To analyze toll-like receptor (TLR)-2 and TLR-4 expression in monocytes of newborns with late-onset sepsis.
MethodsThis prospective study included 27 full-term newborns aged 8 to 29 days, with clinical and laboratory diagnosis of late-onset sepsis. Ten newborns (37%) had positive cultures. Cytokines were measured by cytometric bead array in peripheral blood, while TLR-2, TLR-4 expression, and median fluorescence intensity (MFI) were determined by immunophenotyping peripheral whole blood monocytes, and were analyzed with a BD FACSDiva flow cytometer (Becton, Dickinson and Company, USA). A comparison was performed with healthy adults.
ResultsMicroorganisms were identified in 37% of these septic newborns, and all of them had high levels of pro-inflammatory cytokines (IL-8, IL-6, IL-1β) and anti-inflammatory cytokine (IL-10) corroborating the inflammatory/septic process. In monocytes, the frequency of TLR-4 expression was higher in infected newborns (p = 0.01).
ConclusionThis study investigated the innate immune response in septic newborns. Septic newborns that relied almost exclusively on the innate immune system showed little in vivo response at monocyte activation, suggesting impaired immune response and increased susceptibility to infection.
Analisar a expressão dos TLR-2 e TLR-4 em monócitos de recém-nascidos com sepse tardia.
MétodosTrata-se de um estudo prospectivo com 27 recém-nascidos a termo entre 8 e 29 dias de vida com diagnóstico clínico e laboratorial de sepse tardia dos quais dez (37%) apresentaram cultura positiva. As citocinas foram determinadas por teste de CBA em sangue periférico enquanto que a expressão e MFI (mediana de intensidade de fluorescência) dos TLR-2 e TLR-4 foi determinado por imunofenotipagem em monócitos de sangue periférico total através de análise pelo citômetro de fluxo BD FACSDiva. O grupo usado para comparação foi de adultos saudáveis.
ResultadosMicrorganismos foram identificados em 37% dos pacientes e estes juntamente com os pacientes com sepse clínica tiveram níveis elevados de citocinas pró-inflamatórias (IL-8, IL-6, IL-1β) e de citocina anti-inflamatória (IL-10) corroborando o processo inflamatório/infeccioso. No monócito, a frequência de expressão do TLR-4 foi mais elevada (p = 0,01).
ConclusõesEste estudo analisou a resposta imune inata no recém-nascido com sepse. Recém-nascidos sépticos que dependem quase exclusivamente do sistema imune inato apresentaram pouca resposta in vivo na ativação de monócitos o que sugere uma resposta imune deficiente e maior susceptibilidade à infecção.
Sepsis represents a major cause of morbidity and mortality in the newborn,1,2 whose severity is proportional to the interaction between the host and the causative agent, triggering a cascade of events responsible for the immune response expression.
Late-onset sepsis occurs after 72hours of life, with clinical signs and symptoms that may be subtle and nonspecific early in the infection, which are often misinterpreted or mistaken for other non-infectious clinical conditions. Nevertheless, its evolution can be fulminant, leading to septic shock, disseminated intravascular coagulation, and death within hours.3–5
Differently from the adult, several degrees of deficiency have been described in the newborn regarding the innate and adaptive immune responses.6 At birth, the adaptive immune response is impaired, both by the minimal in utero antigen exposure and by B and T effector cell dysfunction.1 Because of that, the newborn relies on the effectiveness of the innate immune response and the passive protection of maternal antibodies acquired transplacentally.7
A primordial part of the innate immune response trigger corresponds to the activation of the toll-like receptors (TLRs), expressed on the surface of monocytes, macrophages, dendritic cells, lymphocytes, epithelial cells, or in the cytoplasm of different tissue cells. Of the ten types of receptors described in humans, TLR-2 and TLR-4 mainly recognize components of Gram-positive and Gram-negative bacteria, respectively.
