To evaluate the effectiveness of kangaroo mother care (KMC) in reducing the length of hospital stay of preterm and/or low birth weight infants.
SourceCochrane Library, Pubmed, Embase, LILACS, and Scielo. Randomized clinical trials without time or language limit were included. The intervention was the KMC in preterm and/or low birth weight infants born in health facilities compared to conventional care. The article selection was performed by a pair of reviewers independently. The methodological quality assessment was performed using the tool Risk of Bias 2.
Summary of the findingsEight hundred and sixty-four citations were identified and 12 were selected for data extraction. There was a reduction in the length of hospital stay in days in the KMC group compared to the conventional care group, with a statistically significant difference (MD -1.75, 95% CI -3.22 to -0.28). The subgroup that underwent the intervention for more than six hours daily did not show a statistical difference for the length of hospital stay outcome (MD -0.79, 95% CI -2.52 to 0.90), while the subgroup that underwent the intervention for less than six hours daily showed a reduction in this outcome with a statistically significant difference (MD -4.66, 95% CI -7.15 to -2.17).
ConclusionsKMC is a safe and low-cost intervention that has been shown to be effective in reducing the length of hospital stay of preterm and/or low birth weight infants.
Every year, 30 million infants are born in risk conditions worldwide. According to the World Health Organization, these conditions include preterm births, infants small for the gestational age (SGA) or at risk of becoming ill, risk of death, and disabilities.1
Prematurity is a birth that occurs before 37 weeks of gestation and is considered low birth weight (LBW) infants when born with less than 2500 g.1
Most infant deaths still occur in the neonatal period. In 2017, 2.5 million deaths were estimated to have occurred in the first 28 days of life. Approximately 80% of these were LBW infants, while two-thirds of them were preterm infants.1
Reducing neonatal mortality to 12 or less per 1,000 live births and providing the kangaroo mother care (KMC) or other humanized care method to at least 75% of eligible infants are among the objectives of the Every Newborn Action Plan launched by the United Nations International Children's Emergency Fund (UNICEF).1
Since existing methods to prevent infant deaths in the conventional neonatal care involve a high cost and require qualified human resources and permanent logistical support, the KMC is an effective and safe alternative for infants clinically stable,2 especially in developing countries where 12% are preterm infants and 60%, occur in Africa and South Asian countries.1
The KMC was developed by Rey and Martinez in Bogotá, Colombia, in 1978; its aim was to promote the early discharge of LBW, preterm, or SGA infants. It was conceived as a proposal to solve overcrowding, shortage of equipment, absence or unpreparedness of professionals, and high cross-infection rates.2
KMC is a standardized and protocolized care system for LBW and/or preterm infants at birth, based on skin-to-skin contact between the infant and the mother. It seeks to empower the mother and family, gradually transferring the ability and responsibility of being the primary caregivers of their infant, meeting their physical and emotional needs.3 Besides skin-to-skin contact, its other components are exclusive breastfeeding (ideally), starting in the hospital and continuing at home, early discharge, building social support, and follow-up.4
Although the effectiveness of KMC is evidenced in systematic reviews, these are focused on analyzing the reduction in morbidity and mortality,5 the neonatal pain,6-8 and its association with breastfeeding.9-14 Therefore, a synthesis of evidence to assess whether the KMC effectively reduces the length of LBW and/or preterm infants’ hospital stay is still needed.
Having a shorter hospital stay provides families with anticipation of returning home and living with their social support. In addition, optimizing hospital beds and some studies reduce the costs in groups that used the KMC method as intervention.15-18
Thus, the objective of this systematic review and meta-analysis was to evaluate the effectiveness of KMC in reducing the length of hospital stay of preterm and/or LBW infants.
MethodsThis is a systematic review and meta-analysis registered on the International Prospective Register of Systematic Reviews (PROSPERO) platform (https://www.crd.york.ac.uk/prospero) on April 28, 2020, under code CRD42020171496.
Using the PICOS criteria, the following question was used to guide the study: What is the effectiveness of the kangaroo mother care in reducing the length of hospital stay of preterm and/or LBW infants?
P (population): preterm infants (gestational age of less than 37 weeks) and/or infants weighing less than 2500 g at birth;
I (intervention): kangaroo mother care (also known as kangaroo position or skin-to-skin contact);
C (comparison): conventional neonatal care;
O (outcome): length of hospital stay; and
S (study design): randomized clinical trial (RCT).
Inclusion criteriaPopulation of interestInfants with a gestational age of less than 37 weeks and/or weighing less than 2500 g at birth born in health facilities.
Type of interventionIntervention with kangaroo mother care, also known as kangaroo position or skin-to-skin contact.
Control groupStudies with a control group in which infants were submitted to conventional neonatal care were included.
