To summarize the current literature describing high-flow nasal cannula use in children, the components and mechanisms of action of a high-flow nasal cannula system, the appropriate clinical applications, and its role in the pediatric emergency department.
SourcesA computer-based search of PubMed/MEDLINE and Google Scholar for literature on high-flow nasal cannula use in children was performed.
Data summaryHigh-flow nasal cannula, a non-invasive respiratory support modality, provides heated and fully humidified gas mixtures to patients via a nasal cannula interface. High-flow nasal cannula likely supports respiration though reduced inspiratory resistance, washout of the nasopharyngeal dead space, reduced metabolic work related to gas conditioning, improved airway conductance and mucociliary clearance, and provision of low levels of positive airway pressure. Most data describing high-flow nasal cannula use in children focuses on those with bronchiolitis, although high-flow nasal cannula has been used in children with other respiratory diseases. Introduction of high-flow nasal cannula into clinical practice, including in the emergency department, has been associated with decreased rates of endotracheal intubation. Limited prospective interventional data suggest that high-flow nasal cannula may be similarly efficacious as continuous positive airway pressure and more efficacious than standard oxygen therapy for some patients. Patient characteristics, such as improved tachycardia and tachypnea, have been associated with a lack of progression to endotracheal intubation. Reported adverse effects are rare.
ConclusionsHigh-flow nasal cannula should be considered for pediatric emergency department patients with respiratory distress not requiring immediate endotracheal intubation; prospective, pediatric emergency department-specific trials are needed to better determine responsive patient populations, ideal high-flow nasal cannula settings, and comparative efficacy vs. other respiratory support modalities.
Resumir a literatura atual que descreve o uso da cânula nasal de alto fluxo em crianças, os componentes e mecanismos de ação do sistema de cânula nasal de alto fluxo, as aplicações clínicas adequadas e o papel desse sistema no departamento de emergência pediátrico.
FontesRealizamos uma pesquisa informatizada na PubMed/MEDLINE e utilizamos o Google Acadêmico para encontrar literatura sobre o uso da cânula nasal de alto fluxo em crianças.
Resumo dos dadosA cânula nasal de alto fluxo, modalidade de suporte respiratório não invasiva, fornece misturas de gases aquecidas e totalmente umidificadas para pacientes por meio de uma cânula nasal. A cânula nasal de alto fluxo provavelmente auxilia a respiração por meio da redução da resistência inspiratória, eliminação do espaço morto anatômico nasofaríngeo, redução do trabalho metabólico relacionado ao condicionamento de gás, melhora da condutância das vias aéreas e transporte mucociliar e fornecimento de baixos níveis de pressão positiva nas vias aéreas. A maior parte dos dados que descrevem o uso da cânula nasal de alto fluxo em crianças é focada em crianças com bronquiolite, embora a cânula nasal de alto fluxo tenha sido usada em crianças com outras causas de doenças respiratórias. A introdução da cânula nasal de alto fluxo na prática clínica, incluindo o departamento de emergência, foi associada à redução dos índices de intubação endotraqueal. Dados intervencionistas prospectivos limitados sugerem que a cânula nasal de alto fluxo pode ser tão eficaz quanto a pressão positiva contínua nas vias aéreas e mais eficaz do que a oxigenoterapia padrão em alguns pacientes. As características dos pacientes, como melhora da taquicardia e taquipneia, foram associadas a uma ausência de progressão para intubação endotraqueal. Foram raros os efeitos adversos relatados.
ConclusõesA cânula nasal de alto fluxo deve ser considerada para pacientes do departamento de emergência pediátrico com insuficiência respiratória que não precisam de intubação endotraqueal imediata, contudo, são necessários ensaios clínicos prospectivos específicos para o departamento de emergência pediátrico para determinar melhor as populações de pacientes que respondem ao tratamento, as configurações ideais da cânula nasal de alto fluxo e a eficácia comparada a outras modalidades de suporte respiratório.
High-flow nasal cannula (HFNC) is a non-invasive respiratory support modality that provides conditioned (heated and fully humidified) gas mixtures to patients via a nasal cannula interface. There is no universally accepted definition of the minimum flow rate that defines “high” flow. In neonates, high-flow may be defined as flow rates ≥2L/min, whereas for older children, flow rates ≥4–6L/min are commonly considered high.1–3 Over the past decade, HFNC systems have gained increased acceptance and are now widely utilized to support critically-ill patients across the entire age spectrum, from premature neonates to adults. It has also found a role across various hospital sites, including the neonatal intensive care unit (NICU), pediatric intensive care unit (PICU), medical and surgical intensive care units (ICU), intermediate care units, and, more recently, the emergency department (ED). A recent randomized controlled trial has shown that HFNC may be superior to standard low-flow oxygen delivery in preventing treatment failure in children with bronchiolitis,4 while other trials support that HFNC is equivalent to more traditional modalities of non-invasive ventilation support, such as continuous or bi-level positive airway pressure (CPAP or BiPAP).5,6
In this article, the authors review the rationale for utilizing HFNC in children, the basic anatomy of a HFNC system, its mechanisms of action, clinical application, and its role in the pediatric ED.
Rationale for using HFNCOxygen supplementation is a cornerstone of treating children with hypoxemia due to an acute respiratory process, typically through a facemask or a simple nasal cannula. The concentration of oxygen in the inspired gas increases as the flow rate of oxygen is increased and less atmospheric air is entrained during inspiration. Unlike atmospheric air, which is rich in water vapor, medical gases – including oxygen – are stored as a dehydrated substance. Prolonged administration of supplemental oxygen causes dryness and irritation of the mucus membranes and adversely affects mucociliary clearance, unless humidification is added.7 It is routine in the hospital setting for a bubble humidifier filled with sterile water to be used for this purpose. These simple and affordable devices provide some level of hydration to dry medical gases, but this humidification is not adequate for gas flows greater than 5L/m.7,8 When higher gas flows are utilized, it is imperative that the gas mixture be fully saturated with water vapor and heated close to body temperature, as the airway mucosa is unable to independently transfer sufficient heat and humidity at these supraphysiologic flow rates.
The delivery of high-flow therapy is predicated on four important features.
- (1)
“Open” system: gas flow should be delivered through a cannula interface that does not obstruct the nostrils. This is a key distinction compared to pressurized modes of nasal ventilation, such as CPAP and BiPAP. There should be ample opportunity for gas leakage around the cannula, and a standard rule is to size the nasal cannula's prongs to occupy no more than 50% of the cross-sectional area of each nostril.
- (2)
Conditioned gas: gas mixtures delivered through HFNC should be properly heated and humidified to prevent desiccation of the respiratory mucosa.
- (3)
High flows: HFNC should deliver gas mixture flows that are greater than the patient's peak inspiratory flow, so as to prevent significant entrainment of ambient air during inspiration.
- (4)
High velocity: gas delivered at high velocity deeply penetrates the airway, moving the source of fresh gas closer to the carina and providing some level of respiratory support.
Although the composition of an HFNC system varies among medical equipment manufacturers, the basic set up includes the same essential elements: (1) a source of pressurized oxygen and air regulated by a flow-meter/blender; (2) a sterile water reservoir attached to an efficient heater humidifier; (3) an insulated and/or heated circuit that maintains temperature and relative humidity of the conditioned gas as it travels toward the patient; and (4) a non-occlusive cannula interface.
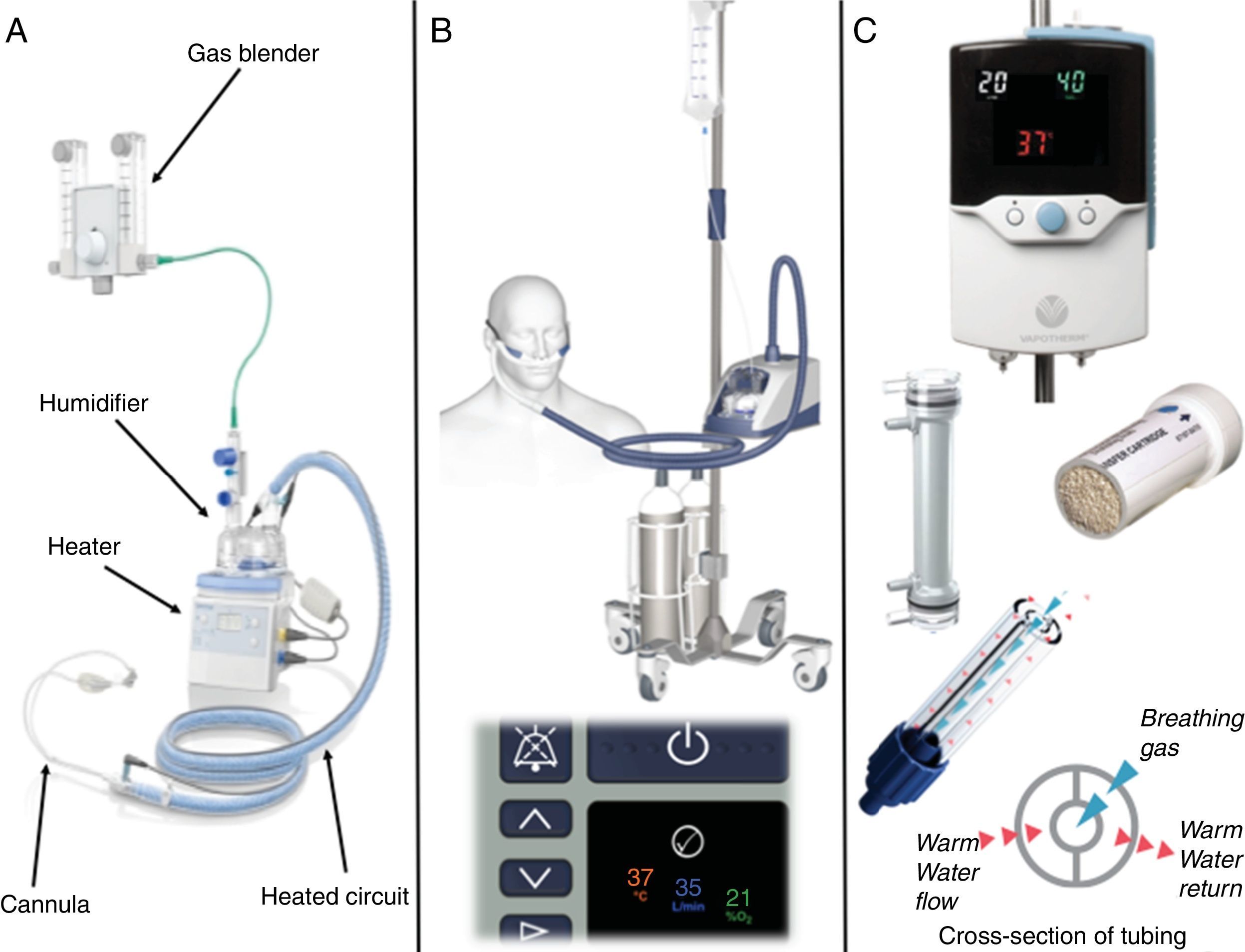
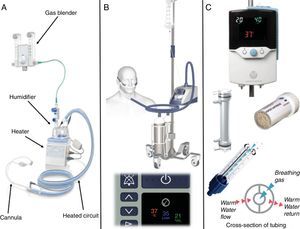
An HFNC system can be assembled from items commonly used in respiratory care and widely available in most units. These systems (Fig. 1A) are composed of a water reservoir heated by a plate heater, such as those used in mechanical ventilators, a high-flow blender to control gas composition and flow, and a circuit fitted with a heated wire to maintain gas temperature and reduce condensation. In the authors’ experience, HFNC support is more commonly delivered through commercial equipment designed specifically for this purpose, such as the Airvo 2 (Fisher & Paykel Healthcare Limited, Auckland, New Zealand) (Fig. 1B) or the Precision Flow system (Vapotherm Inc., Exeter, New Hampshire, United States) (Fig. 1C).
Examples of commercially available devices to deliver high-flow nasal cannula (HFNC) support. Panel A: HFNC system assembled from commonly available components, including a blender, heater/humidifier, heated circuit, and cannula. Panel B: the Airvo 2 HFNC system shown here as a mobile unit with air and oxygen cylinders (top), and a close up of the digital console indicating the set temperature, flow and oxygen concentration of the inspired gas (bottom). Panel C: the Precision Flow HFNC system (top), the internal humidification cartridge and a cutout showing the hollow-fiber configuration (middle), and a cutout of the circuit with a diagram of the warm water insulation system (bottom). Images courtesy of Fisher & Paykel Healthcare Limited (A and B) and Vapotherm Inc (C).