The signal transduction activation recruits several intracellular proteins (MyD88, IRAK, and TRAF-6), which trigger the activation of JNK (Jun amino-terminal kinases) and ERK (extracellular signal-regulated kinases) pathways in the cascade of mitogen-activated protein kinases (MAPKs). This induces the activation of transcription factors activator protein 1 (AP-1) and nuclear factor kappa B (NF-kB) that express several genes directly involved in the production of inflammatory cytokines in response to infection, playing a key role in the amplification of cell immune response.8–11
Monocytes are antigen-presenting cells that act on the inflammatory process and as a source of macrophages and dendritic cells. After activation through TLRs, there is an increase in the expression of costimulatory molecules (CD80 and CD86), which are important in the early adaptive immune response and cytokine production.12
The regulation of the immune system at birth results in a biased TLR neonatal response by stimulating a lower production of pro-inflammatory cytokines and demonstrating lower multi-functionality.13,14 Only in the course of life do cytokine levels become equivalent to those of the adult individual.15 However, in the neonatal period, quantitative and qualitative changes in TLRs and cells participating in the innate immune response have been described when compared to the adult individual, which are proportional to gestational age at birth.16 These differences could elucidate the increased susceptibility to infection observed in the neonatal age group.1,17
Therefore, despite the growing awareness of the importance of the TLR system in protecting newborns against infections,18 much still needs to be clarified about the mechanisms of regulation of TLR responses in the neonatal period. Thus, this study aimed to characterize the expression of TLR-2 and TLR-4 in monocytes of full-term newborns with late-onset sepsis.
MethodsThis was a prospective study, whose convenience sample included 27 full-term newborns transferred to the neonatal intensive care unit (NICU) of the Instituto da Criança- Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), from February of 2011 to January of 2013. Patients whose gestational age ranged from 37 to 42 weeks and showed clinical and/or laboratory symptoms (complete blood count and C-reactive protein) of neonatal sepsis from 72hours up to 30 days of life at the time of admission or during hospitalization, which led to the start of antibiotic therapy, were included in the study.
Exclusion criteria were factors that alone would alter the immune response, such as: diagnosis of congenital infections, inborn errors of metabolism, use of anti-inflammatory drugs (indomethacin, ibuprofen, and steroids), diagnosis of intracranial hemorrhage confirmed by skull ultrasound or computed tomography and surgery in the week before, in addition to those in which the date of sample collection did not permit analysis.
The criteria used to define and classify the diagnosis regarding clinical severity (sepsis, severe sepsis, and septic shock) were those mentioned in the Surviving Sepsis Campaign (2012), adjusted for age range based on the criteria of Goldstein (2005) as determined by the consensus.19,20 The complete blood counts (CBC) analysis was based on the Hematologic Scoring System (HSS) of Rodwell, whereas CRP (C-reactive protein) level > 10mg/L was considered suggestive of infection. The study was approved by the Ethics Committee for Analysis of Research Projects of the HCFMUSP (CAPPesq).
SamplingAfter an informed consent was obtained from the legal guardians of the newborn, blood samples were collected by venipuncture from a peripheral vein for assessment of infectious picture (CBC, CRP, and blood culture) and analysis of cytokines and monocytes. The time of collection was standardized to occur within the first 24hours from the suspected diagnosis of infection and the start of antibiotic therapy. An aliquot of 0.5mL of blood was distributed in a separator tube with gel for cytokine measurement, and 1.5mL was placed in a tube with EDTA (Ethylenediamine tetraacetic acid) for immunophenotyping, which were both performed at the Laboratory of Medical Investigation 36 (LIM 36) of Instituto da Criança of HCFMUSP.
Cytokine analysisCytokines TNF- α, IL-1 β, IL-2, IL-4, IL-6, IL-8, and IL-10 were measured in serum samples using a human cytometric bead array (CBA) set (Cytometric bead array BD OptEIA™ Set Human, Becton, Dickinson and Company, USA) according to the manufacturer's instructions.
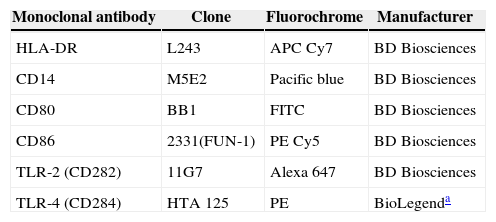
Analysis of TLRsImmunophenotyping was performed with the sample red blood cells, which were collected in tubes containing EDTA after being prepared for addition of pre-established antibodies (Table 1) and processed until they were transferred to be read in the flow cytometer FACS II LRS Fortessa (Becton, Dickinson and Company, USA). The monocyte population was defined by gating of CD14+HLA-DR+ cells using the size (forward scatter - FSC) and cell granularity as parameters (side scatter - SSC) as described in the literature.20
Monocyte cell surface markers.
