Posterior urethral valve is the most common lower urinary tract obstruction in male children. A high percentage of patients with posterior urethral valve evolve to end-stage renal disease. Previous studies showed that cytokines, chemokines, and components of the renin–angiotensin system contribute to the renal damage in obstructive uropathies. The authors recently found that urine samples from fetuses with posterior urethral valve have increased levels of inflammatory molecules. The aim of this study was to measure renin–angiotensin system molecules and to investigate their correlation with previously detected inflammatory markers in the same urine samples of fetuses with posterior urethral valve.
MethodsUrine samples from 24 fetuses with posterior urethral valve were collected and compared to those from 22 healthy male newborns at the same gestational age (controls). Renin–angiotensin system components levels were measured by enzyme-linked immunosorbent assay.
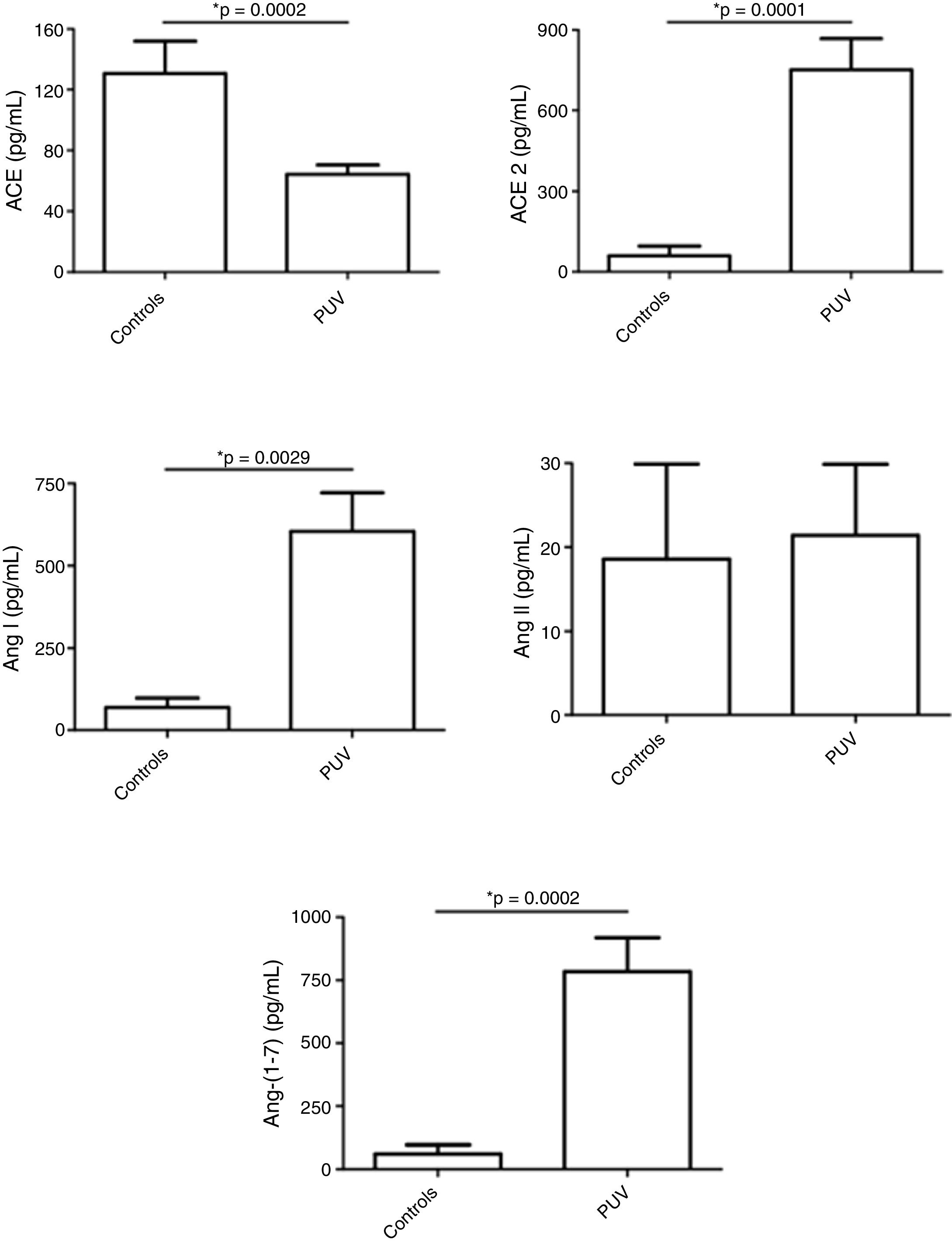
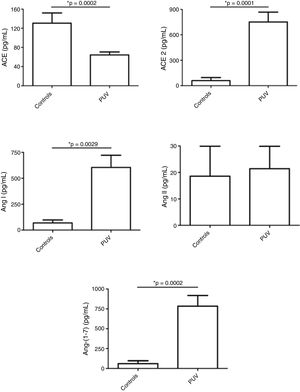
ResultsFetuses with posterior urethral valve presented increased urinary levels of angiotensin (Ang) I, Ang-(1-7) and angiotensin-converting enzyme 2 in comparison with controls. ACE levels were significantly reduced and Ang II levels were similar in fetuses with posterior urethral valve in comparison with controls.
