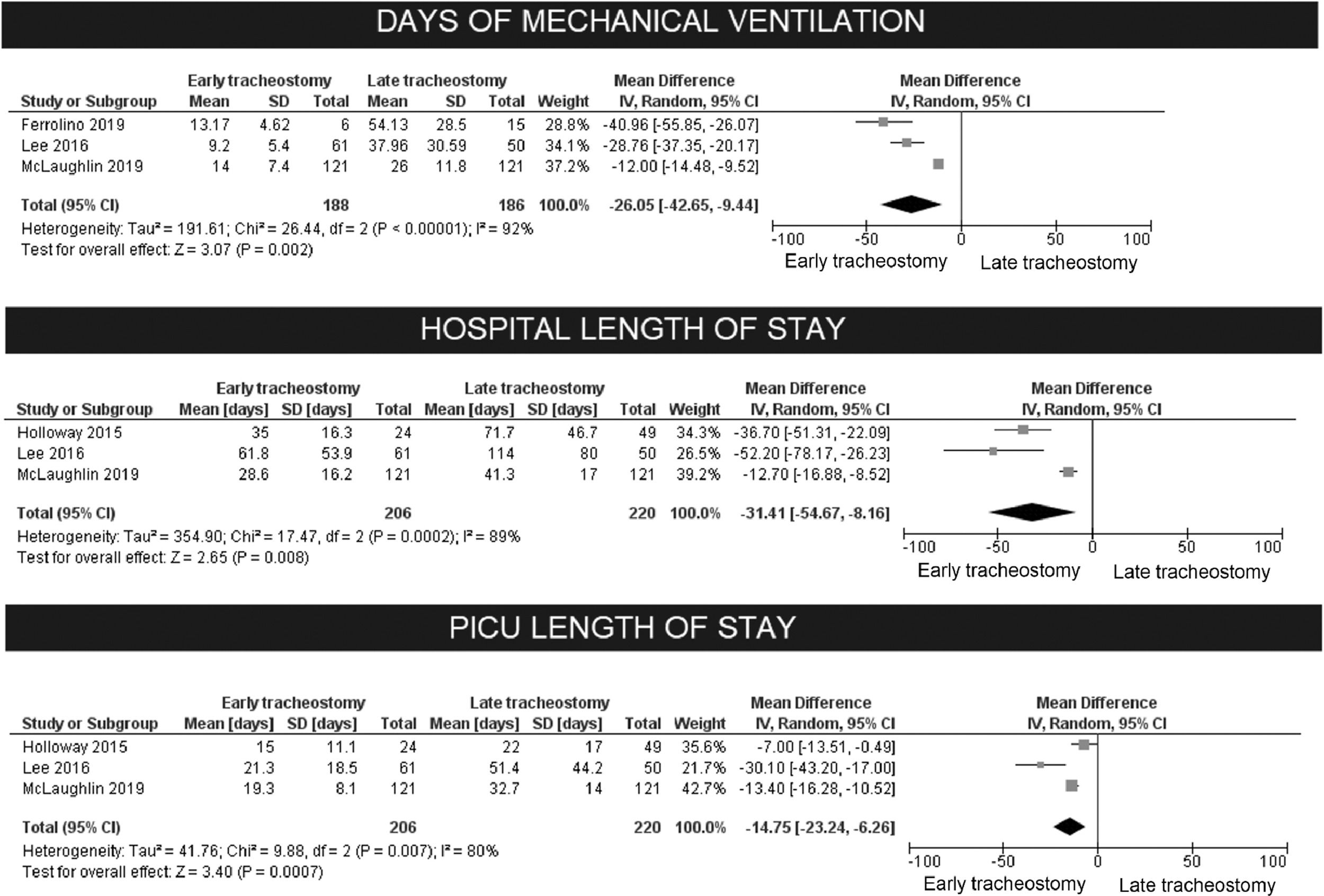
The early tracheostomy before 14 days had a great impact on the days of MV (reduction of 18 days in mean difference, 95% CI 9.4–42.6, p < 0.00001). Substantial heterogeneity was present, although there was consistency in the direction of effect. The exclusion of studies in a sensitivity analysis did not improve the model. To incorporate the heterogeneity, the authors chose to analyze random effects, as the authors had no means to explore its possible causes.
D. The effect of early tracheostomy on length of stay (LOS)
The authors observed also a great reduction in LOS both for PICU and hospital. For hospital LOS, the mean reduction was 31.4 days (95% CI 8.2–54.7, p < 0.008). For the days in PICU, the mean reduction was of 14.7 days (95% CI 6.3–23.2, p < 0.007). For both, there was consistency in the direction of effect, despite the heterogeneity (Fig. 3). These effects were also analyzed for the two studies (Holscher, 2014, and Sheehan, 2019) that defined early tracheostomy as being performed before seven days post-trauma:[63,64] there was a reduction of 11.9 days in MV for the early group (95% CI 1.75–22, p = 0.02). For PICU LOS, the reduction was of 10.8 days for the “early” group (95% CI 4.3–17.3, p = 0.001), and for hospital LOS, there was a reduction of 13.5 days (95% CI 7.4–19.7, p < 0.0001). These results are shown in Fig. 3.
E. Effects on long-term ventilation, decannulation, and discharge to home
For long-term ventilation after tracheostomy, the meta-analysis of Pizza, McLaughlin, and Lee showed a non-significant risk ratio (p = 0.32) for late and early tracheostomy.65–67 For successful decannulation, the meta-analysis of Lee and Pizza also showed no difference (p = 0.98).66,67 Early tracheostomy was associated with higher probabilities of discharge to home in McLaughlin (29.8% versus 14.9%), and Sheehan (50% versus 9.2%, p < 0.001).64,65
F. Other effects and complications related to early or late tracheostomy
Deep vein thromboses were reported by Sheehan (6.3% in the early, 8.1% in the late groups) and McLaughlin (2.7% and 12.9%): the meta-analysis showed a risk ratio of 0.31, non-significant (p = 0.06).64,65 The Sheehan's and McLaughlin cohorts were composed of children with traumatic brain injury; different times were used to define “early tracheostomy” (Table 2).64,65 The authors did not find other comparable cohorts of brain trauma patients.
Holscher et al. reported 13 complications following early or late tracheostomies, in 12 patients: six were tracheitis, which prevalence was not different in the two groups (5% versus 8%). All other complications were seen in the late tracheostomy group: subglottic stenosis, glottis granuloma, tracheomalacia, arytenoid dislocation, vocal cord hypofunction.63
In children who underwent extracorporeal membrane oxygenation, early tracheostomy (defined as being performed ≤ 21days after intensive care unit admission) was associated with a shorter PICU length of stay and ventilator days compared to late tracheostomy, and no different from the no tracheostomy group, in one unique study.71
DiscussionThe authors evaluated data from hundreds of children undergoing tracheostomy. A great deal of empirical knowledge has accumulated over the years, but without providing definitive answers on fundamental questions, as the optimal time for the procedure and how to proceed with decannulation. As the authors show in Table 1, there are a huge number of clinical conditions that require tracheostomy, and the underlying conditions determine the success of decannulation.54 Several studies show that severe neurological conditions can lead to very low decannulation success rates. For many patients with severe or irreversible brain injury or malformation, the discussion about a tracheostomy is a mandatory part of care, and a successful decannulation cannot realistically be expected in most cases.72 Although it was not an objective of the study's revision, the authors observed in the literature that the indication of tracheostomy in children has changed from an emergency procedure during diphtheria and poliomyelitis epidemics to support for children with chronic conditions and dependent on assisted ventilation.73 Higher rates of tracheostomy for weaning from ventilation are reported from quaternary referral centers in developed countries,74 whereas, in a mid-incoming country like Brazil, the most common indications are obstructive airway conditions.75
As for the question about the most suitable time to perform the tracheostomy, the authors did not find any high-quality evidence. It would be perfectly possible to conduct multicenter randomized studies, comparing the procedure at different times. In adults, a meta-analysis of eight randomized, controlled trials (n = 1977 participants) showed lower mortality rates and higher probabilities of ICU discharge on day 28 in the early (≤ 10 days after tracheal intubation) tracheostomy group. Divergent results were observed on the duration of mechanical ventilation and no differences were noted for pneumonia.5 The choice for defining “early” when a tracheostomy was performed up to 14 days of ventilation was empirically based by McLauglin et al. on the natural history of the timing of successful extubation, for their cohort of traumatic brain injury children. Considering that most of the children in this cohort were extubated between 7 and 14 days, a tracheostomy performed before seven days would be aggressive,65 but the generalizability of this observation is unclear. The meta-analyzed studies did not show any effect on mortality, but there was a clear reduction in the duration of MV, length of stay in PICU and hospital, and in the risk of ventilator-associated pneumonia. Early tracheostomy increased the chances of being discharged to home, but care must be taken with these results, because the studies included children with a wide heterogeneity of medical conditions, and the decision to perform a tracheostomy may be due to the baseline condition, and not related to ventilation.
There are great limitations in this meta-analysis. Almost all information available comes from retrospective, single-center cohorts, and there are no randomized, controlled studies. These cohorts are not comparable, due to demographic, regional, and economic differences, which explain the enormous variation observed in the rates of complications and success in decannulation. The study with the highest quality score, by McLaughlin et al., was also retrospective, although improved by the use of a propensity-matched analysis. It was not possible to compare the mean ages between the studies, because the presentation of these data has great variability. Although the fact that all the studies encompass the age groups usually treated in PICU, from one month to 18 years old, the impossibility of analyzing subgroups may be the major weakness of the authors’ work. Many biases may have also been present at the selection of patients for tracheostomy, e.g., patients in the “late” group would be excessively unstable for the procedure or would require very high ventilation parameters. Patients deemed as good candidates for discharge to home without ventilator dependency may have been offered tracheostomy earlier.65
Early tracheostomy may improve patient autonomy and comfort, reduce requirements of sedatives, and enable faster weaning from MV,65 but solid recommendations will only be possible after well-designed, prospective, randomized controlled trials with an adequate sample size. These findings should be analyzed with caution because no high-quality evidence is available.
ConclusionsA meta-analysis of observational studies suggests that tracheostomy performed in the first 14 days of ventilation can reduce the time spent on the ventilator, the length of stay in the PICU and in the hospital, providing a greater possibility of discharge to home. It also appears to reduce the incidence of ventilator-associated pneumonia, with no impact on mortality. These findings should be analyzed with caution because no high-quality evidence is available. In children, different from adults, the decision to perform a tracheostomy early or late may be much more dependent on the baseline disease and comorbidities than just time on mechanical ventilation support.