| Monoclonal antibody | Clone | Fluorochrome | Manufacturer |
|---|---|---|---|
| HLA-DR | L243 | APC Cy7 | BD Biosciences |
| CD14 | M5E2 | Pacific blue | BD Biosciences |
| CD80 | BB1 | FITC | BD Biosciences |
| CD86 | 2331(FUN-1) | PE Cy5 | BD Biosciences |
| TLR-2 (CD282) | 11G7 | Alexa 647 | BD Biosciences |
| TLR-4 (CD284) | HTA 125 | PE | BioLegenda |
HLA-DR, human leukocyte antigen class II-DR locus; CD, cluster of differentiation; TLR, toll-like receptor; APC Cy7, allophycocyanin combined with cyanine dye; Pacific blue, 6,8-difluoro-7-hydroxycoumarin fluorophore; FITC, fluorescein isothiocyanate; PE Cy5, conjugate of phycoerythrin and cyanine dye; Alexa 647, fluorochrome for reading between 594 and 633nm; PE, R-phycoerythrin. Clone, refers to the specified markers of monoclonal antibodies.
The analysis was performed in FlowJo software (Tree Star - Ashland, OR, United States), where each cell population was analyzed separately by gating using cell size and granularity as parameters. The same software provided data representing the median fluorescence intensity (MFI) of the respective markers.
The absolute numbers of populations were calculated by multiplying the percentage indicated for each population in flow cytometry and the absolute number of leukocytes determined by automatic cell counter.
Blood control samples from healthy adultsPeripheral blood samples were obtained from 27 healthy adults and used for test standardization and control. The selection criteria were: healthy adult volunteers; aged 18-35 years; of both genders; with negative serological tests for human immunodeficiency virus (HIV), HTLV I/II (Human T lymphotropic virus type I/II), hepatitis B and C, syphilis, and Chagas disease; and no symptoms of infection during the collection period.
Statistical analysisThe newborn data were recorded on the collection forms and stored in spreadsheets from the statistical package GraphPad Prism®, release 6.0c for Mac OSX (GraphPad Software, La Jolla California, United States). For qualitative variables, absolute and relative frequencies (percentage and number of cases) were calculated, whereas for quantitative variables, the median, the minimum and maximum values, and interquartile range (P25-P75) were calculated for each of the receptors evaluated in each analysis. The Mann-Whitney test was used to compare the median of markers between the groups, while the comparative analysis of the medians of more than two groups used the Kruskal-Wallis test with Dunn's post-test. The tests were performed with 95% confidence intervals and a p-value < 0.05 was considered significant.
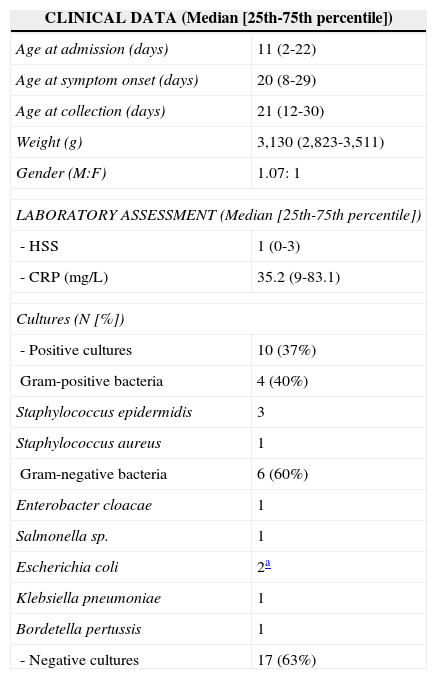
ResultsClinical and laboratory characteristics of the 27 infants included in the study are shown in Table 2. It can be observed that most patients (62.9%) had symptoms compatible with severe sepsis (44.4%) and septic shock (18.5%), which occurred at a median age of 20 days of life; death was observed in only two patients (7.4%). Among the 17 patients who had negative cultures, all showed clear clinical signs and symptoms of infection and compatible laboratory alterations in the CBC and CRP levels at diagnosis and subsequent clinical analysis, and thus were considered patients with clinical sepsis.