Conventional neonatal care refers to care where the infant is kept in an incubator and/or heated crib. The mother is allowed to visit, touch, and breastfeed. The baby is not placed in the kangaroo position (skin-to-skin contact).
Evaluated outcomeLength of hospital stay.
Type of study includedStudies included were RCTs.
Exclusion criteriaStudies with combined interventions, such as massage, other modalities of kangaroo mother care, plastic bags for heating infants, kangaroo mother care beginning in the delivery room, and kangaroo mother care at home, were excluded. There were no restrictions regarding the date and language of publication.
Methods for study identificationBibliographic databasesThe following databases were searched on March 13, 2020: Cochrane Library, Pubmed, Embase, Scielo, and LILACS. The search was updated in the same databases on March 19, 2021.
Search strategyKeywords were defined using DECs (Descriptors in Health Sciences), MeSH (Medical Subject Headings), and Emtree. The terms were combined using the Boolean operators OR and AND. The databases search strategy can be found in the Supplementary Material 1.
Data selection and analysisStudy selection and methodological quality assessmentAfter obtaining the search strategy results, accountability and duplicate exclusion were performed using the platform Covidence (https://www.covidence.org).19 Then, titles and abstracts were read for study eligibility evaluation, which was performed by a pair of reviewers independently, with conflicts being resolved by consensus. After reading the abstracts, the selected articles were read in full. When considered eligible, they were included in the methodological quality assessment stage using the Cochrane Collaboration tool Risk of Bias 220 for assessing the risk of bias in the RCTs.
The process of describing the study selection was conducted based on the PRISMA Flow Diagram (http://www.equator-network.org/reporting-guidelines/prism/).21
Extraction and statistical analysis of data from included studies
The data were extracted according to pre-specified criteria, including information about the study (number of participants, gestational age at birth, birth weight, and assistance types used), intervention characteristics (place, time, type, and frequency), and length of hospital stay in days. The assistance types refer to the description of the intervention used in the KMC group.
The outcome value was obtained at hospital discharge in both groups, being collected using continuous data (mean and standard deviation) and the total number of participants.
The software Review Manager (version 5.3)22 was used to calculate the effect sizes as mean differences (MDs), adopting a 95% confidence interval (CI). A random-effects model was used for meta-analysis.
The statistical heterogeneity between studies was assessed using the I2 statistic. The following classification was applied to heterogeneity indicators: unimportant, between 0% and 40%; moderate, between 30% and 60%; substantial, between 50% and 90%; and considerable, between 75% and 100%.23
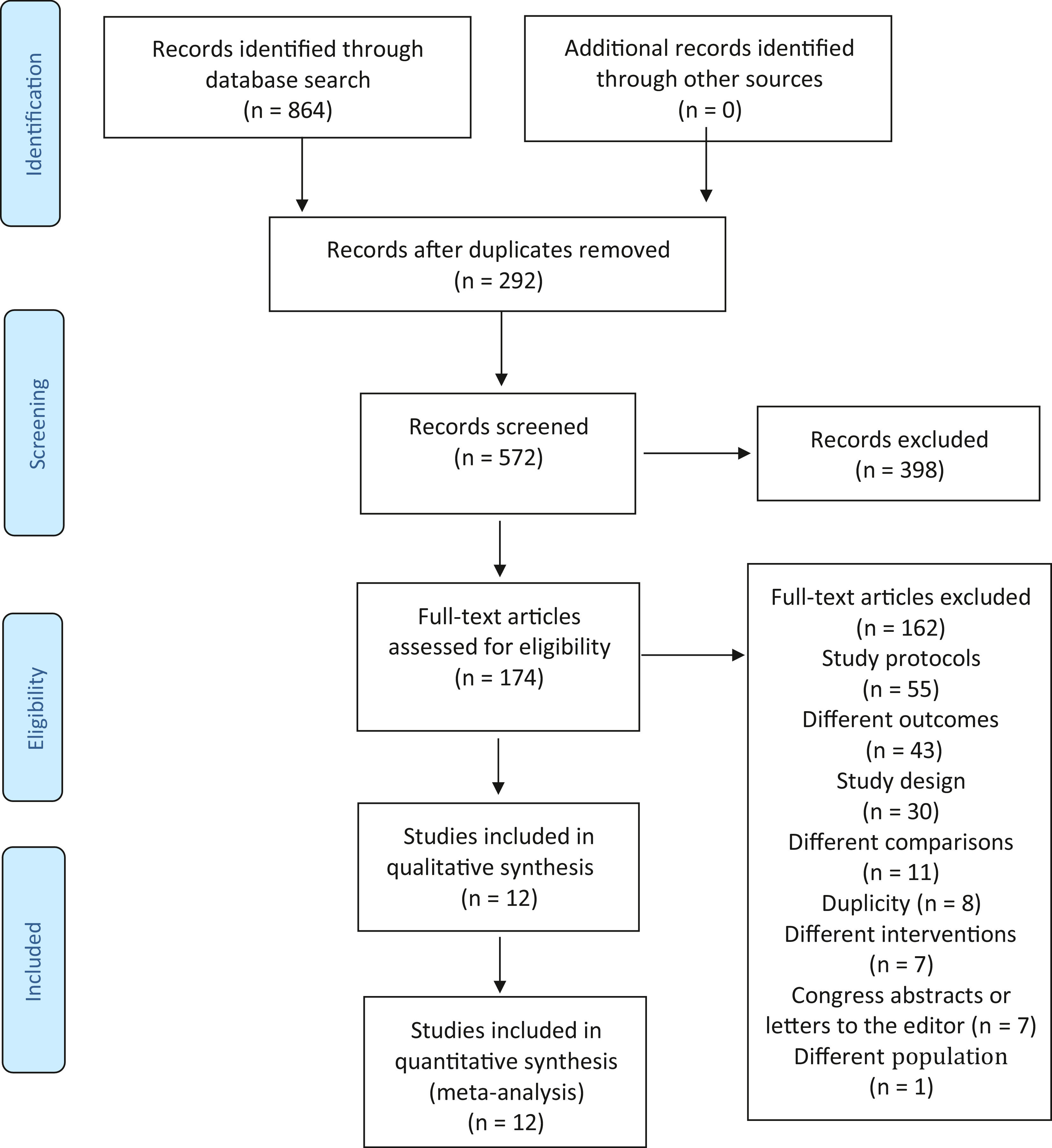
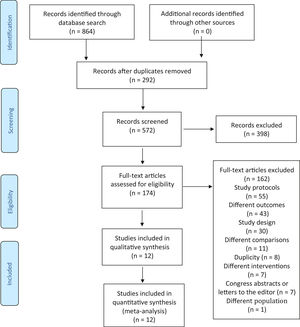
ResultsThe search in the abovementioned databases identified 1636 citations. Of these, 292 were duplicate studies. Based on the titles and abstracts, 572 studies were analyzed and 398 studies were excluded. Thus, 174 studies were read in full. After such full-text reading, 162 studies were excluded for the following reasons: a) they were study protocols (55 studies); b) study outcomes were not of interest to the research (43 studies); c) the study design was not RCT (30 studies); d) comparisons were not of interest to the research (11 studies); e) for duplicity (8 studies); f) interventions were not of interest to the research (7 studies); g) they were just congress abstracts or letters to the editor (7 studies); and h) the population studied was not of interest to the research (1 study). Twelve studies were selected for data extraction, as shown in Fig. 1. The study by Tessier et al.24 was subdivided into three according to the birth weight of participants because there was no result of the three subgroups sum in the full text of the article. Therefore, 14 studies were considered in the analysis, not 12.
Characteristics of the studiesTwelve studies, including 816 infants, met the inclusion criteria. All studies were performed in developing countries: Nepal,25 India,26-31 Malaysia,32 Taiwan,33 Indonesia,34 Kenya,35 and Colombia.24 Samples ranged from 28[29] to 488[24] infants. The characteristics of the studies are shown in the Supplementary Material 2.
Six studies included LBW infants weighing less than 2500 g,24-27,29,30 five studies included LBW infants weighing less than 1500 g,28,31,32,34,35 and one study classified infants according to their gestational age between 34 and 36 weeks.33
In most studies, congenital malformations, perinatal complications, critically ill mothers, or mothers refusing to take part in the research were exclusion criteria. One study used severe perinatal asphyxia as an exclusion criterion.32
Four studies did not report the discharge criteria from where they were conducted.25,30,33,34 Five studies described the minimum weight as a discharge criterion: 1300 g,31 1400 g,28 1700 g,24 1750 g,32 and 1800 g.35 Five studies used weight gain for three consecutive days as a discharge criterion.26,27,29,30,32 Other discharge criteria used included: absence of diseases and of use of intravenous medications,26,28 infants essentially in breastfeeding,26-29 body temperature maintenance without assistance,27,29 mother confidence in baby care at home,27-29 and specific hospital criteria.24,29
In eight studies, the KMC duration was longer than six hours daily,24,25,27,29-31,33,35 while in four studies its duration was shorter than six hours daily.26,28,32,34
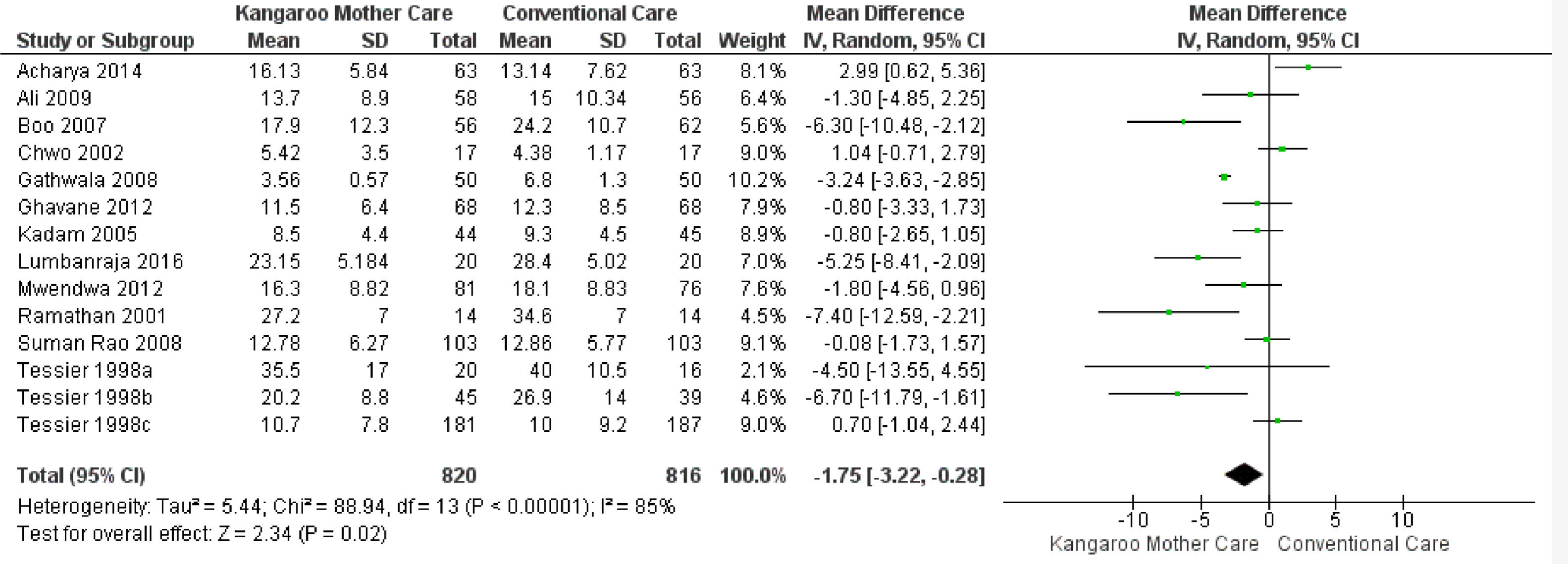
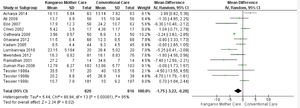
KMC effect on the length of hospital stayTwelve studies reported the length of hospital stay,24-35 totaling 1,636 infants. The data combination showed that KMC had the effect of reducing the hospital stay duration in the group submitted to this intervention compared to the group submitted to conventional care, with a statistically significant difference (MD -1.75, 95% CI -3.22 to -0.28). The heterogeneity was considerable (I² = 85%). Clinically, the length of hospital stay varies from 3 days, 5 hours, and 16 minutes to 6 hours and 43 minutes, with a mean hospital stay 1 day and 18 hours shorter in the intervention group when compared to the control group (Fig. 2).
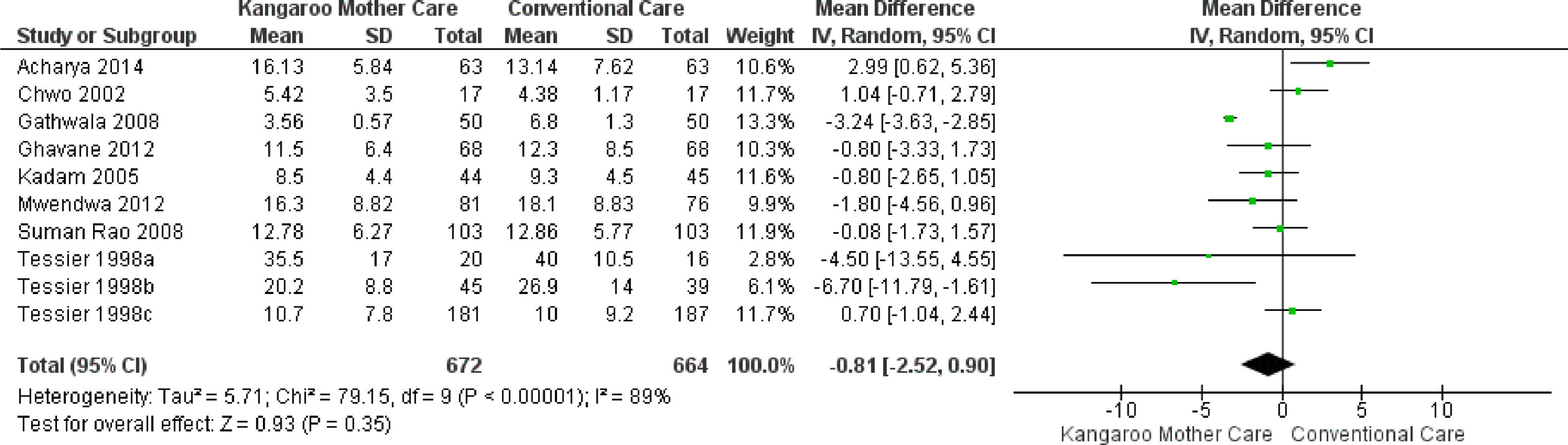
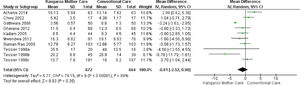
Subgroup analysisKMC effect on the length of hospital stay when its duration was longer than six hours dailyEight studies reported the intervention use for more than six hours daily,24,25,27,29-31,33 totaling 1,336 infants. The analysis of this subgroup showed no statistical difference for the length of hospital stay outcome in the group submitted to the KMC compared to the group submitted to conventional care (MD -0.81, 95% CI -2.52 to 0.90). The heterogeneity was considerable (I² = 89%). Clinically, the length of hospital stay varies from 2 days, 13 hours, and 26 minutes less to 23 hours and 16 minutes more, with a mean hospital stay duration 18 hours and 57 minutes shorter in the intervention group compared to the control group (Fig. 3).
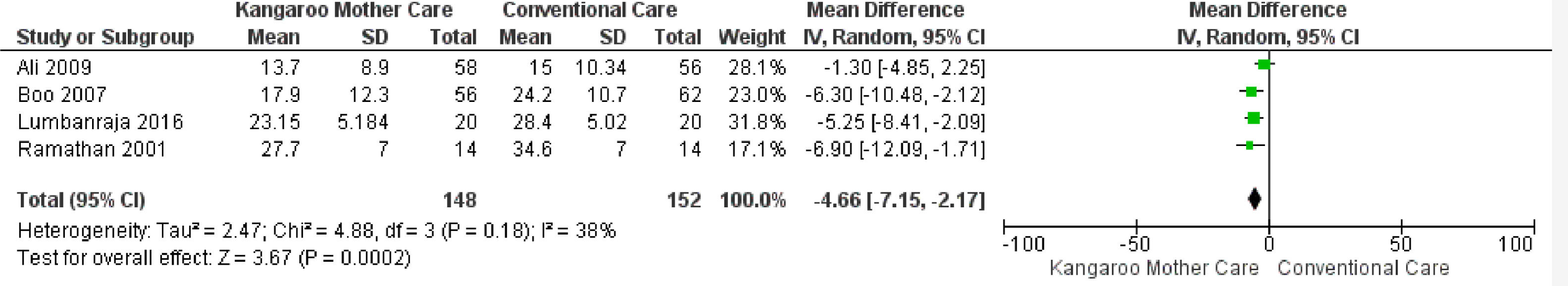
KMC effect on the length of hospital stay when its duration was shorter than six hours dailyFour studies reported the intervention use for less than six hours daily,26,28,32,34 totaling 300 infants. The data combination showed that KMC had the effect of reducing the hospital stay duration in the group submitted to this intervention compared to the group submitted to conventional care, with a statistically significant difference (MD -4.66, 95% IC -7.15 to -2.17). The heterogeneity was unimportant/moderate (I² = 38%). Clinically, the length of hospital stay varies from 7 days and 3 hours to 2 days and 4 hours, with a mean hospital stay duration 4 days, 15 hours, and 50 minutes shorter in the intervention group when compared to the control group (Fig. 4).
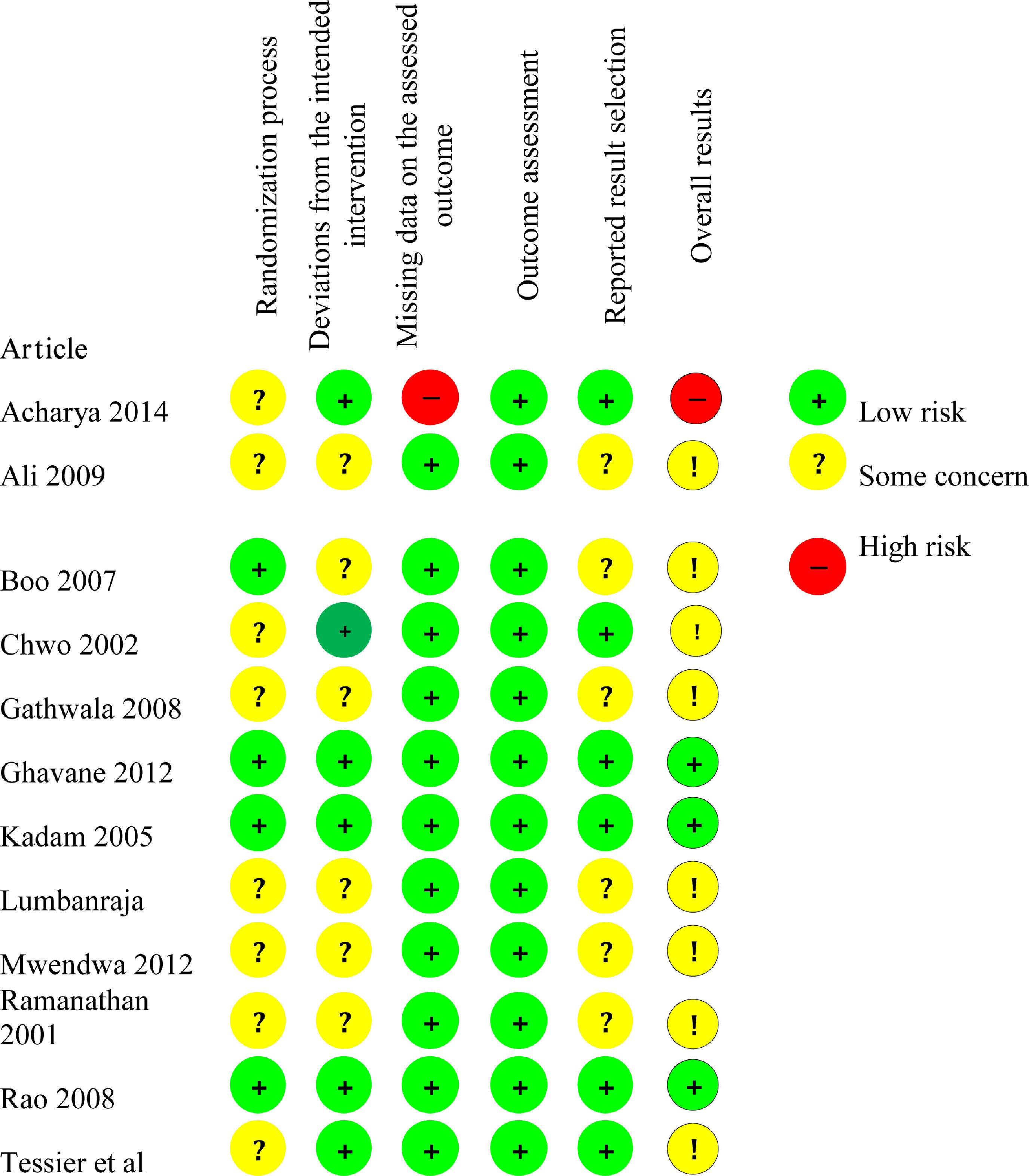
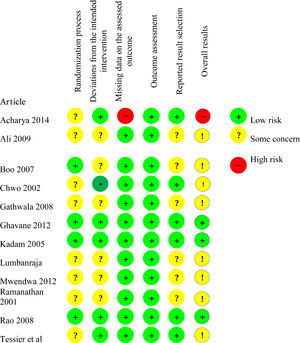
Risk of bias assessmentThe risk of bias assessment is shown in Fig. 5.
Risk of bias arising from the randomization processEight studies26-29,28,30,33-35 presented the result "Some concern" for bias in the randomization process, while four studies27,29,31,32 presented “Low risk” result.
Regarding the allocation sequence, the process implemented in seven studies24,25,28,30-33 was appropriate: table of random numbers,24,25,28,30 randomized blocks randomly mixed before being sealed,29 computerized minimization,33 and computerized generation of random numbers.34 Five studies26,27,29,34,35 did not report how the allocation sequence process was performed.
Regarding the allocation concealment, four studies27,29,31,32 used sealed envelopes. Eight studies24-26,28,30,33-35 did not provide information on the allocation concealment.
None of the studies showed problems of imbalance between the intervention and control groups.
Risk of bias arising from deviations from the intended interventionSix studies were classified as “Low risk”24,25,27,29,31-33 and six studies were classified as “Some concern”24,26,28,30-32 for bias arising from deviations from the intended intervention.
Due to the intervention nature, none of the studies blinded the participants or the health care team. The seven studies classified as “Some concern” did not inform whether there was a deviation from the intended intervention because of the study context. In the other five studies, classified as “Low risk”, changes were consistent with what would occur outside the trial context.
In all studies, a proper analysis was performed to estimate the effect of the assigned intervention.
Risk of bias arising from missing data on the assessed outcomeAll studies,24,26-35 except one,25 were classified as “Low risk of bias” for the length of hospital stay outcome. Data on this outcome were available to all or almost all participants in the studies firstly mentioned above.
The study by Acharya et al.19 was classified as “High risk” for the length of hospital stay outcome because there was no information on whether the data were available to all participants. In addition, there was no evidence of absence of bias in the results.
Risk of bias arising from the outcome assessmentAll studies24-35 were classified as "Low risk" for the length of hospital stay outcome. Although most studies did not report whether the outcome evaluators were blinded, there was no evidence that the measuring method of this outcome was inadequate, that there was a difference in the outcome assessment between groups, or that the outcome assessment was influenced by knowledge of the intervention received.
Risk of bias arising from the reported result selectionSix studies26,28,30,32,34,35 were classified as “Some concern” for the length of hospital stay outcome considering the risk of bias arising from the reported result selection because they did not inform whether the data that produced such result was analyzed according to a pre-established analysis plan. The other six studies24,25,27,29,31,33 were classified as “Low risk” for the outcome studied in this criterion.
DiscussionThrough the systematic review detailed above, evidence was found that KMC is associated with a reduction in the length of hospital stay of preterm and/or LBW infants.
This result meets one of the purposes intended when the KMC was created, which include the following components: 1) the kangaroo position, 2) the kangaroo feeding policy, and 3) the kangaroo discharge policy (early discharge in kangaroo position).16
Early (timely) hospital discharge reduces overcrowding in neonatal units, thus reducing the chances of infants contracting hospital infections and the economic impact of hospital stay imposed on families of infants. Additionally, it could also reduce the overall cost of health services.17,27
Considering the parameters for heterogeneity described in the Cochrane Handbook for Systematic Reviews of Interventions,23 the meta-analysis of the 12 studies showed an I² = 85% (considerable), which represents a high degree of heterogeneity. Thus, a subgroup analysis was performed, where one subgroup was submitted to the intervention for more than six hours daily and the other, for less than six hours daily.
The subgroup that underwent KMC for more than six hours a day presented an I² = 89% (considerable), while the subgroup that underwent this intervention for less than six hours a day presented an I² = 38% (unimportant/moderate).
When analyzing the heterogeneity of the full chart of studies and of the chart of the group that performed the intervention for more than six hours daily, clinical heterogeneity was found, which consists of the difference between the characteristics of the studies.36 This difference between study characteristics is believed to be due to the discharge criteria used –some studies used satisfactory weight gain as a criterion, while others used different criteria, such as the type of delivery. Some infant characteristics also appeared to affect the heterogeneity, since most studies used the birth weight as an inclusion criterion. One study33 used the gestational age as an inclusion criterion.
Eight studies were analyzed in relation to the reported intervention performance for more than six hours daily,24,25,27,29-31,33,35 totaling 1,336 infants. This analysis showed no statistical difference for the length of hospital stay outcome in the group that performed KMC when compared to the group that underwent conventional care. The clinical difference was a length of hospital stay 18 hours and 57 minutes (-0.81 days) shorter than that of the control group, ranging between 2 days, 13 hours, and 26 minutes less to 23 hours and 16 minutes more. When the length of hospital stay is reduced, there is also a reduction in the costs imposed on the infant family27,37 and on the health system,17,37,38 in addition to the bed turnover promotion in neonatology units.
The study by Acharya et al.25 demonstrated a longer hospital stay in the group that received the KMC. The authors state that this outcome is probably related to the discharge criterion (weight greater than 1600 g) and to the fact that the infants in the control group started the study with a weight greater than that of the intervention group infants (1415 g +/- 174.91 g x 1362.3 g +/- 240.14 g). Multivariate analysis was performed to eliminate possible final result confounders for this difference.
Chwo et al.33 also demonstrated a longer hospital stay in the intervention group than in the control group (conventional care). The authors attribute this result to the infant characteristics and to the hospital discharge criterion. Subjects in this study were classified as late preterm infants (34 to 36 weeks) weighing more than 2000 g, which differs from other studies, whose subjects weighed less than 2000 g.25,26,30 The discharge criterion used was maternal discharge according to the postpartum time, being three days for vaginal delivery and five days for cesarean delivery. Additionally, the intervention group had two outliers, although the analysis showed their inclusion did not interfere with the outcome.
The study by Gathwala et al.30 showed that the group submitted to the KMC had a hospital stay significantly shorter than that of the control group. This result is in line with other studies that show similar results.16,32,39,40 The infants studied had birth weights below 1800 g and were followed up until reaching three months of age. They continued receiving the intervention even after hospital discharge.
The studies by Kadam et al.,27 Suman et al.,29 Ghavane et al.,31 and Mwendwa et al.,35 did not show a statistically significant difference, but demonstrated a reduction in hospital stay varying from 1 hour and 55 minutes (-0.08 days)[29] to 1 day and 19 hours (-1.80 days)[35] in favor of the intervention group. In the study by Mwendwa et al.,35 infants were divided by weight, and the group with the highest weight (1500 to 1750 g) had a significantly shorter hospital stay in the intervention group when compared to the control group. All four studies used satisfactory weight gain as a hospital discharge criterion.
In the study by Tessier et al.,24 the infants were subdivided according to their birth weight. Infants weighing between 1201 g and 1500 g receiving KMC had a shorter hospital stay than infants who received conventional care. There was no statistically significant difference between infants weighing less than 1201 g and more than 1500 g.
Four studies reported the intervention for less than six hours daily,26,28,32,34 totaling 300 infants. The data combination analysis of this subgroup showed that KMC had the effect of reducing the length of hospital stay in the group submitted to this intervention compared to the group submitted to conventional care, with a statistically significant difference and low heterogeneity between them.
The studies by Ramanathan et al.28 and Lumbanraja et al.34 demonstrated a statistically significant reduction in the length of hospital stay of infants submitted to the KMC compared to the group that received conventional care. This result confirms the results of other studies.16,30
The study by Ali et al.26 has shown that infants who received conventional care remained hospitalized for longer periods compared to the infants who received the intervention. Although a statistically significant difference has not been demonstrated, the reduction in the hospitalization duration in more than one day in the intervention group contributes to reducing overcrowding in neonatal units.26
The study by Boo and Jamli32 demonstrated a statistically significant difference, despite its short intervention time (one hour daily). This result is consistent with that of other studies using short-duration KMC.26,28,34
Studies show that even when performed for short periods, the KMC is associated with a high breastfeeding rate, which facilitates an early discharge.3
In the systematic review conducted by Conde-Agudelo et al.5 for the length of hospital stay outcome, statistical differences were not found between groups. Three studies included for this outcome compared the infants receiving KMC with the conventional cuddling care.38,39,41,42
Some studies reinforce the physiological benefits of the KMC, like the improvement in physiological parameters, such as increased oxygen saturation and temperature,26,27,43,44 improved weight gain,15,25,28,29,34,35,37 reduced pain responses,43-47 reduced sepsis,26,29,37 reduced apnea episodes,37 and reduced hypothermia.25 Studies have also shown an increase in the exclusive breastfeeding rate, one of the pillars of the KMC.9-14 Weight gain and exclusive or almost exclusive breastfeeding were discharge criteria for most studies included in this systematic review, which explains the reduction in the length of hospital stay.
The meta-analysis performed involving all studies demonstrated the KMC effect in reducing the length of hospital stay compared to the conventional care. Although the heterogeneity is considerable, the reduction of 1 day and 18 hours (ranging from 3 days, 5 hours and 16 minutes to 6 hours and 43 minutes (-1.75 [-3.22 to -0.28]) in the hospitalization duration contributes to the reduction in the costs imposed on the family of infants,15,27 and on the health system.15,17,18
The statistical difference between the subgroups is due to the difference in the characteristics of the studies, especially regarding the discharge criteria. Although there was a statistical difference, the two subgroups showed a clinical reduction in the length of hospital stay.
The limitations found in the studies were mainly related to the risk of bias. Due to the intervention nature, it is not possible to blind the subjects and it is difficult to blind the professional who conducts the intervention. Another limitation refers to the discharge criteria used in the studies, which are believed to have generated a high heterogeneity.
The relevant aspects of this systematic review are the development of a sensitive search strategy, with a comprehensive search of the literature, and the quality screening and assessments performed by two independent authors. In addition, the included studies made it possible to perform a meta-analysis, where the hypothesis of effectiveness of the KMC in reducing the length of hospital stay of preterm and/or LBW infants was established. There was no language limitation in the analysis of primary studies.
The findings in this systematic review show that managers and health professionals working in neonatology units should support and encourage actions to implement the KMC method in their facilities. Regarding the research implications, further studies are suggested to evaluate other benefits of the KMC method in addition to those already described in the literature to establish the ideal time of performance of KMC within the health units.
ConclusionThe KMC is a safe and low-cost intervention that has been shown to be effective in reducing the length of hospital stay of preterm and/or LBW infants.