The Oxford Classification for Immunoglobulin A nephropathy (IgAN) identifies pathological variables that may predict the decline of renal function. This study aimed to evaluate the Oxford Classification variables as predictors of renal dysfunction in a cohort of Brazilian children and adolescents with IgAN.
MethodsA total of 54 patients with IgAN biopsied from 1982 to 2010 were assessed. Biopsies were re-evaluated and classified according to the Oxford Classification. Multivariate analysis of laboratory and pathological data was performed. The primary outcomes were decline of baseline estimated glomerular filtration rate (eGFR) greater than or equal to 50%.
ResultsMean follow-up was 7.6±5.0 years. Mean renal survival was 13.5±0.8 years and probability of decline ≥50% in baseline eGFR was 8% at five years of follow-up and 15% at ten years. Ten children (18.5%) had a decline of baseline eGFR≥50% and five (9.3%) evolved to end-stage renal disease. Kaplan–Meier analysis showed that baseline proteinuria, proteinuria during follow-up, endocapillary proliferation, and tubular atrophy/interstitial fibrosis were associated with the primary outcome. Multivariate Cox analysis showed that only baseline proteinuria (HR, 1.73; 95% CI, 1.20–2.50, p=0.003) and endocapillary hypercellularity (HR, 37.18; 95% CI, 3.85–358.94, p=0.002) were independent predictors of renal dysfunction. No other pathological variable was associated with eGFR decline in the multivariate analysis.
ConclusionThis is the first cohort study that evaluated the predictive role of the Oxford Classification in pediatric patients with IgAN from South America. Endocapillary proliferation was the unique pathological feature that independently predicted renal outcome.
A Classificação Oxford para a Nefropatia por Imunoglobulina A (IgAN) identificou variáveis patológicas de risco para disfunção renal. O presente estudo teve como objetivo avaliar as variáveis da Classificação de Oxford como preditores de disfunção renal em crianças Brasileiras com IgAN.
MétodosForam analisados 54 pacientes com diagnóstico de IgAN entre 1982-2010. As biópsias renais foram reavaliadas pela Classificação de Oxford. Foram realizadas análises uni e multivariada das variáveis clínicas e patológicas. O desfecho primário foi queda da taxa de filtração glomerular (TFG) ≥50% da filtração basal.
ResultadosO acompanhamento médio foi 7,6±5,0 anos. A sobrevida renal média foi 13,5±0,8 anos e a probabilidade de atingir o desfecho primário foi 8% em 5 anos e 15% em 10 anos de seguimento. Dez crianças (18,5%) apresentaram queda na TFG basal ≥50% e 5 (9,3%) evoluiu para doença renal crônica terminal. A análise de Kaplan–Meier mostrou que a proteinúria basal e de seguimento, a proliferação endocapilar e a atrofia tubular/fibrose intersticial foram associadas com o desfecho primário. A análise multivariada de Cox mostrou que a proteinúria basal (HR=1.73; IC95% 1.20-2.50, p=0.003) e a proliferação endocapilar (HR=37.18; IC95% 3.85-358.94, p=0.002) foram preditores independentes de disfunção renal. Nenhuma outra variável patológica foi associada com declínio da TFG na análise multivariada.
ConclusãoEste é o primeiro estudo brasileiro que avaliou a Classificação Oxford em crianças com IgAN. A proliferação endocapilar foi a única característica patológica capaz de predizer independentemente o declínio da função renal.
IgA nephropathy (IgAN) is one of the leading causes of glomerulonephritis worldwide. The predominant/co-dominant presence of IgA1 in mesangial immune deposits is essential to diagnose IgAN.1 IgAN is characterized by a highly variable course, extending from a very mild disease2 to end-stage renal disease (ESRD).3 The disease usually progresses insidiously and the search for predictors of renal outcome may enable individualized decision-making, early patient care, and appropriate treatment with less adverse effects.4–6
In this regard, the analysis of kidney tissue may also contribute additional prognostic information. In 2009, the International IgA Nephropathy Network developed the Oxford Classification, in which four histological variables with prognostic importance were identified as predictors of renal outcome in IgAN patients (MEST score): mesangial hypercellularity (M), endocapillary proliferation (E), segmental sclerosis or adhesion (S), and tubular atrophy/interstitial fibrosis (T).7,8 The Oxford Classification encompasses analysis of data from patients with a wide range of age. The predictive value of each specific lesion on renal survival appeared not to be distinct between children and adults with IgAN in the Oxford Classification study.9 In contrast, previous studies had shown that the histological features of IgAN in children and adults were different.10–13 Compared with adults, children with IgAN showed significantly more mesangial and endocapillary proliferation and less chronic tubulointerstitial and vascular damage.10–13 Only three studies assessed the performance of the Oxford Classification exclusively in pediatric patients.14–16 Le et al. reported that tubular atrophy/interstitial fibrosis was the only feature independently associated with renal outcomes in Chinese children with IgAN.15 In the Swedish study, mesangial hypercellularity, endocapillary proliferation, or tubular atrophy/interstitial fibrosis were each associated with a poor outcome, but segmental sclerosis or adhesion did not reach statistical significance.14 The Japanese study showed that only mesangial hypercellularity score, tubular atrophy, and crescents were significant predictors of renal outcome.16 Hence, whether the classification system has similar predictive power for children with IgAN in different populations still needs to be evaluated. Although geographically distinct, none of these studies was conducted in South America. For the first time, this study aimed to evaluate the predictive value of clinical, laboratory, and the four histological variables of the Oxford Classification on renal outcome in a cohort of Brazilian pediatric patients.
Patients and methodsPatientsThe records of 54 patients with biopsy-proven IgAN were included in the analysis of this retrospective cohort study. Inclusion criteria were patients between 2 and 18 years of age with biopsy-proven IgAN who were admitted to the Pediatric Nephrology Unit (PNU), Clinics Hospital, Federal University of Minas Gerais, Brazil, from 1982 to 2010, and had at least nine months of follow-up. Diagnostic criteria for IgAN were based on the finding of dominant/codominant mesangial deposition of IgA on immunofluorescence of kidney tissue.17 This PNU was established in 1969 and has followed-up numerous children with IgAN according to a well-established protocol. Briefly, the protocol included investigation of disease etiology, assessment of clinical course and laboratory alterations, and application of treatment according to international guidelines.18 Pediatric patients admitted to the PNU with estimated glomerular filtration rate (eGFR) below 60mL/min/1.73m2 or who had Henoch-Schönlein purpura, diabetes, or liver or systemic diseases were excluded from analysis.
Ethical aspectsThe local ethics committee approved the study (CAAE 18196713.4.0000.5149). Informed consent was obtained from all parents or persons legally responsible for pediatric patients with IgAN. The research protocol did not interfere with any medical recommendations or prescriptions.
Baseline and follow-up covariatesThe date of renal biopsy was considered the baseline time point. The follow-up period was calculated as the time between renal biopsy and last outpatient visit, death, or 50% reduction of baseline eGFR. Variables included in the analysis were: sex, ethnicity, age at baseline, eGFR, proteinuria, hypertension, weight-for-age Z-score, height-for-age Z-score, body mass index (BMI), and serum levels of creatinine. In order to avoid the overestimation of weight in patients with edema, only the dry weight was considered for the variables weight and BMI. Treatment was also evaluated according to medical prescription, regardless the duration of use. Renin-angiotensin system blockers (RASB) referred to prescription of angiotensin-converting enzyme inhibitors (ACEi) and/or angiotensin receptor blockers (ARB), and immunosuppressive drugs included corticosteroids and cyclophosphamide.
Histologic studiesRenal biopsy was performed in all 54 children. Less than one-third of the cohort (29%) was biopsied before 1990. Biopsies were re-examined by a single renal pathologist, who was blinded to patient outcome at the time of scoring. Biopsies were analyzed based on 2μg periodic acid Schiff-stained sections. No specimens had less than six glomeruli.
Biopsy specimens were classified and standardized according to the Oxford Classification,7,8 in which the number of glomeruli was assessed and their mesangial hypercellularity (M) scored as ≤0.5 or >0.5, if more or less than half of the glomeruli showed hypercellularity, defined as four or more mesangial cells/mesangial area, endocapillary proliferation (E, absent or present), segmental glomerulosclerosis (S, absent or present), and tubular atrophy/interstitial fibrosis (T, 0=0–25%, 1=26–50%, and 2=>50%). Glomeruli with cellular and fibrocellular crescents and arterial intimal thickening were also determined.
DefinitionsBlood pressure was measured and evaluated according to the Fourth Task Force on Blood Pressure in Children19 and the 95th percentile was used as the cutoff point. Proteinuria was expressed in grams per day per 1.73m2. When 24-h urine protein measurements were not available, urine protein-to-creatinine ratio (uP/Cr) was used to estimate 24-h urine protein excretion by adjusting the values for body surface area.16 Absence of proteinuria or mild proteinuria was considered when below 1g/day/1.73m2 or uP/Cr lower than 1g/g. Moderate proteinuria was defined as a non-nephrotic proteinuria of more than 1g/day/1.73m2 or uP/Cr between 1 and 3g/g. Severe or nephrotic range proteinuria was considered when above 3g/day/1.73m2 or when uP/Cr>3g/g.9,16 For each year of follow-up, the average value for all measurements of proteinuria was determined. The variable proteinuria during follow-up was the mean of all annual average values. Hematuria was diagnosed when five or more red blood cells were found in at least two urinary sediment exams. Weight-for-age and height-for-age Z-scores were used to assess weight and stature. These measures were calculated using the public-domain software Epi Info (version 3.4.1). Since creatinine measurements were made by Jaffe method until November 2011 in this institution, glomerular filtration rate was estimated by the conventional formula of Schwartz20 for data obtained until this period. Accordingly, the value for the constant (K) was 0.55 for children under 13 years old and for adolescent girls. For adolescent boys at least 13 years of age, the K value was 0.70. After November 2011, creatinine was measured by IDMS (isotope dilution mass spectrometry) traceable method. Therefore, the modified Schwartz formula21 was adopted to estimate GFR rather than the conventional Schwartz formula.
OutcomeDecline of baseline eGFR greater than or equal to 50% was assigned as the dependent variable. Renal survival was measured from the date of renal biopsy to the date of first estimation of GFR 50% lower than baseline eGFR.
Statistical analysisStatistical analysis was performed using (SPSS Statistics, version 19.0, IL, USA). Continuous variables were expressed as mean±SD. Categorical variables were described as percentages and analyzed using Pearson's chi-squared test. Student's t-test (normally distributed variables) and Mann–Whitney or Kruskal–Wallis (nonparametric variables) were used to compare continuous variables. Univariate analysis of continuous variables was performed using Cox regression, whereas categorical variables were analyzed using Kaplan–Meier and log-rank methods. Variables at baseline included in the univariate analysis were: gender, ethnicity, age at baseline, hypertension, and histopathology features. Laboratory tests at baseline were also included: eGFR, serum creatinine, and proteinuria. The Cox proportional hazards model was applied to identify variables that were independently associated with a decline ≥ 50% from baseline eGFR. Only variables that were associated with the event of interest by univariate analysis (p<0.25) were included in the multivariate Cox regression. All reported p-values were two sided. Values of p lower than 0.05 were considered statistically significant. Confidence intervals (CI) included 95% of the predicted values.
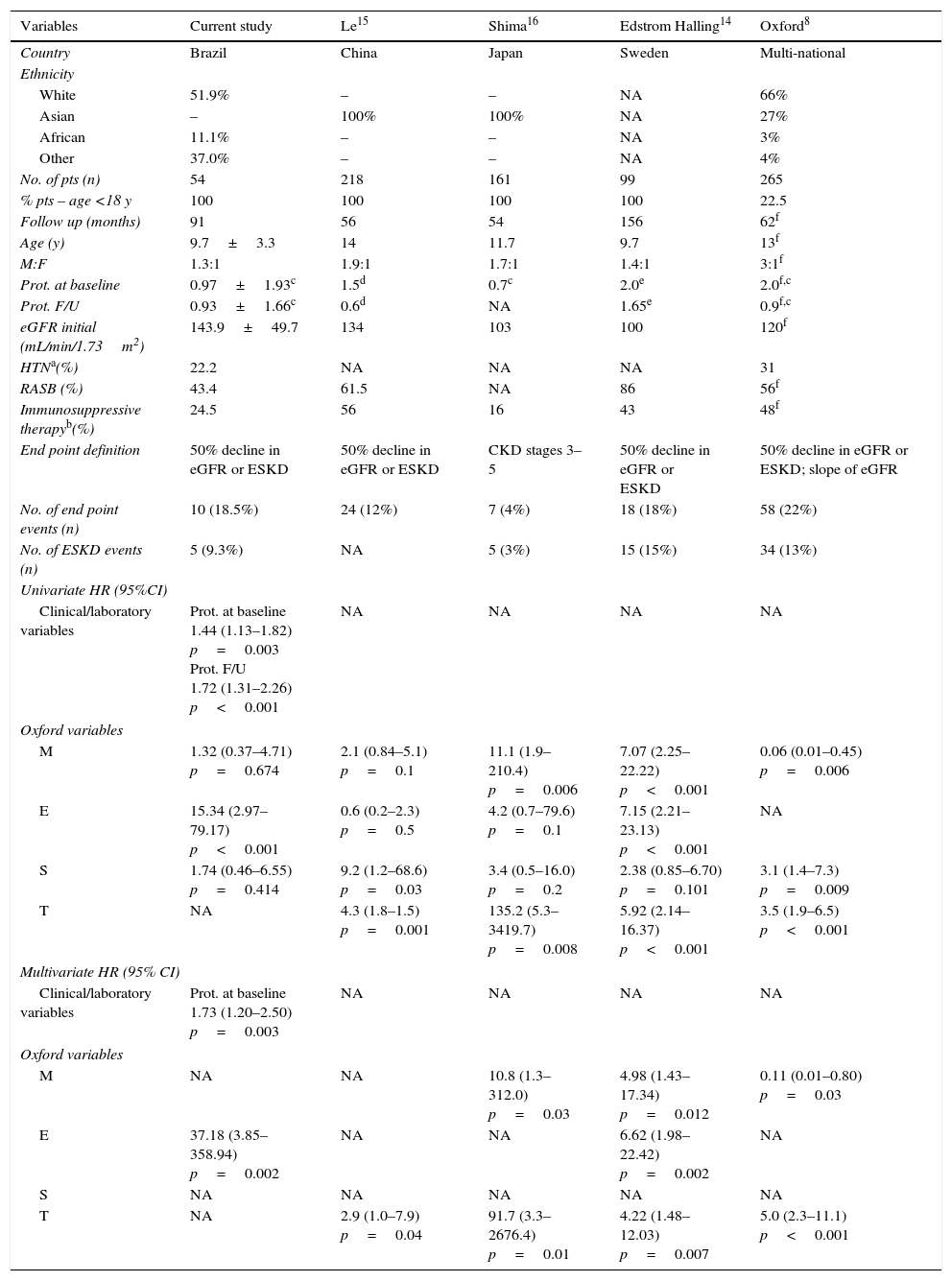
ResultsClinical and pathological characteristicsA total of 54 patients (31 boys) with IgAN were analyzed. The majority were classified as white (51.9%). The mean age was 9.7±3.3 years. At the time of renal biopsy, mean eGFR was 143±49.7mL/min/1.73m2. The mean baseline proteinuria was 0.97±1.93g/day per 1.73m2. Four patients (7.4%) had baseline proteinuria in the nephrotic range and six patients (11.1%) presented with moderate proteinuria. Twelve patients (22.2%) had arterial hypertension or were receiving antihypertensive therapy at the time of renal biopsy. Table 1 summarizes clinical and demographic data at the time of renal biopsy. These findings were also compared with four other pediatric cohort studies.
Clinical variables at the time of initial biopsy and the end point of follow-up. Comparison of current data with other pediatric cohort studies.
| Variables | Current study | Le15 | Shima16 | Edstrom Halling14 | Oxford8 |
|---|---|---|---|---|---|
| Country | Brazil | China | Japan | Sweden | Multi-national |
| Ethnicity | |||||
| White | 51.9% | – | – | NA | 66% |
| Asian | – | 100% | 100% | NA | 27% |
| African | 11.1% | – | – | NA | 3% |
| Other | 37.0% | – | – | NA | 4% |
| No. of pts (n) | 54 | 218 | 161 | 99 | 265 |
| % pts – age <18 y | 100 | 100 | 100 | 100 | 22.5 |
| Follow up (months) | 91 | 56 | 54 | 156 | 62f |
| Age (y) | 9.7±3.3 | 14 | 11.7 | 9.7 | 13f |
| M:F | 1.3:1 | 1.9:1 | 1.7:1 | 1.4:1 | 3:1f |
| Prot. at baseline | 0.97±1.93c | 1.5d | 0.7c | 2.0e | 2.0f,c |
| Prot. F/U | 0.93±1.66c | 0.6d | NA | 1.65e | 0.9f,c |
| eGFR initial (mL/min/1.73m2) | 143.9±49.7 | 134 | 103 | 100 | 120f |
| HTNa(%) | 22.2 | NA | NA | NA | 31 |
| RASB (%) | 43.4 | 61.5 | NA | 86 | 56f |
| Immunosuppressive therapyb(%) | 24.5 | 56 | 16 | 43 | 48f |
| End point definition | 50% decline in eGFR or ESKD | 50% decline in eGFR or ESKD | CKD stages 3–5 | 50% decline in eGFR or ESKD | 50% decline in eGFR or ESKD; slope of eGFR |
| No. of end point events (n) | 10 (18.5%) | 24 (12%) | 7 (4%) | 18 (18%) | 58 (22%) |
| No. of ESKD events (n) | 5 (9.3%) | NA | 5 (3%) | 15 (15%) | 34 (13%) |
| Univariate HR (95%CI) | |||||
| Clinical/laboratory variables | Prot. at baseline 1.44 (1.13–1.82) p=0.003 Prot. F/U 1.72 (1.31–2.26) p<0.001 | NA | NA | NA | NA |
| Oxford variables | |||||
| M | 1.32 (0.37–4.71) p=0.674 | 2.1 (0.84–5.1) p=0.1 | 11.1 (1.9–210.4) p=0.006 | 7.07 (2.25–22.22) p<0.001 | 0.06 (0.01–0.45) p=0.006 |
| E | 15.34 (2.97–79.17) p<0.001 | 0.6 (0.2–2.3) p=0.5 | 4.2 (0.7–79.6) p=0.1 | 7.15 (2.21–23.13) p<0.001 | NA |
| S | 1.74 (0.46–6.55) p=0.414 | 9.2 (1.2–68.6) p=0.03 | 3.4 (0.5–16.0) p=0.2 | 2.38 (0.85–6.70) p=0.101 | 3.1 (1.4–7.3) p=0.009 |
| T | NA | 4.3 (1.8–1.5) p=0.001 | 135.2 (5.3–3419.7) p=0.008 | 5.92 (2.14–16.37) p<0.001 | 3.5 (1.9–6.5) p<0.001 |
| Multivariate HR (95% CI) | |||||
| Clinical/laboratory variables | Prot. at baseline 1.73 (1.20–2.50) p=0.003 | NA | NA | NA | NA |
| Oxford variables | |||||
| M | NA | NA | 10.8 (1.3–312.0) p=0.03 | 4.98 (1.43–17.34) p=0.012 | 0.11 (0.01–0.80) p=0.03 |
| E | 37.18 (3.85–358.94) p=0.002 | NA | NA | 6.62 (1.98–22.42) p=0.002 | NA |
| S | NA | NA | NA | NA | NA |
| T | NA | 2.9 (1.0–7.9) p=0.04 | 91.7 (3.3–2676.4) p=0.01 | 4.22 (1.48–12.03) p=0.007 | 5.0 (2.3–11.1) p<0.001 |
CI, confidence interval; CKD, chronic kidney disease; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; ESKD, end stage kidney disease; F/U, follow-up; HR, Hazard ratio; HTN, hypertension; M, mesangial hypercellularity; M:F, male:female; NA, not available; prot, proteinuria; pts, patients; RASB, renin–angiotensin-system blockade; S, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis.
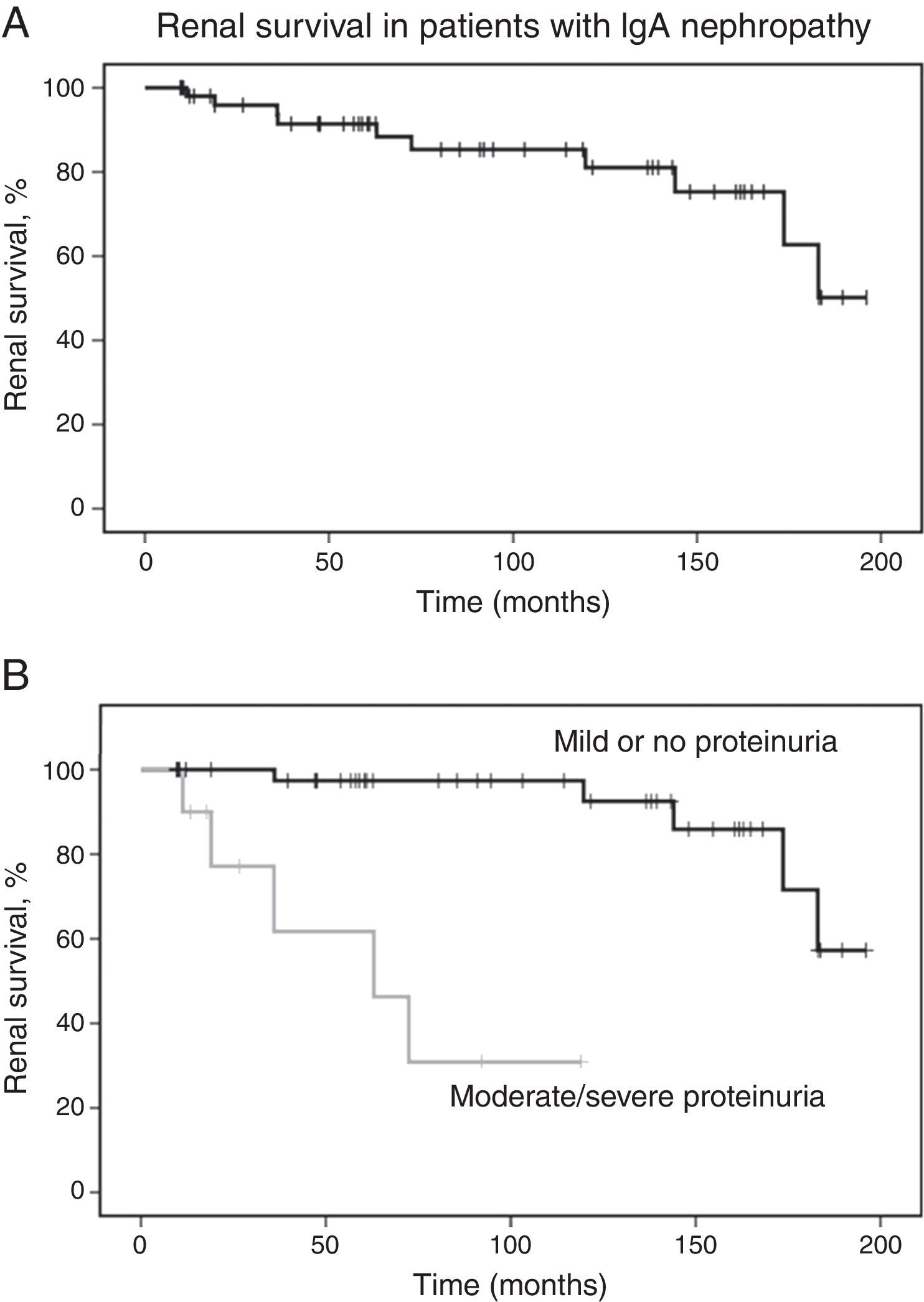
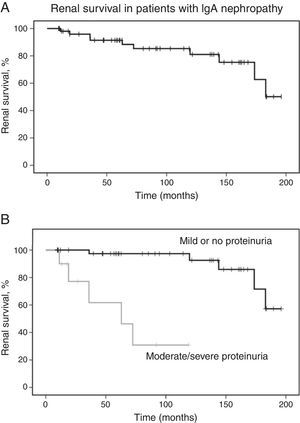
The mean follow-up time was 90±60 months. Of all patients, ten (18.5%) developed 50% decline in renal function, with mean endpoint eGFR of 70±43mL/min/1.73m2. Five patients (9.3%) developed ESRD in 109±85 months. Survival analysis estimated that the probability of 50% decline in renal function was 8% at five years and 15% at ten years. Laboratory evaluation at last visit revealed mean eGFR of 143.9±49.7mL/min/1.73m2 for patients who did not achieve the primary outcome. Renal survival analysis is shown in Fig. 1.
Renal survival in patients with IgA nephropathy. (A) Kaplan–Meier curves show the probability of renal survival (decline of baseline eGFR ≥50%) for patients with IgA nephropathy (n=54). (B) Kaplan–Meier curves show the lower probability of renal survival for patients with moderate to severe proteinuria (n=10) as compared with patients with mild or no proteinuria (n=44). eGFR, estimated glomerular filtration rate.
Pathological analysis showed a mean of 23 glomeruli per biopsy. There were 18 patients with mesangial proliferation (M1), five patients with endocapillary proliferation (E1), 13 patients with segmental sclerosis/adhesion lesion (S1), and only two patients with tubulointerstitial fibrosis, moderate in one (T1) and severe in the other (T2). Due to the low frequency of tubulointerstitial fibrosis, T1 and T2 were combined for the following analysis. The frequency of crescents was also low (7.4%) and vascular lesions were found in just one case. Vascular lesions were not included in statistical analysis.
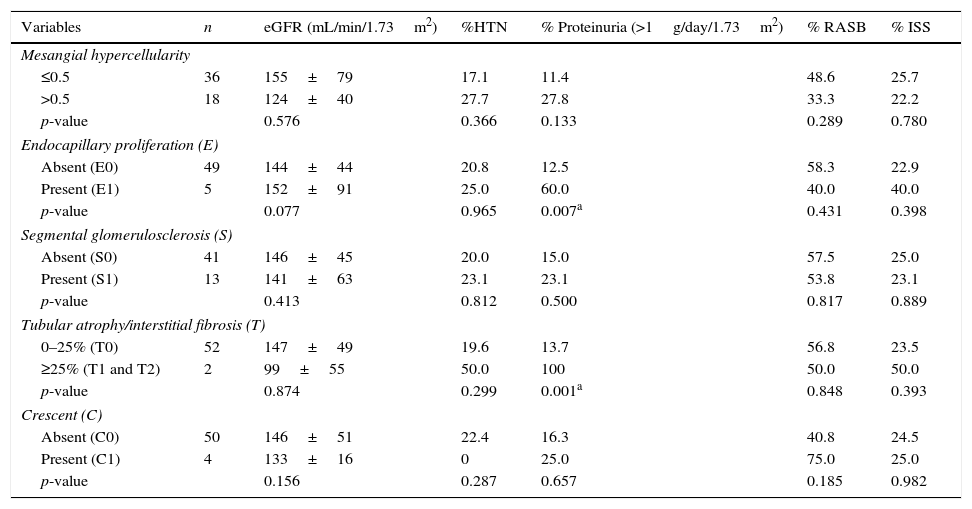
Associations between pathological lesions, baseline clinical features, and treatmentPossible associations between clinical findings, therapeutic approach, and renal histology of the patients are shown in Table 2. None of the lesions were significantly associated with eGFR or hypertension at the time of biopsy. Endocapillary proliferation and tubular atrophy/interstitial fibrosis (≥25%) were associated with moderate/severe proteinuria at baseline. Mesangial hypercellularity, segmental glomerulosclerosis, and extracapillary hypercellularity were not associated with any of the clinical features. The therapeutic approach with RASB and immunosuppressors was not significantly associated with pathological variables.
Association between pathological and clinical variables at the time of renal biopsy.
| Variables | n | eGFR (mL/min/1.73m2) | %HTN | % Proteinuria (>1g/day/1.73m2) | % RASB | % ISS |
|---|---|---|---|---|---|---|
| Mesangial hypercellularity | ||||||
| ≤0.5 | 36 | 155±79 | 17.1 | 11.4 | 48.6 | 25.7 |
| >0.5 | 18 | 124±40 | 27.7 | 27.8 | 33.3 | 22.2 |
| p-value | 0.576 | 0.366 | 0.133 | 0.289 | 0.780 | |
| Endocapillary proliferation (E) | ||||||
| Absent (E0) | 49 | 144±44 | 20.8 | 12.5 | 58.3 | 22.9 |
| Present (E1) | 5 | 152±91 | 25.0 | 60.0 | 40.0 | 40.0 |
| p-value | 0.077 | 0.965 | 0.007a | 0.431 | 0.398 | |
| Segmental glomerulosclerosis (S) | ||||||
| Absent (S0) | 41 | 146±45 | 20.0 | 15.0 | 57.5 | 25.0 |
| Present (S1) | 13 | 141±63 | 23.1 | 23.1 | 53.8 | 23.1 |
| p-value | 0.413 | 0.812 | 0.500 | 0.817 | 0.889 | |
| Tubular atrophy/interstitial fibrosis (T) | ||||||
| 0–25% (T0) | 52 | 147±49 | 19.6 | 13.7 | 56.8 | 23.5 |
| ≥25% (T1 and T2) | 2 | 99±55 | 50.0 | 100 | 50.0 | 50.0 |
| p-value | 0.874 | 0.299 | 0.001a | 0.848 | 0.393 | |
| Crescent (C) | ||||||
| Absent (C0) | 50 | 146±51 | 22.4 | 16.3 | 40.8 | 24.5 |
| Present (C1) | 4 | 133±16 | 0 | 25.0 | 75.0 | 25.0 |
| p-value | 0.156 | 0.287 | 0.657 | 0.185 | 0.982 | |
eGFR, estimated glomerular filtration rate; HTN, hypertension; RASB, renin-angiotensin-system blockade; ISS, immunosuppressive therapy.
Values are expressed as mean±SD for eGFR and as proportion for HTN, proteinuria, RASB, and ISS.
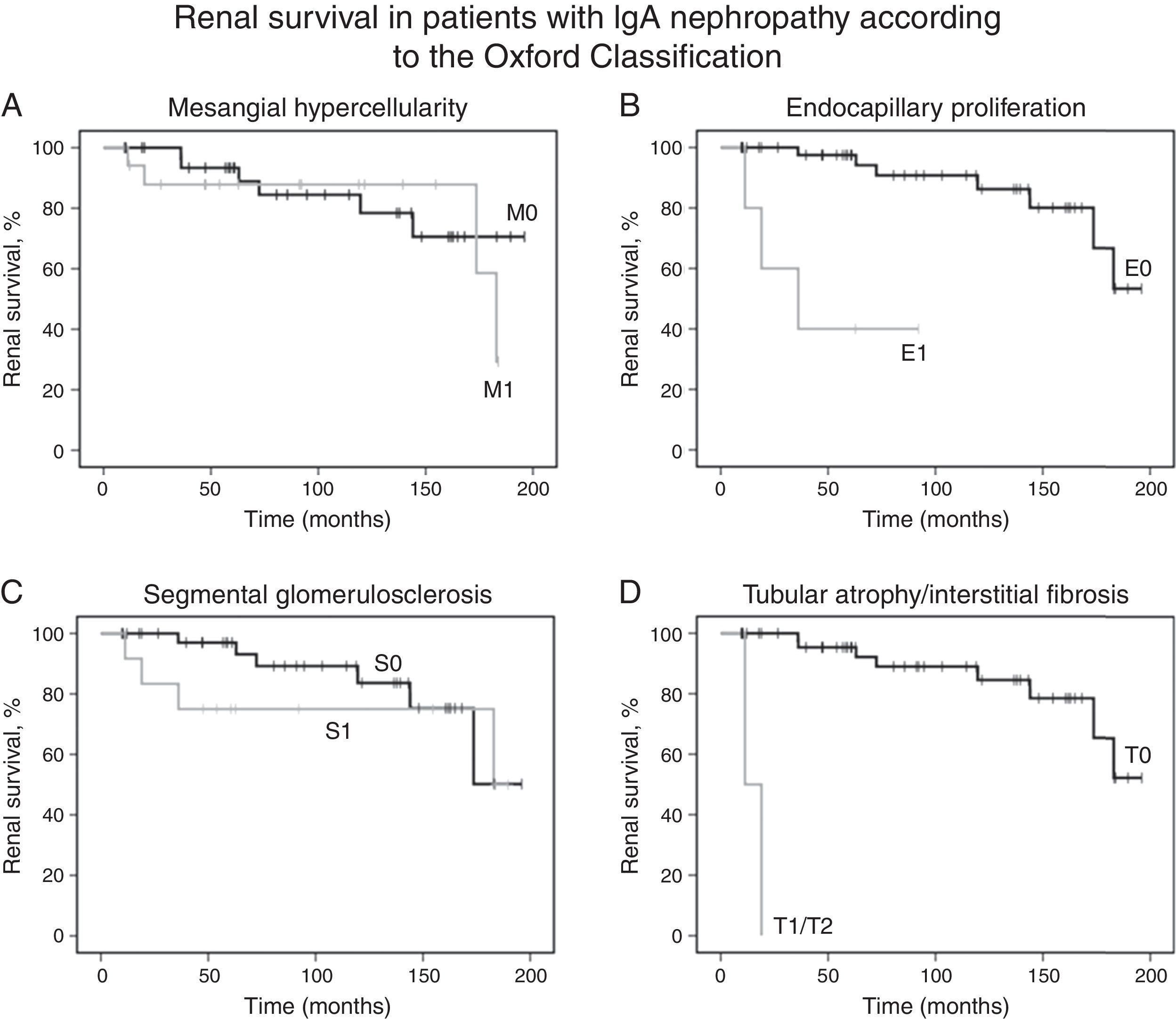
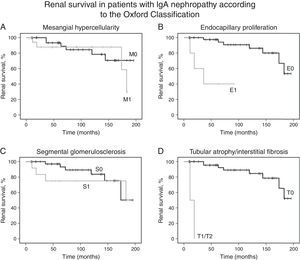
Kaplan–Meier analysis showed that moderate/severe proteinuria at the time of biopsy was significantly associated with a decline of eGFR ≥50% (chi-squared 25.7; p<0.001, log-rank [Mantel–Cox], Fig. 1B). Regarding pathological variables of the Oxford Classification, Kaplan–Meier revealed that endocapillary hypercellularity (chi-squared 18.52; p<0.001, log-rank [Mantel–Cox]), and tubular atrophy/interstitial fibrosis (chi-squared 62.96; p<0.001, log-rank [Mantel–Cox]) were associated with renal survival. Mesangial hypercellularity (chi-squared 0.18; p=0.673, log-rank [Mantel–Cox]), segmental glomerulosclerosis (chi-squared 0.68; p=0.409, log-rank [Mantel–Cox]), and extracapillary hypercellularity (chi-squared 1.11; p=0.292, log-rank [Mantel–Cox]) were not associated with primary outcome. Fig. 2 shows renal survival by Kaplan–Meier analysis according to the Oxford Classification.
Renal survival of patients with IgA nephropathy according to the Oxford Classification. (A) Kaplan–Meier curves show the same probability of renal survival (decline of baseline eGFR ≥50%) for patients with mesangial hypercellularity M1 (n=18) and without mesangial hypercellularity M0 (n=36). (B) Kaplan–Meier curves show the lower probability of renal survival for patients with endocapillary proliferation E1 (n=5) as compared with patients without endocapillary proliferation E0 (n=49). (C) Kaplan–Meier curves show the same probability of renal survival for patients with segmental glomerulosclerosis S1 (n=13) and without segmental glomerulosclerosis S0 (n=41). (D) Kaplan–Meier curves show the lower probability of renal survival for patients with tubular atrophy/interstitial fibrosis ≥25% T1 or T2 (n=2) as compared with patients with tubular atrophy/interstitial fibrosis equal or lower than 25% T0 (n=52). eGFR, estimated glomerular filtration rate.
Univariate Cox regression analysis showed that baseline proteinuria (hazard ratio [HR], 1.44; 95% CI, 1.13–1.82; p=0.003), proteinuria during follow-up (HR, 1.72; 95% CI, 1.31–2.26; p<0.001) and presence of moderate/severe proteinuria at the time of biopsy (HR, 34.45; 95% CI, 3.98–298.12; p=0.001) were associated with a decline of baseline eGFR equal or higher than 50%. Univariate Cox regression analysis also revealed that only endocapillary proliferation was associated with renal survival (HR, 15.34; 95% CI, 2.97–79.17, p<0.001). Patients with tubular atrophy/interstitial fibrosis greater than 25% had a decline of baseline eGFR of at least 50% during the first two years after diagnosis, but significance was not found due to small number of patients with tubulointerstitial fibrosis (n=2). Also, mesangial hypercellularity (HR 1.32; 95% CI, 0.37–4.71, p=0.674), segmental glomerulosclerosis (HR 1.74; 95% CI, 0.46–6.55, p=0.414) and extracapillary hypercellularity (HR 0.04; 95% CI, 0.00–416.77, p=0.497) were not associated with primary outcome. No other variable was associated with primary outcome.
Baseline variables associated with the renal survival were proteinuria at the time of first renal biopsy and endocapillary hypercellularity. In multivariate Cox analysis, after adjustment by Cox regression model, proteinuria at baseline (HR, 1.73; 95% CI, 1.20–2.50, coefficient 0.55; p=0.003) and endocapillary hypercellularity (HR, 37.18; 95% CI, 3.85–358.94, coefficient 3.62; p=0.002) remained as independent predictors of the decline of baseline eGFR of at least 50%.
DiscussionThis study investigated possible predictive factors associated with decline of eGFR in pediatric patients with IgAN. The main findings were related to the prognostic role of proteinuria and of endocapillary proliferation. Therefore, children who had a decline of 50% in eGFR presented higher levels of proteinuria at baseline and during follow-up. On the other hand, patients with mild or no proteinuria had better renal survival compared with children with moderate/severe proteinuria. These findings are consistent with recent results showing a good prognosis for patients with mild proteinuria.22–24 Among pathological variables, endocapillary proliferation (E1) at first renal biopsy was also significantly associated with the outcome. In addition, interstitial fibrosis/tubular atrophy (≥25%) at first renal biopsy might be related to renal function deterioration, although without reaching statistical significance due to small sample size. In contrast, mesangial hypercellularity, segmental glomerulosclerosis, and extracapillary hypercellularity showed no significance. After adjustment by the Cox proportional hazard model, only two variables remained as independent predictors of renal outcome: baseline proteinuria (as continuous variable), and endocapillary proliferation (E1) at first renal biopsy. Possibly, variations in sample size may lead to differences in variables behavior after adjustment by the proportional hazard model.
As shown in Table 1, only three studies adopting the Oxford Classification were previously published exclusively with pediatric patients.14–16 Compared with Chinese,15 Swiss,14 and original Oxford8 cohorts, the present sample is very similar. As expected, high proportions of males and of white children (51.9%) among IgAN patients were observed. Although there are clear geographical and ethnic differences, IgAN is more common in Asian and Caucasian children.12 It should be pointed out that all of the patients had a baseline eGFR above 60mL/min/1.73m2. In addition, they presented less severe baseline proteinuria. In comparison to the Oxford cohort, fewer patients exhibited nephrotic range proteinuria at baseline (7.4%). Furthermore, the number of patients with no medication during follow up (RASB or immunosuppressive therapy) seemed to be higher in the present cohort. Accordingly, only eight patients received corticosteroids, and one was also treated with cyclophosphamide. These findings may indicate that this cohort included milder cases of IgAN than the Oxford study. In agreement, the lower incidence of primary outcome in this cohort (18.5% vs. 22% in the Oxford study) might be explained at least in part by the occurrence of milder cases of IgAN.
In regard to histological variables in exclusively pediatric studies, Edström Halling et al. found that segmental glomerulosclerosis (S) was not associated with the progression of IgAN in the Swiss cohort of 99 children.14 Shima et al. examined 161 children in Japan and reported that only mesangial hypercellularity (M) and interstitial fibrosis/tubular atrophy (T) are prognostic markers in that population.16 In China, the analysis of a 218 children revealed that only T remains as a prognostic marker after multivariate analysis.15 Two other large adult cohorts of IgAN also included pediatric patients.22,23 Herzenberg et al. evaluated 187 North American patients including 45 children and observed that each histological variable has the same predictive value found in Oxford original studies, except M, which behaves as a weaker predictor.22 More recently, the European study VALIGA retrospectively analyzed 1147 European patients, including 174 children. Segmental glomerulosclerosis (S), mesangial hypercellularity (M), tubular atrophy/interstitial fibrosis (T), but not endocapillary proliferation (E), were identified as predictors of renal outcome in this cohort.23 More recently, a retrospective study from Great Britain showed that proteinuria, endocapillary proliferation (E1 versus E0), and interstitial fibrosis/tubular atrophy (T2 versus T0) were able to predict ESRD in a cohort of 147 adults with IgAN receiving no immunosuppression.25 Similarly, proteinuria at baseline and endocapillary proliferation in renal tissue predicted renal function deterioration in the present study. On the other hand, the Oxford Classification study showed no significance of E as a predictor of renal survival in children.9 Endocapillary proliferation probably interacted with immunosuppressive therapy in IgAN patients.9 The same was observed in the VALIGA study.23 In a metanalysis with IgAN cohorts, E was not related to the outcome either.3 It should be pointed out that recent studies have showed a benefit of immunosuppressive therapy on glomerular endocapillary proliferation in patients with IgAN.22 Shen et al. reported that endocapillary proliferation could be reversed by immunosuppressive therapy in patients with IgAN.26 The reversal of these lesions may explain the lack of correlation of these lesions with clinical outcomes.26 Although endocapillary proliferation is not a uniform predictor of renal dysfunction in the Oxford validation studies with children, the present study showed that this variable may predict renal dysfunction in IgAN children with less severe disease and low levels of immunosuppression.
It is noteworthy that the present study evaluated, for the first time, the performance of Oxford Classification in a cohort of pediatric patients from South America. However, this study did not aim to validate the Oxford Classification in South American children with IgA. Rather, the objective was to describe risk factors associated with the decline of renal function in Brazilian pediatric patients with IgAN. In addition, the limitations of this study must be recognized. First, the retrospective design made difficult the control over the variables measured. Second, the small sample size might contribute to the lack of predictive value for some pathological variables. Third, it was not possible to systematically analyze some time-dependent variables, including hypertension. However, some features of this study may increase the strength of these findings, including the large set of data collected over many years and long-term follow-up by the same team with a well-established clinical protocol, as well as the careful re-evaluation of all renal biopsies by a single expert pathologist blinded to clinical data and outcome.
In conclusion, endocapillary proliferation in renal tissue was the single pathological variable independently associated with renal outcome in this Brazilian pediatric IgAN cohort. Proteinuria at baseline was also of prognostic relevance. However, the low prevalence of other histopathological lesions – particularly segmental glomerulosclerosis, interstitial fibrosis/tubular atrophy, and extracapillary hypercellularity – hampered the appraisal of their value. Although the results certainly require confirmation by other South American pediatric IgAN cohorts, the adoption of the Oxford Classification may enable the comparison of pathological variables between different studies.
FundingFundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of interestThe authors declare no conflicts of interest.
The authors dedicate this work to the late Professor José Silvério Santos Diniz, founder of the Pediatric Nephrology Unit, Hospital das Clínicas, Universidade Federal de Minas Gerais, Brazil. The authors also thank Dr. Luiz Sérgio Bahia Cardoso, Dr. Maria Goretti Moreira Guimarães Penido, and other colleagues who processed medical records, managed patients, performed renal biopsies, and assisted with follow-up.
Please cite this article as: Fabiano RC, Araújo SA, Bambirra EA, Oliveira EA, Simões e Silva AC, Pinheiro SV. The Oxford Classification predictors of chronic kidney disease in pediatric patients with IgA nephropathy. J Pediatr (Rio J). 2017;93:389–97.