Distribution of 27 newborns according to the clinical and laboratory data.
| CLINICAL DATA (Median [25th-75th percentile]) | |
|---|---|
| Age at admission (days) | 11 (2-22) |
| Age at symptom onset (days) | 20 (8-29) |
| Age at collection (days) | 21 (12-30) |
| Weight (g) | 3,130 (2,823-3,511) |
| Gender (M:F) | 1.07: 1 |
| LABORATORY ASSESSMENT (Median [25th-75th percentile]) | |
| - HSS | 1 (0-3) |
| - CRP (mg/L) | 35.2 (9-83.1) |
| Cultures (N [%]) | |
| - Positive cultures | 10 (37%) |
| Gram-positive bacteria | 4 (40%) |
| Staphylococcus epidermidis | 3 |
| Staphylococcus aureus | 1 |
| Gram-negative bacteria | 6 (60%) |
| Enterobacter cloacae | 1 |
| Salmonella sp. | 1 |
| Escherichia coli | 2a |
| Klebsiella pneumoniae | 1 |
| Bordetella pertussis | 1 |
| - Negative cultures | 17 (63%) |
| CYTOKINES pg/mL[Median (25th-75th percentile)] | Positive culture | Negative culture | p |
|---|---|---|---|
| - IL-6 | 147.7 (72.2-385.1) | 132.3 (6.3-332) | 0.6066 |
| - IL-8 | 137.9 (61.1-411.8) | 58.1 (16.3-55.4) | 0.5414 |
| - IL-1β | 3.0 (1.07-15.8) | 2.55 (1.15-5.62) | 0.5349 |
| - IL-10 | 8.05 (4.12-19.9) | 3.5 (1.8-76.15) | 0.4516 |
| - TNF- α | 1.2 (0.45-1.7) | 1.75 (1.0-2.47) | 0.1188 |
| - IL-2 | 1.7 (1.4-1.9) | 2.4 (1.6-4.55) | 0.1014 |
| - IL-4 | 2.2 (0.85-2.8) | 0.4 (0.3-1.1) | 0.0714 |
HSS, hematologic scoring system of Rodwell; CRP, C-reactive protein; IL, interleukin; TNF-α, tumor necrosis factor alpha.