The Airvo 2 is a versatile HFNC device, capable of delivering a wide range of conditioned gas flows. It comprises a humidifier chamber resting on a plate heater, a digital console for setting gas flow (2–60L/min) and temperature (31, 34, or 37°C), a breathing circuit with dual spiral heating elements fitted with an integrated temperature sensor, and contoured malleable cannulas with soft prongs for added comfort and size options ranging from small neonates to adults. Supplemental oxygen can be blended into the circuit and regulated through an external flow meter, while a built-in ultrasonic oxygen sensor analyzes the fraction of inspired oxygen (FiO2) and displays it on the digital console.
The Precision Flow is a fully integrated device that uses a disposable high-efficiency hollow-fiber cartridge humidification system. Gas transits through the lumen of the hollow fibers while heated water vapor is forced through its small particle (0.005μm) pores. An intuitive single button interface controls gas temperature (33–43°C, adjustable in 1°C increments), FiO2 (0.21–1, adjustable in 0.01 increments), and gas flow (1–40L/min). The cartridge designed for newborns and infants has an operational flow range between 1 and 8L/min, while the cartridge for children and adults operates between 5 and 40L/min; both are cleared for 30-days continuous use on a single patient. Conditioned gas transits from the device to the cannula interface through the center lumen of a heated water-insulated tube that maintains gas temperature and minimizes condensation. A wide array of cannula sizes fit the entire age spectrum, including a single prong cannula to prevent occlusion of the nasal passages in small neonates who also require a nasoenteric tube.
Mechanisms of actionA growing body of evidence indicates that HFNC exerts potentially beneficial effects though several different mechanisms, which include: (1) reduced inspiratory resistance, (2) washout of the nasopharyngeal anatomical dead space, (3) reduced metabolic work related to gas conditioning, (4) improved airway conductance and mucociliary clearance, and (5) provision of low levels of positive airway pressure.
- (1)
Reduced inspiratory resistance: The nostrils and the nasal passages are the points of highest resistance in the human airway.7,9 The use of a flow that meets or exceeds an individual's inspiratory demand through a properly positioned nasal cannula helps offset that inspiratory resistance by effortlessly delivering fresh gas further down the airway, thus bypassing the area of highest resistance and decreasing the work of breathing.
- (2)
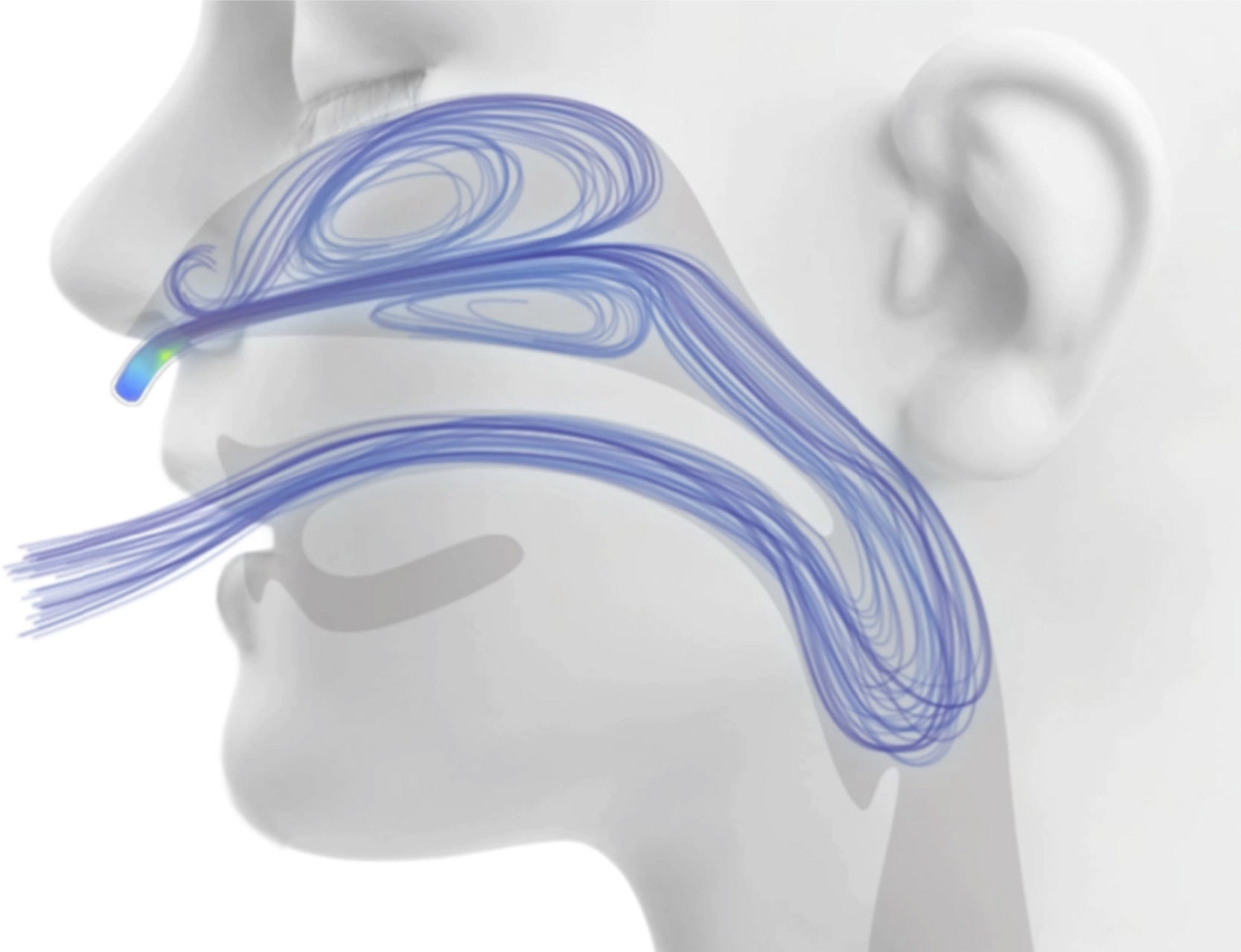
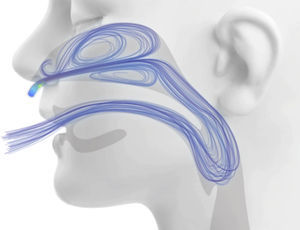
Washout of the nasopharyngeal anatomical dead space: During normal breathing, the nasopharynx contains carbon-dioxide rich gas at the end of exhalation. This gas is then rebreathed during the next respiratory cycle, which reduces the efficiency of gas exchange. When an HFNC system is used, the fresh gas rapidly occupies the nasal cavity and pharynx, washing out carbon dioxide-rich gas from the nasopharyngeal dead space via the aforementioned “open” system (Fig. 2).10 This is equivalent to using the nasopharyngeal anatomical dead space as a reservoir of fresh gas, thus reducing rebreathing and effectively decreasing the contribution of the anatomical dead space to breathing inefficiency. Therefore, a patient supported by HFNC can employ a reduced respiratory effort and lower respiratory rate to maintain the same level of alveolar ventilation and PaCO2. This mechanism is particularly important in small children, considering that the extrathoracic anatomical dead space of a newborn is as high as 3mL/kg and does not approximate that of an adult (0.8mL/kg) until after 6 years of age.11
- (3)
Reduced metabolic work related to gas conditioning: HFNC supplies fully conditioned gas to the airway, thus reducing insensible water losses and the energy cost of heating the inspired gas to body temperature.10
- (4)
Improved airway conductance and mucociliary clearance: Inhalation of heated and humidified gas prevents desiccation of respiratory secretions,7 decreases dyspnea and the sensation of oropharyngeal dryness,12 and has potentially salutary effects on the mucociliary apparatus function.13,14
- (5)
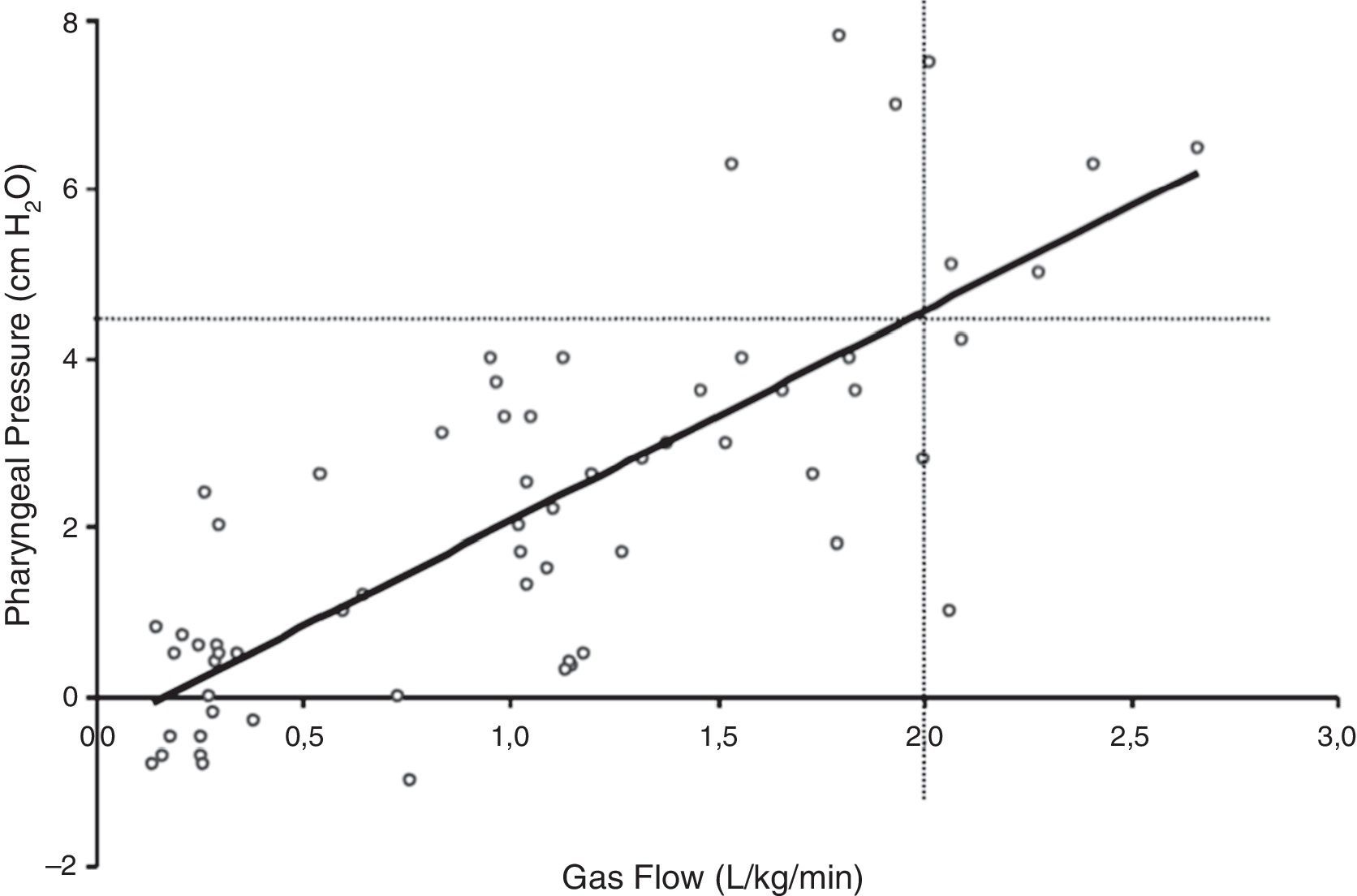
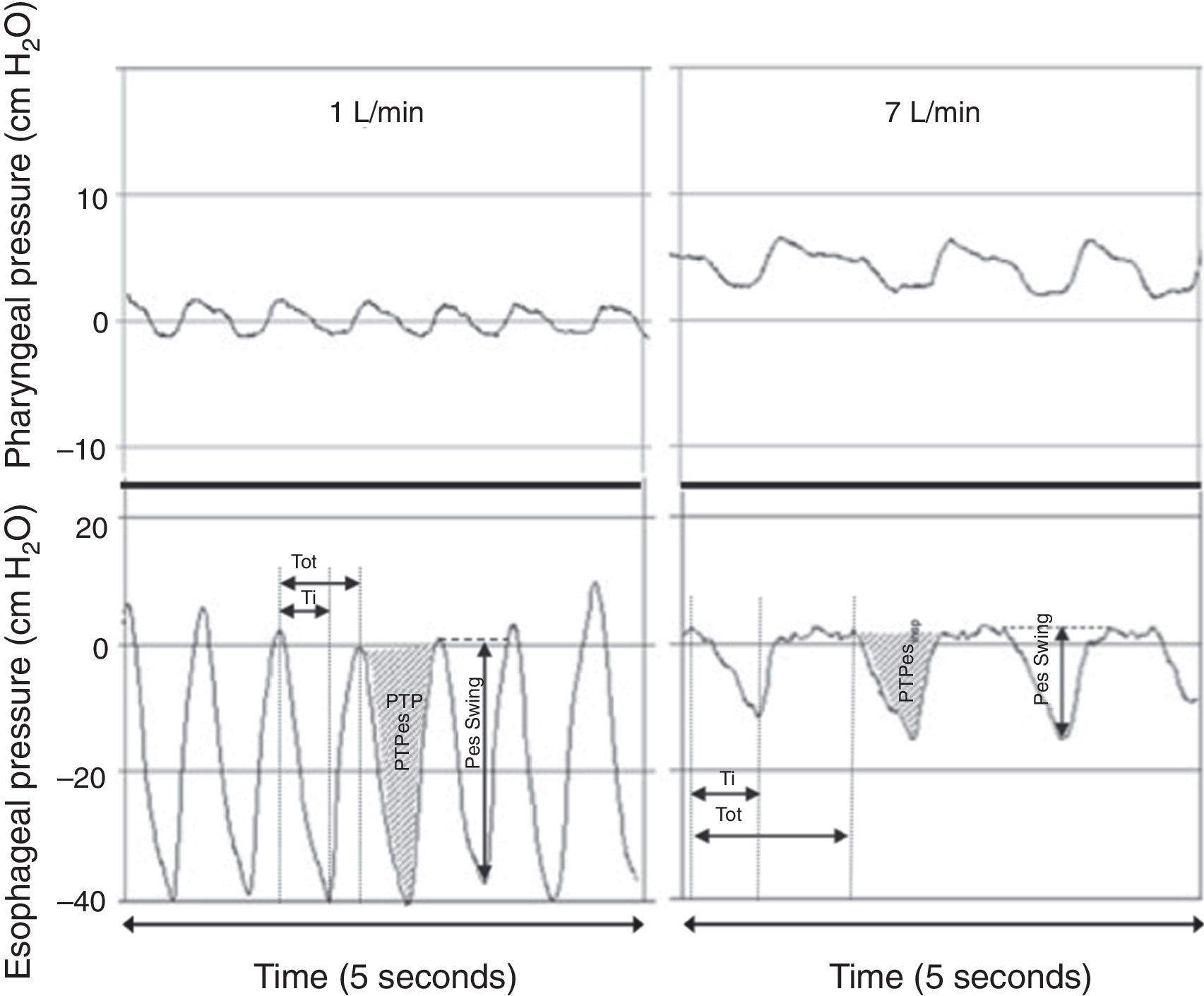
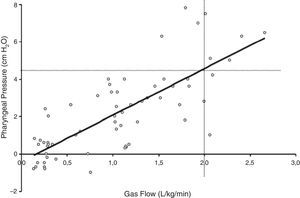
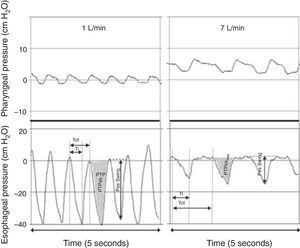
Provision of low levels of airway pressure: HFNC creates a low level of positive pharyngeal pressure that might assist in reducing the dynamic inspiratory airway resistance and provide some degree of continuous positive airway pressure.10 The degree of observed positive airway pressure is directly related to HFNC flow rate, affected by mouth opening, and dependent on the pressure measurement site.10 Three studies have used pressures measured in the nasopharynx as a surrogate for lower positive end-expiration pressures. Milési et al.15 studied 21 infants <6 months with bronchiolitis and found that increasing HFNC to 6–7L/min leads to pharyngeal pressures that increase to ∼6cmH2O and are weight-dependent, with flows of ≥2L/kg/min required to achieve a pharyngeal pressure of ≥4cmH2O (Fig. 3). Arora et al.16 reported lower pharyngeal pressures of ∼3cmH2O in 25 patients with bronchiolitis on 6–8L/min, and pressures were even lower if the subject's mouth was open. Spentzas et al.17 reported nasopharyngeal pressures of 4–5cmH2O in infants on 8–12L/min and pressures of ∼2cmH2O in older patients on 20–30L/min. Pressures measured through a nasopharyngeal probe are potentially affected by direct impression of the inspired gas jet and likely overestimate the actual pressure transmitted to the distal intrathoracic airway, leading other authors to use an esophageal balloon catheter to measure esophageal pressure (Pes).18–20 In one study of 11 infants with bronchiolitis, end-expiratory Pes was ∼7cmH2O on 8L/min.20 In another study of 24 infants with bronchiolitis or recovering from cardiac surgery, end-expiratory Pes was ∼4cmH2O on 2L/kg/min and not significantly different than Pes measured on 2L/min of flow.19 Similarly, a study of 25 critically-ill children with a variety of respiratory diseases found that end-expiratory Pes was ∼5cmH2O on 5–8L/min, only ∼1cm higher than measurements obtained while on a standard nasal cannula at 2L/min.18 Considered together, the available evidence supports the theory that HFNC generates very modest increases in positive end-expiratory pressure relative to standard nasal cannula, although the actual amount is dependent on the HFNC flow and patient size. Regardless of mechanism, HFNC has been shown to significantly reduce the work of breathing, mostly by attenuating the negative intrathoracic inspiratory pressure as evidenced by a decreased esophageal pressure swings (Fig. 4) and electrical activity of the diaphragm.15,19
Figure 3.Relationship between pharyngeal pressure and gas flow during HFNC support. Adapted from Milési et al.15
Figure 4.Simultaneous recordings of pharyngeal (top) and esophageal (bottom) pressures from an infant receiving 1L/min (left) and 7L/min (right) through a nasal cannula. Although there is a notable increase in pharyngeal pressure during higher flow conditions, virtually no increase is observed in end-expiratory pressure measured at the thoracic level. The application of 7L/min flow significantly decreased the intrathoracic pressure swings through an attenuation of the negative inspiratory pressure. Adapted from Milési et al.15
When initiating HFNC therapy, the clinician must control three main variables: gas temperature, FiO2, and flow rate. At our institution, temperature is typically set at approximately 1–2°C below body temperature, and adjusted as needed for patient comfort. In the authors’ experience, older children and young adults describe an uncomfortable and somewhat claustrophobic feeling when gas temperature is at or above body temperature, akin to what is experienced while breathing inside a steam sauna or on a very hot and humid summer day.
HFNC is usually initiated with an FiO2 of 0.6 for the hypoxemic patient, provided there are no physiologic contraindications to the use of these high concentrations of supplemental oxygen (e.g., patients recovering from Norwood stage I palliation for hypoplastic left heart syndrome). FiO2 is then rapidly adjusted up or down over the next few minutes to achieve the target oxygen saturation (SPO2), typically 92%–97%. Although most patients treated with HFNC receive a gas mixture enriched with supplemental oxygen, this is not necessarily the case for all patients. Patients with respiratory distress without hypoxemia can still benefit from the effects of HFNC on respiratory mechanics while receiving conditioned air without the addition of oxygen.
The choice of gas flow rate is based on patient size and the perceived magnitude of respiratory support needed. In general terms, older/larger patients and more dyspneic patients will require higher flows. There is no consensus on ideal HFNC flows. Some authors report using age-based protocols, such as 2L/min for patients <6 months, 4L/min for 6–18 months and 8L/min for those aged 18–24 months21; or 8–12L/min for infants and 20–30L/min for children.17 Others report weight-based dosing, such as 1L/kg/min22 or 2L/kg/min,23 and emerging data support that the effects of HFNC are dependent on weight.15 Modest support can be initially provided with 0.5–1.0L/kg/min, and increasing the flow to up to 1.5–2.0L/kg/min may further attenuate intrathoracic pressure swings and reduce the work of breathing.24 Flows>2L/kg/min may not be additionally efficacious.24 In our practice, a neonate might have HFNC started with flows of 4–5L/min, while a young child starts with a flow of 5–15L/min (Table 1). Initial flow rates of 50L/min have been used in prospective studies of critically ill adults and may be reasonable for adult-sized PICU patients.5
Typical starting flows for initiation of HFNC and clinical flow ranges according to age group and size.
| Age | Weight (kg) | Cannula | Typical starting flow (L/min) | Typical flow range (L/min) |
|---|---|---|---|---|
| 0–30 days | <4 | Neonate | 4–5 | 2–8 |
| 1 month to 1 year | 4–10 | Infant | 4–10 | 2–20 |
| 1–6 year | 10–20 | Pediatric small | 5–15 | 5–30 |
| 6–12 year | 20–40 | Pediatric | 10–20 | 5–40 |
| >12 year | >40 | Pediatric large/adult | 20–30 | 5–50 |
L, liters; min, minutes.
Considering the success of HFNC in the treatment of critically-ill neonates, children, and adults – especially the reported reduction in the need for intubation of infants with bronchiolitis treated in the PICU – the natural next step for clinicians was to consider earlier initiation of HFNC in patients while still in the ED. With its broad range of potentially appropriate patient populations, ease of use, portability, and favorable patient safety and comfort profile, HFNC is rapidly becoming an important adjunct modality in the treatment of acute respiratory failure in the pediatric ED.25,26 While there is a paucity of robust pediatric ED-specific data, the authors recommend considering the use of HFNC in infants and children with acute hypoxemic respiratory failure who need support beyond a standard nasal cannula but do not require endotracheal intubation. HFNC may be initiated following a failed trial of regular nasal cannula or as a primary respiratory support modality.
The burgeoning literature describing HFNC use in children focuses primarily on those with bronchiolitis, although HFNC use has also been reported in children with other causes of respiratory distress, including pneumonia, asthma, croup, and other forms of upper airway obstruction; neuromuscular disease; and convalescence from cardiac surgery.25–29 Several studies have evaluated whether introduction of HFNC into clinical care was associated with a reduced need for invasive mechanical ventilation. Two small retrospective studies of PICU patients with moderate to severe bronchiolitis reported that making HFNC available for clinical use was associated with a decreased overall need for intubation and mechanical ventilation.27,30 A larger study of PICU patients with various etiologies of respiratory distress similarly showed reduced intubation rates after introduction of HFNC.31 While these studies are limited by their retrospective design and use of historical controls, similar results were found when HFNC was implemented in the pediatric ED.25 Wing et al. studied 848 children with acute respiratory insufficiency requiring PICU admission.25 Overall intubation rates decreased from 15.8% to 8.1% (p=0.006) with introduction of HFNC and establishment of a guideline for use, including a decrease from 21% to 10% (p=0.03) among children with bronchiolitis.25 The overall decrease was largely accounted for by a decreased intubation rate in the pediatric ED, from 10.5% to 2.2% (p<0.001), while rates of intubation after transfer to the PICU remained steady.25
Other studies have compared intubation rates of children treated with HFNC or CPAP. In one prospective randomized controlled trial of PICU patients younger than 6 months of age with bronchiolitis, no difference was observed regarding need for invasive mechanical ventilation among subjects treated with HFNC at 2L/kg/min when compared with those treated with CPAP at 7cmH2O, although HFNC was associated with more frequent worsening of dyspnea.32 In a prospective randomized controlled trial of children under 5 years of age with pneumonia, subjects treated with HFNC had similar rates of clinical deterioration, intubation, and death when compared with those treated with CPAP.33 Retrospective studies of PICU patients with bronchiolitis22 and varied forms of respiratory failure28 report similar intubation rates between subjects treated with CPAP and those receiving HFNC. However, there are no published prospective interventional trials of pediatric ED patients specifically designed to test whether HFNC reduces the need for mechanical ventilation, so these data should be generalized to ED patients with caution. Nevertheless, due to its relative safety, comfort and ease of use, the authors recommend consideration of HFNC as a mode of respiratory support for children presenting to the ED with moderate to severe respiratory distress.
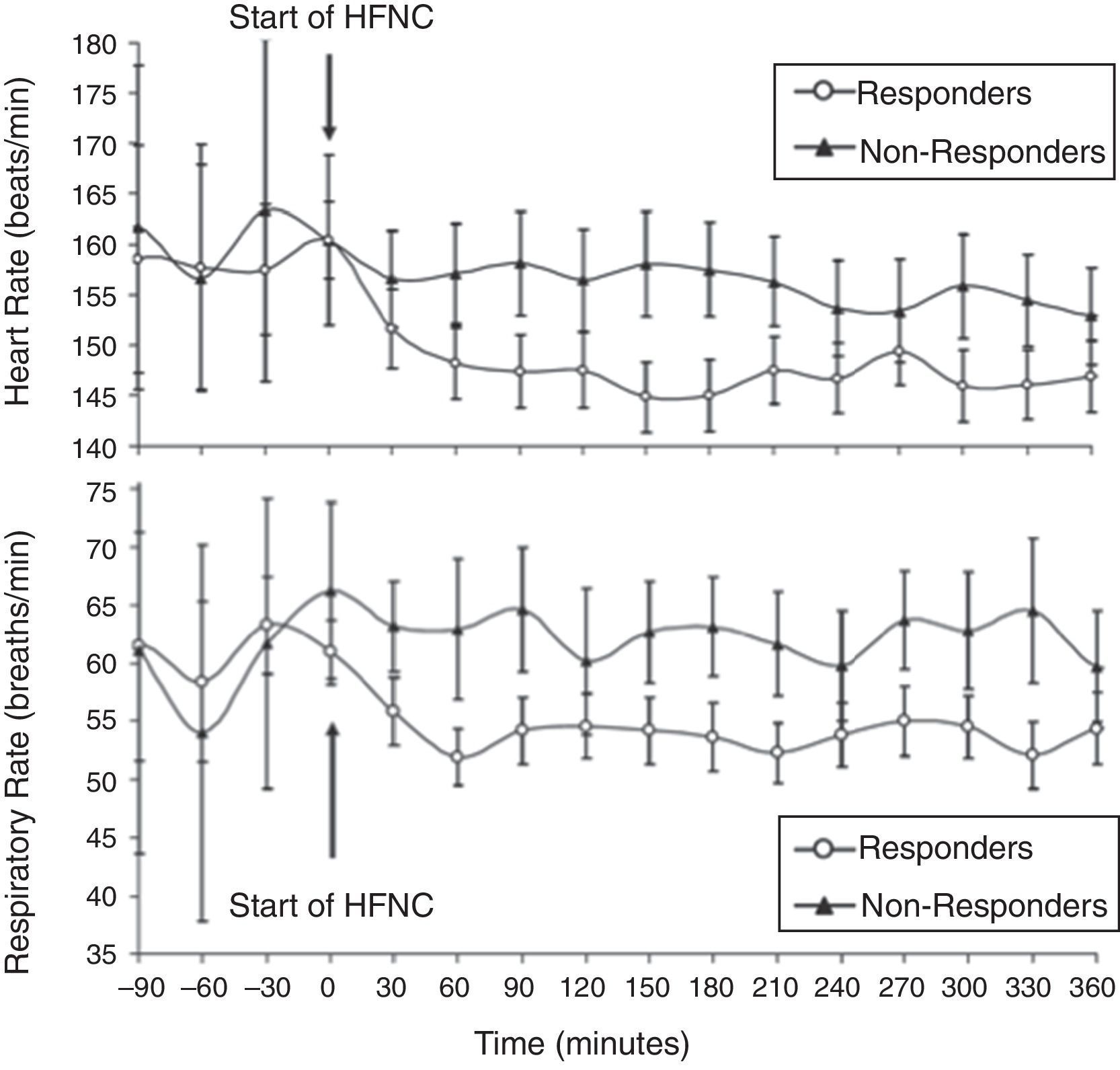
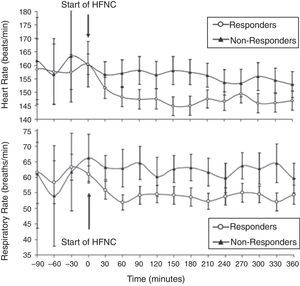
With this strategy, children who have not responded to HFNC and require a higher level of respiratory support must be quickly identified. Several small retrospective studies conducted in the pediatric ED and PICU have sought to identify demographic and physiologic factors that could discriminate between HFNC success and failure in children with bronchiolitis.23,26,27,34 Predictors of success include a significant decrease in heart rate from baseline within 60min of HFNC initiation,23,27 and similarly significant improvement in respiratory rate27,34 (Fig. 5). Infants and children who failed HFNC, variably defined as a need for PICU admission, or escalation to noninvasive or invasive ventilation, were younger,26 smaller,34 experienced no improvement in heart rate23,27 or respiratory rate34; they were found to be sicker upon presentation, with worse initial respiratory rate,26 respiratory acidosis,26,34 and severity of illness scores.27,34
Heart rate (top) and respiratory rate (bottom) over time in infants with acute viral bronchiolitis. A notable reduction in heart rate and respiratory rate at 60min following initiation of HFNC support separates responders from non-responders in this cohort. Adapted from Schibler et al.27
For less ill children, HFNC has been compared with standard nasal cannula (NC). In one prospective observational study of pediatric ED patients with bronchiolitis, 18 children were treated with HFNC and 18 children were treated with standard NC because no HFNC system was available.35 Although the groups had similar baseline characteristics, HFNC was associated with faster improvement in dyspnea and shorter duration of hospitalization.35 In another observational study of 94 bronchiolitis patients initiated on HFNC in the ED, need for PICU transfer was four-fold lower than 33 contemporary controls receiving standard NC therapy.23 There have also been several interventional trials of HFNC vs. standard NC. In one study, children under 18 months of age undergoing extubation following cardiac surgery were randomized to HFNC at 2L/kg/min or 2L/min of standard NC.36 HFNC was associated with increased PaO2:FiO2 ratio and less need for non-invasive positive pressure ventilation, but no difference in PaCO2 or re-intubation.36 A small prospective pilot study of 19 children with bronchiolitis found no difference in duration of oxygen therapy or hospitalization with HFNC vs. head-box oxygen.37 In a much larger, open, randomized controlled trial comparing HFNC and standard NC in children with moderate bronchiolitis admitted to the general ward, Kepreotes et al.4 also found no difference in length of hospitalization, duration of oxygen therapy, or estimated room costs between the two groups. However, the authors estimated the pragmatic trial design, which allowed children in the standard NC oxygen group who experienced treatment failure to escalate to HFNC therapy while staying on the general care ward, was ultimately cost-effective. This is important, given that hospital charges associated with bronchiolitis are rising and efforts toward limiting costs are recommended.38
A key factor in the cost of HFNC therapy is the location in the hospital in which it is provided. Many hospital systems only use HFNC in the ED and PICU. Other centers have reported HFNC use on the general wards with reasonable safety profiles.21,23,37,39–41 Allowing HFNC use on the general ward was associated with decreased median length of hospitalization and total hospital charges in one retrospective study of children with bronchiolitis initially treated with HFNC in the PICU.21 Reducing hospital costs by providing HFNC on the general wards must be balanced with patient safety concerns, especially given a ∼10% rate of eventual intubation reported in several studies of varying patient populations.17,21,22,26,27,30,33,34,40
When HFNC is started in the ED, consideration should also be given to the availability of portable HFNC equipment, so as to avoid the need to interrupt therapy (and the associated risk of clinical deterioration) while transporting a patient from the ED to an inpatient unit. Although some of these patients might tolerate the delivery of non-heated non-humidified gas through a simple cannula or even interruption of HFNC delivery for a short period of time during transport, the authors prefer to deploy self-contained portable transport HFNC devices in the ED so that patients can be transported to the inpatient site without interruptions in treatment, when needed.
Assessment of clinical response to HFNCAs more hospitals and more sites within a hospital utilize HFNC, it is imperative that usage guidelines be developed, including how, on who, and when to initiate HFNC, protocols for titration and weaning, frequency and type of serial clinical assessment, and clear definitions as to what constitutes treatment failure with the need to escalate to other forms of non-invasive ventilation (CPAP or BiPAP) or endotracheal intubation.
The clinical impact of HFNC is subjectively obvious the patient within minutes of its initiation, as evidenced by the reports of reduced dyspnea and improved comfort expressed by adult patients and verbal children. Standard objective signs of clinical response, such as heart rate, respiratory rate, nasal flaring, accessory muscle use, and SpO2, used individually or as part of a respiratory distress score, are often used to gauge clinical response to treatment. Several studies show that initiation of HFNC is associated with improvements in respiratory effort as measured by improvements in vital signs, clinical respiratory scores, and gas exchange.17,19,30,35,39–41 In the authors’ clinical experience, responders can usually be discerned from non-responders within 60min of initiating HFNC, and often even sooner than that. Some centers have attempted to asses work of breathing in a more objective way than mere direct clinical observation by measuring intrathoracic pressure swings during the respiratory cycle through a pressure sensor or balloon placed in the mid-distal intrathoracic portion of the esophagus.15,18,24 When considered in conjunction with the patient's respiratory rate (pressure/rate product), estimates of intrathoracic pressure measured in the esophagus can be a valuable tool to evaluate clinical response to HFNC and even help titrate support.15,18,24 However, the need for insertion of the esophageal transducer and special pressure transducing equipment mostly relegates this monitoring technique to use in research.
Patients who show no clinical improvement or continue to deteriorate despite initiation of HFNC should be considered for elective escalation of therapy (i.e., BiPAP or endotracheal intubation) prior to the development of acute cardiorespiratory collapse. Conversely, patients who show significant clinical improvement still warrant close observation in a monitored environment due to the cyclic nature of many respiratory conditions (i.e., bronchiolitis) and the possibility that the improvement observed after initiation of HFNC might only be temporary.
Adverse eventsHFNC is generally safe, provided it is used within its accepted clinical parameters. Adverse events are generally mild, such as epistaxis, skin irritation caused by the cannula interface, or aerophagia. Serious adverse events are extremely rare, but pneumocephalus has been reported in a premature neonate,42 and cases of pulmonary air leak (i.e., pneumothorax or pneumomediastinum)43 have been reported in older children receiving HFNC support. These adverse events underscore the importance of delivering HFNC through an “open system” with a properly sized non-occlusive cannula that allows for ample gas leak between the prongs and the surrounding nostrils. An open system ensures that the nasal cannula prongs are non-occlusive, thus reducing the risk of sudden increases in airway pressure as a result of inadvertent obstruction. It is important to note that the incidence of pulmonary air leak observed in randomized controlled trials in vulnerable preterm neonates was not different between patients treated with HFNC or nasal CPAP.6 Furthermore, the incidence of skin breakdown in patients treated with HFNC was significantly lower than in patients treated with a nasal CPAP interface that requires pressure onto the skin surface to create an occlusive seal.6
Feeding patients on HFNCIt is not uncommon for patients with respiratory distress, especially infants with viral bronchiolitis, to have inadequate oral intake in the hours or even days leading up to the ED visit. In addition, the discomfort from hunger often contributes to a patient's agitation, which can aggravate respiratory distress. For these reasons and the high value of resuming adequate nutrition, consideration is often given as to whether or not a patient receiving HFNC can be fed. Although this concern might not be pertinent to the ED environment in institutions where patients on HFNC are quickly transitioned to the PICU, delays in definitive placement of a patient on HFNC might take many hours or even days in other centers.
Although some practitioners consider HFNC use to be a contraindication for oral feeding, it has been the authors’ personal experience that adults can swallow liquids and solids without difficulty when subjected to flows as high as 40L/min. This group and others have shown that feeding selected patients on HFNC can be done safely and with few adverse events.44,45 In this institution, patients on HFNC who have experienced significant and sustained clinical improvement of their respiratory distress are generally feed, regardless of the HFNC flow rate. The authors’ practice has been to disregard HFNC as a factor in the decision to initiate enteral feeds and apply the same criteria that would be used for any patient in respiratory distress (with or without HFNC): a patient who has shown sufficient improvement of various markers of respiratory distress will be tried on enteral feeds regardless of HFNC flow, while enteral nutrition will be withheld from a patient who continues to struggle and is at risk of requiring escalation of support and/or aspiration. The authors believe that the combination of HFNC support and the comfort of enteral feeding (for patients who meet the criteria for such) can go a long way toward reducing the distress experienced by infants with acute viral bronchiolitis being treated in the acute in-hospital setting.
ConclusionHFNC has found a well-defined role in the treatment of children with acute hypoxemic respiratory failure, bridging the gap between the delivery of low-flow supplemental oxygen and traditional non-invasive ventilation (i.e., CPAP, BiPAP). Considering its ease of use, comfort, and the growing body of clinical evidence supporting its clinical equivalence to other non-invasive ventilation modalities, the use of HFNC is expected to continue to expand beyond the confines of the neonatal and pediatric ICUs. Retrospective data and anecdotal reports showing a reduction in the need for intubation and mechanical ventilation when HFNC is initiated in the ED are encouraging, and more definitive data from large randomized clinical trials are eagerly awaited to determine the exact role of HFNC in various patient subsets presenting to the pediatric ED in respiratory distress.46
Conflicts of interestDr. Rotta has received honoraria for the development of educational materials and for lectures sponsored by Vapotherm, Inc. and by BD/Carefusion. He also receives royalties from Elsevier for editorial work on a pediatric critical care textbook. The other authors declare no conflicts of interest.
Please cite this article as: Slain KN, Shein SL, Rotta AT. The use of high-flow nasal cannula in the pediatric emergency department. J Pediatr (Rio J). 2017;93:36–45.