ConclusionsIncreased urinary levels of angiotensin-converting enzyme 2 and of Ang-(1-7) in fetuses with posterior urethral valve could represent a regulatory response to the intense inflammatory process triggered by posterior urethral valve.
A válvula de uretra posterior é a obstrução do trato urinário inferior mais comum em crianças do sexo masculino. Uma alta porcentagem de pacientes com válvula de uretra posterior evolui para doença renal em estágio final. Estudos anteriores mostraram que citocinas, quimiocinas e componentes do sistema renina-angiotensina contribuem para o dano renal em uropatias obstrutivas. Recentemente, descobrimos que amostras de urina de fetos com válvula de uretra posterior tinham níveis aumentados de moléculas inflamatórias. O objetivo deste estudo foi medir as moléculas de renina-angiotensina e investigar sua correlação com marcadores inflamatórios previamente detectados nas mesmas amostras de urina de fetos com válvula de uretra posterior.
MétodosAmostras de urina de 24 fetos com válvula de uretra posterior foram coletadas e comparadas com amostras de urina de 22 recém-nascidos saudáveis de mesma idade gestacional (controles). Os níveis dos componentes de SRA foram medidos por ensaio de imunoabsorção enzimática.
ResultadosOs fetos com válvula de uretra posterior apresentaram níveis urinários aumentados de angiotensina (Ang) I, Ang-(1-7) e enzima conversora de angiotensina 2 em comparação com os controles. Os níveis de enzima conversora de angiotensina eram significativamente menores e os níveis de Ang II eram semelhantes nos fetos com válvula de uretra posterior em comparação com os controles.
ConclusõesO aumento dos níveis urinários de enzima conversora de angiotensina 2 e de Ang-(1-7) em fetos com válvula de uretra posterior poderia representar uma resposta regulatória ao intenso processo inflamatório desencadeado pela válvula de uretra posterior.
Posterior urethral valve (PUV) is a membranous structure in posterior urethra that obstructs the urinary outflow from the bladder1 and can lead to chronic kidney disease.2 PUV can be diagnosed in utero; antenatal ultrasound findings of PUV include bilateral hydronephrosis, megacystis, and dilated posterior urethra in male fetuses.3 The obstruction of urinary outflow can result in oligohydramnios, which cause pulmonary hypoplasia, leading to early death.4,5
Advances in diagnosis and management of PUV resulted in a dramatic decrease in mortality.6 A retrospective cohort of fetuses with severe lower urinary tract obstruction (LUTO) attending two centers between January 1990 and August 2013 showed that the antenatal diagnosis and interventions in PUV were effective in improving the survival rate and renal function.7 These findings were further corroborated by another study showing that antenatal interventions resulted in a 13-fold increase in the six-month chance of survival.6
PUV is frequently associated with lesions in renal parenchyma, including dysplasia or hypoplasia.8 As a result, PUV patients may evolve to end-stage renal disease (ESRD).9 The outcomes appear to be dependent on the kind and extent of prenatal kidney damage. The functional outcome of patients with prenatally detected PUV was described as considerably better than clinically presenting, i.e., the long-term prognosis of intermediate severity PUV might be improved by prenatal diagnosis.10 It is worth mentioning that a significant proportion of PUV cases are not yet detected antenatally, presenting outside the neonatal period.11 Altogether, these facts highlight the relevance of finding antenatal PUV diagnosis biomarkers.
The precise pathophysiology of ESRD due to PUV is not completely understood. This research team is investigating the mechanisms of renal damage in obstructive uropathies. First, inflammatory molecules in urine samples from fetuses with PUV were measured; it was observed that fetuses with PUV present higher urinary levels of inflammatory molecules than controls.12 The present study is the continuation of the abovementioned investigation. Herein, the authors measured renin–angiotensin system (RAS) components in the same urine samples. Experimental studies showed that RAS components may interact with inflammatory molecules in obstructive nephropathies.13,14 Obstructed kidneys also exhibited an elevation in angiotensin (Ang) II activity, which, in turn, decreases renal blood flow and causes ischemia and kidney growth arrest.15 Therefore, the current study was designed to measure RAS molecules and to investigate its correlation with previously detected inflammatory markers12 in the same urine samples of fetuses with PUV.
MethodsThis study included 24 pregnant women whose fetuses were diagnosed with LUTO by antenatal ultrasonography. All fetuses underwent a detailed ultrasound scan aimed at detecting renal abnormalities, other malformations, and markers of aneuploidy, as detailed elsewhere.5 The exclusion criteria were: (i) pregnant women with formal contraindication for invasive procedures; (ii) fetuses with chromosomal abnormalities; and (iii) fetuses presenting other malformation findings at ultrasonography. The study also included a control group of 22 healthy male preterm newborns matched by gestational age and by race/ethnicity with the fetuses with PUV at the time of urine collection. Preterm newborns with congenital malformations, infections and/or any acute illness at the time of urine sampling were excluded. The institutional Ethics Committee approved the study and all subjects (i.e., pregnant women or mothers of newborns) provided written informed consent.
Following the protocol, all fetuses with LUTO were submitted to bladder puncture in order to measure renal function parameters, including urinary osmolality, creatinine and β2-microglobulin. Fetal urine samples were collected using a long length spinal needle (BD Biosciences – San Jose, United States). Renal function markers helped clinical decisions regarding pregnancy and the need of fetal interventions as previously described.12 Briefly, fetal intervention was considered when there was severe LUTO at gestational ages between 16 and 34 weeks, in the presence of oligohydramnios (after 18 weeks) and normal renal function parameters (18–30 weeks of gestation), including the absence of bilateral renal dysplasia or renal cysts on ultrasonography and/or acceptable values in urinary biochemistry analysis.16–18
Urine samples from healthy male newborns were collected at the fifth day of life (control group) using a newborn urinary bag collector. All urine samples were centrifuged (1800g, 10min, 4°C) immediately after collection, and the supernatant was aliquoted and stored at −80°C until assayed.
Samples were then thawed and urine levels of Ang I, Ang II, Ang-(1-7), angiotensin-converting enzyme (ACE), and ACE2 were measured by enzyme-linked immunosorbent assay (ELISA), according to the procedures supplied by the manufacturer (MyBioSource, San Diego, CA, United States). All kits applied sandwich ELISA technique, except for ACE measurement whose kit applied competitive ELISA method. The sensitivity of the assays was 1.0pg/mL for ACE and ACE2; 3.9pg/mL for Ang I; 2.0pg/mL for Ang-(1-7); and 18.75pg/mL for Ang II. The biochemical assessments were performed blindly regarding the clinical diagnosis.
The software SPSS version 22.0 (SPSS Inc., Chicago, IL, United states) was used for statistical analysis. Gaussian distribution was assessed using the Shapiro–Wilk test. Patients and controls were compared with Mann–Whitney or Student's t-tests, when appropriate. Spearman's correlation analyses assessed the relationship between urinary levels of RAS components and previous measurements of inflammatory molecules in the same samples.12 All statistical tests were two-tailed with significance level set at p<0.05.
ResultsAt the time of urine collection, PUV fetuses (n=24) had a mean gestational time of 22±5 weeks, and male preterm newborns (n=22) were matched for gestational age (23±4 weeks). Regarding ethnicity, 14 PUV fetuses were white (58.3%), nine were mixed-race (37.5%), and one was black (4.2%). Among controls, 13 were white (59.1%), eight were mixed-race (36.4%) and one was black (4.5%). No statistical difference between cases and controls were found regarding gestational age (p=0.76) and ethnicity (p=0.85). As previously reported,12 most of the patients with PUV had bilateral kidney hyperechogenicity (78%), oligohidramnio or anihidramnio (83%) in antenatal ultrasonography; 17 (71%) died at the neonatal period due to pulmonary hypoplasia. Patients with PUV who survived (n=7) underwent vesicostomy (n=5) or endoscopic valve ablation (n=2) within the first weeks after birth. All patients presented higher urinary levels of creatinine, β2-microglobulin and osmolality than controls.12
Fetuses with PUV presented decreased urinary levels of angiotensin-converting enzyme (ACE) with no difference in Ang II levels in comparison with controls. In turn, ACE2, Ang I and, notably, Ang-(1-7) levels were higher in the urine of fetuses with PUV than controls (Fig. 1).
Levels of renin–angiotensin system (RAS) components in urine samples from fetuses with posterior urethral valve (PUV) and in a control group comprised of healthy male newborns. Fetuses with PUV presented decreased urinary levels of angiotensin-converting enzyme (ACE) with no change in angiotensin (Ang) II levels in comparison with controls. In turn, ACE2, Ang I and, notably, Ang-(1-7) levels were higher in the urine of fetuses with PUV than controls. These results show an activation of the counter-regulatory arm of the RAS in PUV. Each figure shows the mean and the standard error of the mean (SEM). *p<0.05, Mann–Whitney test.
Correlations were found between RAS components and inflammatory mediators. Among controls, urinary levels of ACE were positively correlated with interleukin (IL)-2 levels (rho=0.790, p=0.020). ACE2 levels were significantly associated with eotaxin/CCL11 (rho=0.807, p=0.015) and interferon gamma-inducible protein (IP-10)/CXCL10 (rho=0.707, p=0.050) levels. In addition, Ang-(1-7) levels were negatively correlated with ACE levels (rho=−0.810, p=0.015) and positively correlated with ACE2 levels (rho=0.946, p=0.000).
Regarding patients with PUV, urinary levels of ACE correlated negatively with β2-microglobulin (rho=−0.491, p=0.033) and soluble tumor necrosis factor receptor (sTNFR)1 (rho=−0.466, p=0.038) levels. ACE2 levels were positively correlated with IL-4 levels (rho=0.512, p=0.025). In addition, Ang I levels were significantly associated with IL-1β (rho=−0.455, p=0.044) and tumor necrosis factor (TNF) (rho=−0.546, p=0.013) levels.
DiscussionFetal urine has been regarded as an important pool of peptides that can predict postnatal renal function.19 Klein et al. identified and validated 12 fetal urinary peptides (called 12PUV) as predictors of postnatal renal function with high sensitivity and specificity, outperforming routine methods.19 The present study aimed not only at searching for biomarkers but also to investigate potential involvement of RAS molecules in PUV. It was observed that the profile of RAS components significantly differed in PUV fetuses when compared with healthy neonates. PUV fetuses had higher urinary concentrations of ACE2 and of Ang-(1-7) than healthy neonates, whereas ACE levels were lower in the urine of PUV fetuses than in the urine of healthy neonates.
Currently, it has been proposed that RAS has two major arms: (i) the classical arm formed by ACE, Ang II, and AT1 receptor, and (ii) the counter-regulatory arm composed by ACE2, Ang-(1-7), and Mas receptor.20 While the classical arm mainly activates pathways related to tissue injury and inflammation; in sharp contrast, the counter-regulatory RAS axis has anti-inflammatory effects.21 Therefore, the observed increase of components of the protective RAS arm may represent a regulatory response to the intense inflammatory process triggered by PUV.12 The present study not only demonstrated that patients with PUV present increased levels of ACE2 and Ang-(1-7) in tandem with decreased levels of ACE, but also observed significant correlations between the urinary levels of RAS components and inflammatory mediators. Noteworthy, ACE2 levels were associated with IL-4 levels and ACE levels significant correlated with sTNFR1 levels in urine samples of fetuses with PUV.
Altogether, the present data reinforces the hypothesis that changes in the RAS occur in parallel with the inflammatory process triggered by PUV. Indeed, some evidence linked RAS to the pathophysiology of PUV. Chowdhury et al.22 showed that serum pro-renin was higher in PUV cases than in controls and declined after corrective surgery. There is experimental evidence that the classical RAS arm contributed to tubulointerstitial scarring in obstructive uropathies.23
Mice with genetic deletion of AT1a receptors presented less severe tubulointerstitial fibrosis induced by unilateral ureteral obstruction, suggesting a role for the classical RAS axis in renal injury.24 More recently, two experimental studies supported a protective role for Ang-(1-7).25,26 The first showed that, in obstructed kidneys from mice with genetic deletion of Mas receptor, apoptosis and macrophage infiltration were increased when compared with wild-type mice.25 In the second, Kim et al.26 reported that the infusion of Ang-(1-7) in rats who underwent unilateral ureteral obstruction decreased pro-apoptotic and pro-fibrotic protein expression. Taken together, previous and the present findings suggest that inflammatory and fibrotic pathways triggered by obstructive uropathies might stimulate local production of Ang-(1-7) as a compensatory mechanism in fetuses with PUV.
A possible mechanism by which Ang-(1-7) may protect against kidney injury due to obstruction could be the reduction of AT1 receptors expression through Mas receptor activation. In this regard, it was reported that mRNA expression for AT1 receptor was higher in Mas knockout mice.27 Indeed, Mas receptor acts as an AT1 receptor antagonist through the constitutive formation of hetero-oligomeric complexes.28 Another possible explanation for the elevation of ACE2 and Ang-(1-7) in the urine of fetuses with PUV could be a dysfunction or reduced expression of the Mas receptor at kidney tissue in PUV fetuses. In this regard, Ng et al. reported that Mas receptor expression is reduced in the kidneys of CKD rats and the administration of the uremic toxin indoxyl sulfate induced down-regulation of Mas receptor probably via up-regulation of TGF-β1 in proximal tubules.29 Therefore, it can be hypothesized that an alteration in Mas receptor expression and/or signaling may contribute to renal injury in PUV fetuses.
The present study has limitations, including its relatively small sample size, cross-sectional design, and the use of urine samples from preterm newborns as control. Since urine collection from fetuses is an invasive procedure, it was impossible to collect urine from healthy fetuses due to ethical reasons. One study has found increased urinary angiotensinogen-to-creatinine ratio in preterm neonates when compared with that in full-term neonates. The urinary angiotensinogen-to-creatinine ratio decreased significantly with increasing gestational age.30 It is important to emphasize that, in order to rule out the effects of gestational age in RAS components concentrations, in the present study the control group was composed of preterm newborns matched by gestational age with fetuses with PUV at the time of urine collection.
In conclusion, urinary tract obstruction leads to complex molecular interactions in an early timing; in utero interventions are sometimes performed when renal lesions were already irreversible. Many studies evidenced the role of RAS in obstructive uropathies. However, up to now, the protective RAS arm has never been investigated in fetuses with PUV. The present findings support a role for ACE2-Ang-(1-7)-Mas receptor in PUV that deserve further investigation.
FundingThis study was supported by FAPEMIG (Grant # PPM-00555-15), CAPES and CNPq (Grant # 470472/2014-6 and Grant # 460334/2014-0), Brazil.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge the participation of volunteers in this study and are indebted to the pregnant women and parents of the fetuses/newborns for their magnificent support. They also would like to thanks the members of LIIM for their skilled comments.
Please cite this article as: Rocha NP, Bastos FM, Vieira ÉL, Prestes TR, Silveira KD, Teixeira MM, et al. The protective arm of the renin–angiotensin system may counteract the intense inflammatory process in fetuses with posterior urethral valves. J Pediatr (Rio J). 2019;95:328–33.