Of these patients with clinical sepsis, eight (47%) had a clinical picture compatible with sepsis, five (29.5%) with severe sepsis, and four (23.5%) with septic shock. Moreover, the measurement of pro-inflammatory cytokines was similar in infected patients regardless of positive cultures, as shown in Table 2. Culture positivity in material collected from sterile fluids (blood, urine, and CSF) occurred in ten patients (37%), with distribution according to the isolated agent as shown in the same table.
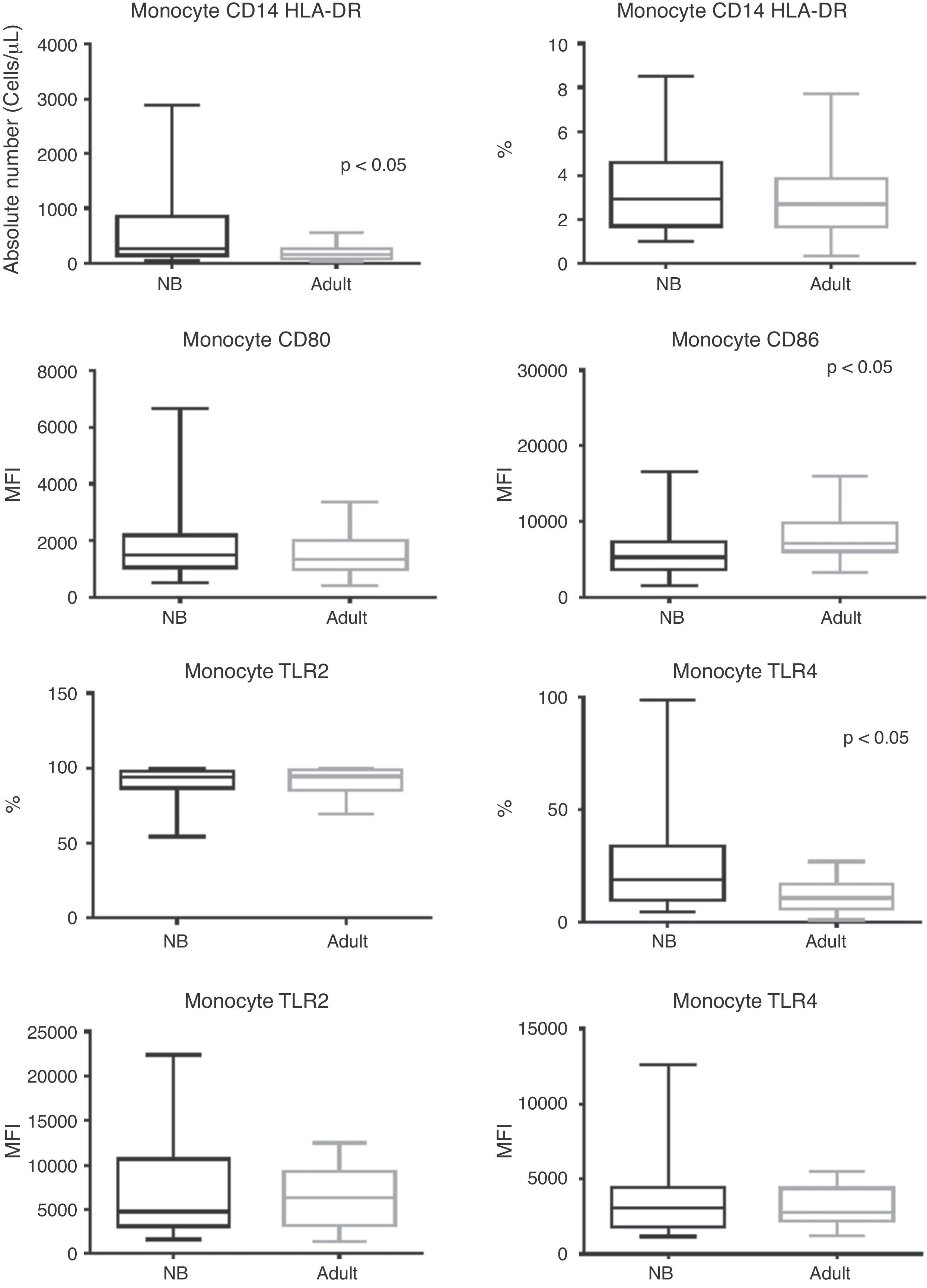
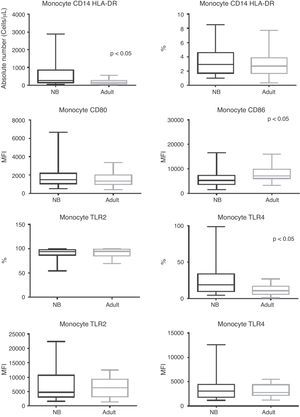
Regarding monocytes, it can be observed that despite the fact that their total absolute number was higher in newborns when compared to adults (p < 0.0001), there was a similar frequency of monocytes between groups (Fig. 1).
Box plot of absolute numbers (cells/μL), frequency (%), and median fluorescence intensity (MFI) of CD80, CD86, TLR-2, and TLR-4 activation molecules. Values expressed as median ± interquartile range (P25-P75); p refers to statistical significance. Sample number of the “newborn” and “adult” groups = 27.
NB, newborn; HLA-DR, human leukocyte antigen class II-DR locus; CD, cluster of differentiation; TLR, toll-like receptor.
MFI for activation molecules CD80, CD86; and frequency and MFI for TLR-2 and TLR-4 are shown in Fig. 1. In addition to a lower MFI for CD86 in newborns with infection, these patients showed maintenance of CD80 and TLR-2 expression (p = 0.822 and p = 0.825, respectively), while there was a higher frequency for TLR-4 in septic newborns when compared to adults (p = 0.0043).
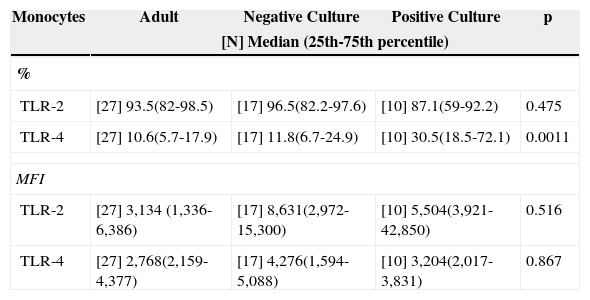
Culture positivity was associated to a higher frequency of TLR-4 when compared to adults and patients with negative cultures (Table 3), while there was a similar frequency between groups in the evaluation of TLR-2.
Median frequency (%) and MFI of toll-like receptors 2 and 4 in peripheral blood monocytes of infected newborns (positive or negative culture), and adult peripheral blood.
| Monocytes | Adult | Negative Culture | Positive Culture | p |
|---|---|---|---|---|
| [N] Median (25th-75th percentile) | ||||
| % | ||||
| TLR-2 | [27] 93.5(82-98.5) | [17] 96.5(82.2-97.6) | [10] 87.1(59-92.2) | 0.475 |
| TLR-4 | [27] 10.6(5.7-17.9) | [17] 11.8(6.7-24.9) | [10] 30.5(18.5-72.1) | 0.0011 |
| MFI | ||||
| TLR-2 | [27] 3,134 (1,336-6,386) | [17] 8,631(2,972-15,300) | [10] 5,504(3,921-42,850) | 0.516 |
| TLR-4 | [27] 2,768(2,159-4,377) | [17] 4,276(1,594-5,088) | [10] 3,204(2,017-3,831) | 0.867 |
p refers to statistical significance using the Kruskal-Wallis test.
MFI, median fluorescence intensity; TLR, toll-like receptor.
Despite the identification of some bacteria, the analysis between culture positivity according to the type of bacteria identified and the types of TLR was not performed due to the sample number.
DiscussionThis study described the in vivo response of neonatal peripheral blood monocytes in the presence of an infectious picture, which showed similar or lower expression of activation molecules, in addition to increased expression of TLR-4 in newborns with infection caused by Gram-positive and Gram-negative bacteria, and increased expression of TLR-2 in patients with clinical sepsis.
Monocytes are phagocytic cells, and play an important role in innate and acquired immunity through their effects on the inflammatory process or as a source of macrophages and dendritic cells. The behavior of TLRs in the newborn, either in healthy or infected infants, is controversial. According to Levy et al., although the basal expression of TLRs in monocytes of full-term newborns is similar to that of adult individuals, the functional consequences of activation are quite different, as a lower production of cytokines and lower expression of co-stimulatory molecules are observed in newborns.18
It was observed that in the presence of an infectious stimulus, the population of assessed newborns showed a lower number of activated cells (lower MFI for CD86 than in the adult individual and MFI for CD80 similar to adult individuals without infection) in peripheral blood, suggesting a lower activity potential of this cell type in newborns, which is in accordance with the literature.18–21
Regarding TLR-2, it was observed that it was widely expressed in neonatal monocytes (93.9%) and monocytes of adult individuals without infection (94.6%). The same similarity was observed regarding MFI for this receptor (MFI = 4,748 and 6,386, respectively) regardless of culture positivity, contradicting findings in the literature that demonstrate an up-regulation in the expression of TLR-2 in monocytes isolated from patients with sepsis, as well as significant changes in TLR-2 expression.22,23 Thus, in the present study, in which all newborns had clinical and laboratory signs of infection, it was observed that despite presence of the necessary tools to recognize the invading antigen (TLR and co-stimulatory molecules), they did not undergo the up-regulation expected in the overall analysis.
Meanwhile, contrary to what was reported by Viemann et al.,1 the presence of a positive culture was associated with a higher frequency in TLR-4 expression in relation to the adult individual with a negative culture, probably secondary to the fact that most patients with positive cultures had Gram-negative bacteria (60%) isolated from the culture. As for the similar expression of the same receptor in newborns with negative cultures and in adult patients, it may suggest a tendency to immunoparalysis that would have shifted the median to lower levels, similar to the levels found in healthy adult individuals, as four newborns from this group progressed to septic shock.
The present study has limitations that should be emphasized, such as the sample size and the pointwise characteristic of the infectious picture assessment. However, the results still suggest that at the time of diagnostic hypothesis of infection in the newborns, TLR-2 and TLR-4 receptors had a higher expression in infants with infection, but with maintenance of expression of costimulatory molecules, indicating a possible deficiency in the cell activation process in vivo.
Thus, although larger extrapolations cannot be made, the authors believe it would be intriguing to assess the response of TLR as well as that of cell activation in newborns during the evolution of a clinical infection.
FundingFundação de Amparo à Pesquisa do Estado São Paulo - FAPESP.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank FAPESP for their financial support.
Please cite this article as: Redondo AC, Ceccon ME, Silveira-Lessa AL, Quinello C, Palmeira P, Carvalho WB, et al. TLR-2 and TLR-4 expression in monocytes of newborns with late-onset sepsis. J Pediatr (Rio J). 2014;90:472–8.
Study conducted at Instituto da Criança, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil.