To assess the impact of asthma and its treatment (inhaled corticosteroids and other control medications) on growth.
Data sourcesThe authors searched PubMed (up to August 24, 2018) and screened the reference lists of retrieved articles. Systematic reviews and meta-analysis were selected. If there was no such article, the authors selected either randomized clinical trials or observational studies.
Data synthesisA total of 37 articles were included in this review. The findings from 21 studies suggest that asthma per se, especially more severe and/or uncontrolled cases, can transitorily impair child's growth. Two Cochrane reviews of randomized clinical trials showed a small mean reduction in linear growth (−0.91cm/year for beclomethasone, −0.59cm/year for budesonide, and −0.39cm/year for fluticasone) in the first year of treatment with inhaled corticosteroids in prepubertal children with persistent asthma. The effects were likely to be molecule- and dose-dependent. A recent review showed that most of “real-life” observational studies had not found significant effects of inhaled corticosteroids on growth in asthmatic children. Fifteen studies showed that the maintenance systemic corticosteroids could cause a dose-dependent growth suppression in children with severe asthma, but other controllers (cromones, montelukast, salmeterol, and theophylline) had no significant adverse effects no growth.
ConclusionsSevere and/or uncontrolled asthma can transitorily impair child's growth. Regular use of inhaled corticosteroids may cause a small reduction in linear growth in children with asthma, but the well-established benefits of inhaled corticosteroids in controlling asthma outweigh the potential adverse effects on growth. Use of the minimally effective dose of inhaled corticosteroids and regular monitoring of child's height during inhaled corticosteroids therapy are recommended.
Avaliar o impacto da asma e seu tratamento (corticosteroides inalados e outros medicamentos de controle) no crescimento.
Fontes de dadosUma busca foi feita no PubMed (até 24 de agosto de 2018) e foram triadas as listas de referência dos artigos recuperados. Revisões sistemáticas e metanálises foram selecionadas. Se não houvesse tal artigo, ensaios clínicos randomizados ou estudos observacionais eram selecionados.
Síntese dos dadosTrinta e sete artigos foram incluídos nesta revisão. Os achados de 21 estudos sugerem que a asma por si só, especialmente os casos mais graves e/ou descontrolados, podem prejudicar o crescimento da criança. Duas revisões Cochrane de ensaios clínicos randomizados mostraram uma pequena redução média no crescimento linear (−0,91cm/ano para beclometasona, −0,59cm/ano para budesonida e −0,39cm/ano para fluticasona) no primeiro ano de tratamento com corticosteroides inalados em crianças pré-púberes com asma persistente. Os efeitos pareciam ter efeito dose- e molécula-dependente. Uma revisão recente mostrou que a maioria dos estudos observacionais da “vida real” não encontrou efeitos significativos dos corticosteroides inalados no crescimento de crianças asmáticas. Quinze estudos mostraram que a manutenção de corticosteroides sistêmicos poderia causar uma supressão do crescimento dose-dependente em crianças com asma grave, mas outros controladores (cromonas, montelucaste, salmeterol e teofilina) não tiveram efeitos adversos significativos no crescimento.
ConclusõesA asma grave e/ou descontrolada pode prejudicar o crescimento da criança. O uso regular de corticosteroides inalados pode causar uma pequena redução no crescimento linear em crianças com asma, mas os benefícios bem estabelecidos dos corticosteroides inalados no controle da asma superam os potenciais efeitos adversos no crescimento. Recomenda-se o uso de doses minimamente eficazes de corticosteroides inalados e o monitoramento regular da altura da criança durante a terapia com corticosteroides inalados.
The impact of asthma and its treatment on child's growth has been and continues to be a matter of concern. Numerous publications have addressed the growth of children with asthma. The earliest studies in the 1940s reported that allergic disorders including asthma, especially uncontrolled cases, could lead to impaired growth and delayed sexual maturation.1,2 Since the late 1950s, attention has been drawn to growth impairment related to maintenance systemic corticosteroids, but short stature had been observed in children with severe asthma before starting corticosteroid therapy.3–5 These data support the earliest observation that asthma per se could adversely impact growth. In the early 1970s, the introduction of inhaled corticosteroids (ICS) revolutionized the treatment of asthma. ICS are currently the most effective anti-inflammatory medications for the treatment of asthma in adults and children, and will probably remain so for the foreseeable future. Although ICS are generally considered safe and highly effective treatment for children with asthma, the potential systemic adverse effects related to regular use of these drugs, especially the effects on growth, have drawn considerable concerns from healthcare providers and parents.6 The unjustified fear of drug side effects has been recognized as one of the reasons for underuse and non-adherence to ICS treatment, and consequently poor asthma control in children.7
This review article aimed to summarize the currently available evidence regarding the impact of asthma and its treatment on the growth of asthmatic children. The review focused on three key questions: (1) What are the effects of asthma itself on the growth? (2) What are the effects of ICS on the growth? (3) What are the effects of non-ICS asthma controllers on the growth? These evidences would help healthcare providers and parents to draw a more comprehensive and precise picture of the effects of asthma and its treatment on child's growth.
MethodsThe authors searched PubMed from inception until August 24, 2018 to retrieve the articles that addressed one of three key review questions. The following search strategy was adopted: asthma AND (“linear growth” OR height OR length). The reference lists of all retrieved articles were also screened for additional relevant studies.
Systematic reviews and meta-analyses were selected when such articles were available. Otherwise, individual studies were selected, either randomized clinical trials (RCTs) or observational studies that provided available data regarding the effects of asthma or its treatment (ICS and other controller medications) on child's growth. Studies that used parent-reported height rather than that measured by stadiometer were excluded. Two authors (LZ, LL) independently selected the articles, and any disagreement was resolved by discussion. The data from the selected articles were extracted by LZ and checked by LL.
The search in PubMed identified 3096 records, of which 25 articles1–3,5,6,8–27 were selected. Twelve additional articles28–39 were retrieved from checking the reference lists of retrieved papers. Thus, a total of 37 articles were included in this review, of which 171–3,8–16,18–20,22,23 for question 1, five6,25–27,29 for question 2, ten30–39 for question 3, four5,18,22,28 for both question 1 and 3, and one24 for both question 2 and 3. The data from individual studies were not suitable for meta-analysis due to substantial heterogeneity in study design, outcome measures and interventions. Thus the data synthesis involved the use of narrative text and tables. Fourteen additional articles4,7,40–51 were selected and used as the references for the comments and final considerations.
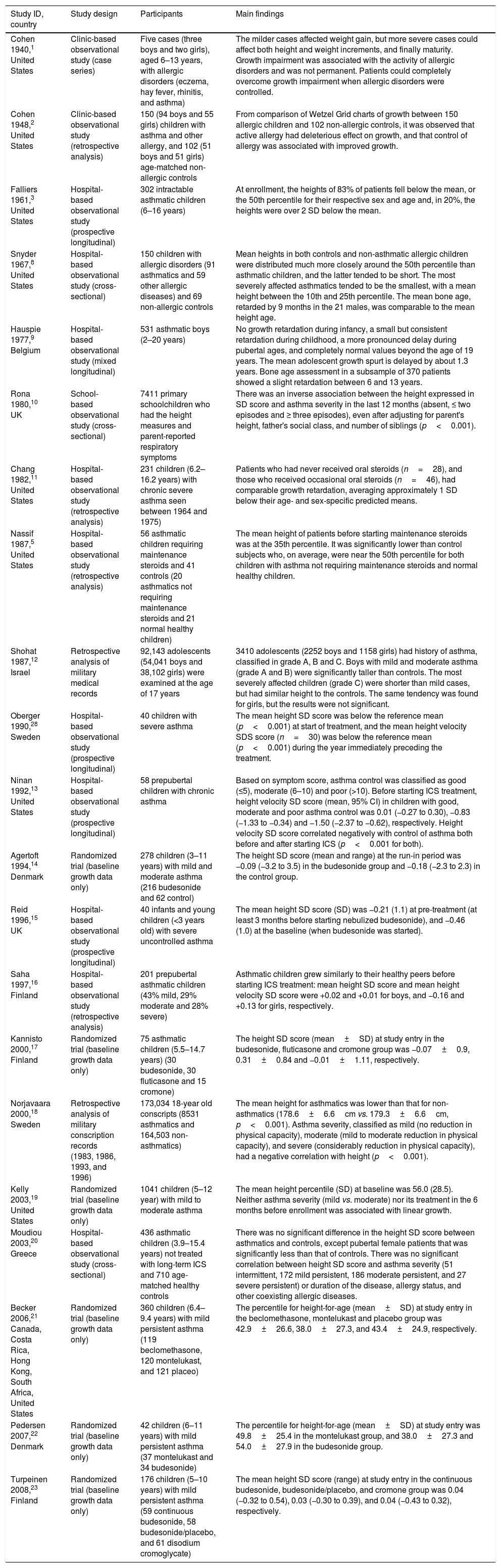
Main findings and commentsEffects of asthma itself on child's growthThe search retrieved 21 studies1–3,5,8–23,28 that presented useful data regarding the effects of asthma on child's growth. Table 1 summarizes the characteristics and main findings of the 21 studies. These studies were classified into three categories: clinic or hospital-based observational studies (n=12), community-based studies (n=3), and randomized trials (n=6). The authors used only baseline growth data before starting maintenance corticosteroid therapy in seven studies designed to assess the effects of corticosteroids on growth.3,5,13,15,16,20,28
Summary of studies that provided data regarding the effects of asthma on child's growth.
| Study ID, country | Study design | Participants | Main findings |
|---|---|---|---|
| Cohen 1940,1 United States | Clinic-based observational study (case series) | Five cases (three boys and two girls), aged 6–13 years, with allergic disorders (eczema, hay fever, rhinitis, and asthma) | The milder cases affected weight gain, but more severe cases could affect both height and weight increments, and finally maturity. Growth impairment was associated with the activity of allergic disorders and was not permanent. Patients could completely overcome growth impairment when allergic disorders were controlled. |
| Cohen 1948,2 United States | Clinic-based observational study (retrospective analysis) | 150 (94 boys and 55 girls) children with asthma and other allergy, and 102 (51 boys and 51 girls) age-matched non-allergic controls | From comparison of Wetzel Grid charts of growth between 150 allergic children and 102 non-allergic controls, it was observed that active allergy had deleterious effect on growth, and that control of allergy was associated with improved growth. |
| Falliers 1961,3 United States | Hospital-based observational study (prospective longitudinal) | 302 intractable asthmatic children (6–16 years) | At enrollment, the heights of 83% of patients fell below the mean, or the 50th percentile for their respective sex and age and, in 20%, the heights were over 2 SD below the mean. |
| Snyder 1967,8 United States | Hospital-based observational study (cross-sectional) | 150 children with allergic disorders (91 asthmatics and 59 other allergic diseases) and 69 non-allergic controls | Mean heights in both controls and non-asthmatic allergic children were distributed much more closely around the 50th percentile than asthmatic children, and the latter tended to be short. The most severely affected asthmatics tended to be the smallest, with a mean height between the 10th and 25th percentile. The mean bone age, retarded by 9 months in the 21 males, was comparable to the mean height age. |
| Hauspie 1977,9 Belgium | Hospital-based observational study (mixed longitudinal) | 531 asthmatic boys (2–20 years) | No growth retardation during infancy, a small but consistent retardation during childhood, a more pronounced delay during pubertal ages, and completely normal values beyond the age of 19 years. The mean adolescent growth spurt is delayed by about 1.3 years. Bone age assessment in a subsample of 370 patients showed a slight retardation between 6 and 13 years. |
| Rona 1980,10 UK | School-based observational study (cross-sectional) | 7411 primary schoolchildren who had the height measures and parent-reported respiratory symptoms | There was an inverse association between the height expressed in SD score and asthma severity in the last 12 months (absent, ≤ two episodes and ≥ three episodes), even after adjusting for parent's height, father's social class, and number of siblings (p<0.001). |
| Chang 1982,11 United States | Hospital-based observational study (retrospective analysis) | 231 children (6.2–16.2 years) with chronic severe asthma seen between 1964 and 1975) | Patients who had never received oral steroids (n=28), and those who received occasional oral steroids (n=46), had comparable growth retardation, averaging approximately 1 SD below their age- and sex-specific predicted means. |
| Nassif 1987,5 United States | Hospital-based observational study (retrospective analysis) | 56 asthmatic children requiring maintenance steroids and 41 controls (20 asthmatics not requiring maintenance steroids and 21 normal healthy children) | The mean height of patients before starting maintenance steroids was at the 35th percentile. It was significantly lower than control subjects who, on average, were near the 50th percentile for both children with asthma not requiring maintenance steroids and normal healthy children. |
| Shohat 1987,12 Israel | Retrospective analysis of military medical records | 92,143 adolescents (54,041 boys and 38,102 girls) were examined at the age of 17 years | 3410 adolescents (2252 boys and 1158 girls) had history of asthma, classified in grade A, B and C. Boys with mild and moderate asthma (grade A and B) were significantly taller than controls. The most severely affected children (grade C) were shorter than mild cases, but had similar height to the controls. The same tendency was found for girls, but the results were not significant. |
| Oberger 1990,28 Sweden | Hospital-based observational study (prospective longitudinal) | 40 children with severe asthma | The mean height SD score was below the reference mean (p<0.001) at start of treatment, and the mean height velocity SDS score (n=30) was below the reference mean (p<0.001) during the year immediately preceding the treatment. |
| Ninan 1992,13 United States | Hospital-based observational study (prospective longitudinal) | 58 prepubertal children with chronic asthma | Based on symptom score, asthma control was classified as good (≤5), moderate (6–10) and poor (>10). Before starting ICS treatment, height velocity SD score (mean, 95% CI) in children with good, moderate and poor asthma control was 0.01 (−0.27 to 0.30), −0.83 (−1.33 to −0.34) and −1.50 (−2.37 to −0.62), respectively. Height velocity SD score correlated negatively with control of asthma both before and after starting ICS (p<0.001 for both). |
| Agertoft 1994,14 Denmark | Randomized trial (baseline growth data only) | 278 children (3–11 years) with mild and moderate asthma (216 budesonide and 62 control) | The height SD score (mean and range) at the run-in period was −0.09 (−3.2 to 3.5) in the budesonide group and −0.18 (−2.3 to 2.3) in the control group. |
| Reid 1996,15 UK | Hospital-based observational study (prospective longitudinal) | 40 infants and young children (<3 years old) with severe uncontrolled asthma | The mean height SD score (SD) was −0.21 (1.1) at pre-treatment (at least 3 months before starting nebulized budesonide), and −0.46 (1.0) at the baseline (when budesonide was started). |
| Saha 1997,16 Finland | Hospital-based observational study (retrospective analysis) | 201 prepubertal asthmatic children (43% mild, 29% moderate and 28% severe) | Asthmatic children grew similarly to their healthy peers before starting ICS treatment: mean height SD score and mean height velocity SD score were +0.02 and +0.01 for boys, and −0.16 and +0.13 for girls, respectively. |
| Kannisto 2000,17 Finland | Randomized trial (baseline growth data only) | 75 asthmatic children (5.5–14.7 years) (30 budesonide, 30 fluticasone and 15 cromone) | The height SD score (mean±SD) at study entry in the budesonide, fluticasone and cromone group was −0.07±0.9, 0.31±0.84 and −0.01±1.11, respectively. |
| Norjavaara 2000,18 Sweden | Retrospective analysis of military conscription records (1983, 1986, 1993, and 1996) | 173,034 18-year old conscripts (8531 asthmatics and 164,503 non-asthmatics) | The mean height for asthmatics was lower than that for non-asthmatics (178.6±6.6cm vs. 179.3±6.6cm, p<0.001). Asthma severity, classified as mild (no reduction in physical capacity), moderate (mild to moderate reduction in physical capacity), and severe (considerably reduction in physical capacity), had a negative correlation with height (p<0.001). |
| Kelly 2003,19 United States | Randomized trial (baseline growth data only) | 1041 children (5–12 year) with mild to moderate asthma | The mean height percentile (SD) at baseline was 56.0 (28.5). Neither asthma severity (mild vs. moderate) nor its treatment in the 6 months before enrollment was associated with linear growth. |
| Moudiou 2003,20 Greece | Hospital-based observational study (cross-sectional) | 436 asthmatic children (3.9–15.4 years) not treated with long-term ICS and 710 age-matched healthy controls | There was no significant difference in the height SD score between asthmatics and controls, except pubertal female patients that was significantly less than that of controls. There was no significant correlation between height SD score and asthma severity (51 intermittent, 172 mild persistent, 186 moderate persistent, and 27 severe persistent) or duration of the disease, allergy status, and other coexisting allergic diseases. |
| Becker 2006,21 Canada, Costa Rica, Hong Kong, South Africa, United States | Randomized trial (baseline growth data only) | 360 children (6.4–9.4 years) with mild persistent asthma (119 beclomethasone, 120 montelukast, and 121 placeo) | The percentile for height-for-age (mean±SD) at study entry in the beclomethasone, montelukast and placebo group was 42.9±26.6, 38.0±27.3, and 43.4±24.9, respectively. |
| Pedersen 2007,22 Denmark | Randomized trial (baseline growth data only) | 42 children (6–11 years) with mild persistent asthma (37 montelukast and 34 budesonide) | The percentile for height-for-age (mean±SD) at study entry was 49.8±25.4 in the montelukast group, and 38.0±27.3 and 54.0±27.9 in the budesonide group. |
| Turpeinen 2008,23 Finland | Randomized trial (baseline growth data only) | 176 children (5–10 years) with mild persistent asthma (59 continuous budesonide, 58 budesonide/placebo, and 61 disodium cromoglycate) | The mean height SD score (range) at study entry in the continuous budesonide, budesonide/placebo, and cromone group was 0.04 (−0.32 to 0.54), 0.03 (−0.30 to 0.39), and 0.04 (−0.43 to 0.32), respectively. |
In 1940, Cohen et al.1 firstly illustrated the importance of anthropometry in following allergic children. They reported repeated anthropometric measures (height and weight) and maturity status over a period from 13 to 47 months in 5 children (6–13 years) with allergic disorders, including asthma. The milder cases affected only weight gain, but more severe cases could affect both height and weight, and finally physical maturity. Growth impairment could be completely recovered once allergic disorders were controlled. Later, Cohen et al.2 compared the growth, as measured by the Wetzel Grid, between 150 allergic children from the Asthma and Hay Fever Clinic and 102 age-matched non-allergic controls. They confirmed their earlier findings that active allergy had deleterious effect on growth, and that control of active allergy was associated with the improvement in growth. These are the two only clinic-based studies before the steroid era that assessed the effects of asthma on child's growth.
Ten hospital-based observational studies were published after the introduction of systemic corticosteroids for the treatment of asthma.3,5,8,9,11,13,15,16,20,28 Three studies described the growth pattern in patients not exposed or minimally exposed to corticosteroids.8,9,11 One hospital-based cross-sectional study showed that asthmatic children (n=91) were shorter than both non-asthmatic allergic children (n=59) and normal controls (n=69).8 The most severely affected asthmatics (n=29) tended to be the smallest, with a mean height between the 10th and 25th percentile. Bone age was assessed in 24 severe asthmatic children (21 males and three females), and a mean retardation of 9 months was observed in 21 boys. Another hospital-based retrospective study reported growth retardation in 28 children with chronic severe asthma who had never received oral steroids, with an average approximately 1 SD below their age- and sex-specific predicted means.11 One hospital-based longitudinal study described growth trajectory of 531 asthmatic boys from childhood to early adult life.9 No patient had been treated with corticosteroids during follow-up, and only 3.5% of the patients received long-term corticosteroids before study entry. This study reported no growth retardation during infancy, a small but consistent retardation during childhood, a more pronounced delay during pubertal ages, and completely normal stature beyond the age of 19 years. Peak height velocity was delayed by approximately 1.3 years, giving the impression of reduced growth in the asthmatic boys during adolescence, but that growth continued to an older age. Another two longitudinal studies described the similar growth trajectory, i.e., transitory growth retardation, in asthmatic children.40,41 One hospital-based study in 66 children with chronic asthma reported normal growth until approximately 10 years of age, pubertal delay with decelerating growth velocity in half the patients, and attainment of the predicted adult height once puberty had commenced.40 Another study of randomly selected schoolchildren (280 asthmatics and 62 controls) showed that those with the most severe asthma were significantly shorter at age 14 years; however, by 21 years of age, their height was no different to mild or moderate asthmatics, or normal controls.41 These two studies were not included in the present review, because a considerable proportion of participants had received corticosteroids.
The remaining seven hospital-based observational studies provided baseline growth data before starting the maintenance therapy with corticosteroids. Four studies in children with severe or intractable asthma reported impaired baseline growth.3,5,13,28 In contrast, another three studies showed normal baseline growth in asthmatic children, of which the majority had mild-to-moderate disease.15,16,20 Six more recent RCTs that aimed to assess the effects of ICS on growth also showed normal baseline growth in children with mild-to-moderate asthma.14,17,19,21–23
Three large school or military community-based studies provided useful insight regarding the growth in children and adolescents with asthma.10,12,18 One study in 7411 primary schoolchildren who had height measures and parent-reported respiratory symptoms showed an inverse association between the height SD score and asthma severity in the last 12 months, even after adjusting for parent's height, father's social class, and number of siblings.10 Two military community-based studies analyzed retrospectively medical records. One study18 with 173,034 conscripts (8531 asthmatics) showed a mean reduction of 0.7cm in the height at 18 years of age in asthmatic conscripts, compared to those without asthma. The severity of asthma had a negative correlation with the height. Another study12 with 92,143 17-year-old conscripts (3410 asthmatics) found comparable growth (height, weight, and body mass index) between adolescents with childhood asthma and those without asthma. There was a slight inverse association between all three growth variables and asthma severity, especially in boys. Caution is needed in interpreting the results from community-based studies, because the effects of the treatments on growth could not be excluded due to lack of such data from the participants.
Taken together, there is a substantial body of evidence suggesting that asthma per se, especially more severe and/or uncontrolled asthma, can adversely affect child's growth. There are several possible explanations for association between growth retardation and childhood asthma, including delayed puberty, severity and control of the disease, and impaired endocrine function.42
Effects of ICS on child's growthICS were introduced in the early 1970s as an alternative to the oral formulation for the treatment of asthma. Numerous studies have confirmed the benefits of ICS in controlling asthma symptoms, reducing exacerbations and hospitalizations, decreasing airway hyperresponsiveness and airway inflammation, improving pulmonary function, improving quality of life, and reducing asthma-related deaths.6 These drugs are currently considered to be first-line treatment for persistent asthma, both in adults and in children. However, concerns remain regarding the systematic side effects of ICS, such as impaired growth in children. Steroid phobia is still common among parents, which may lead to non-adherence to ICS therapy and poor asthma control in children.7
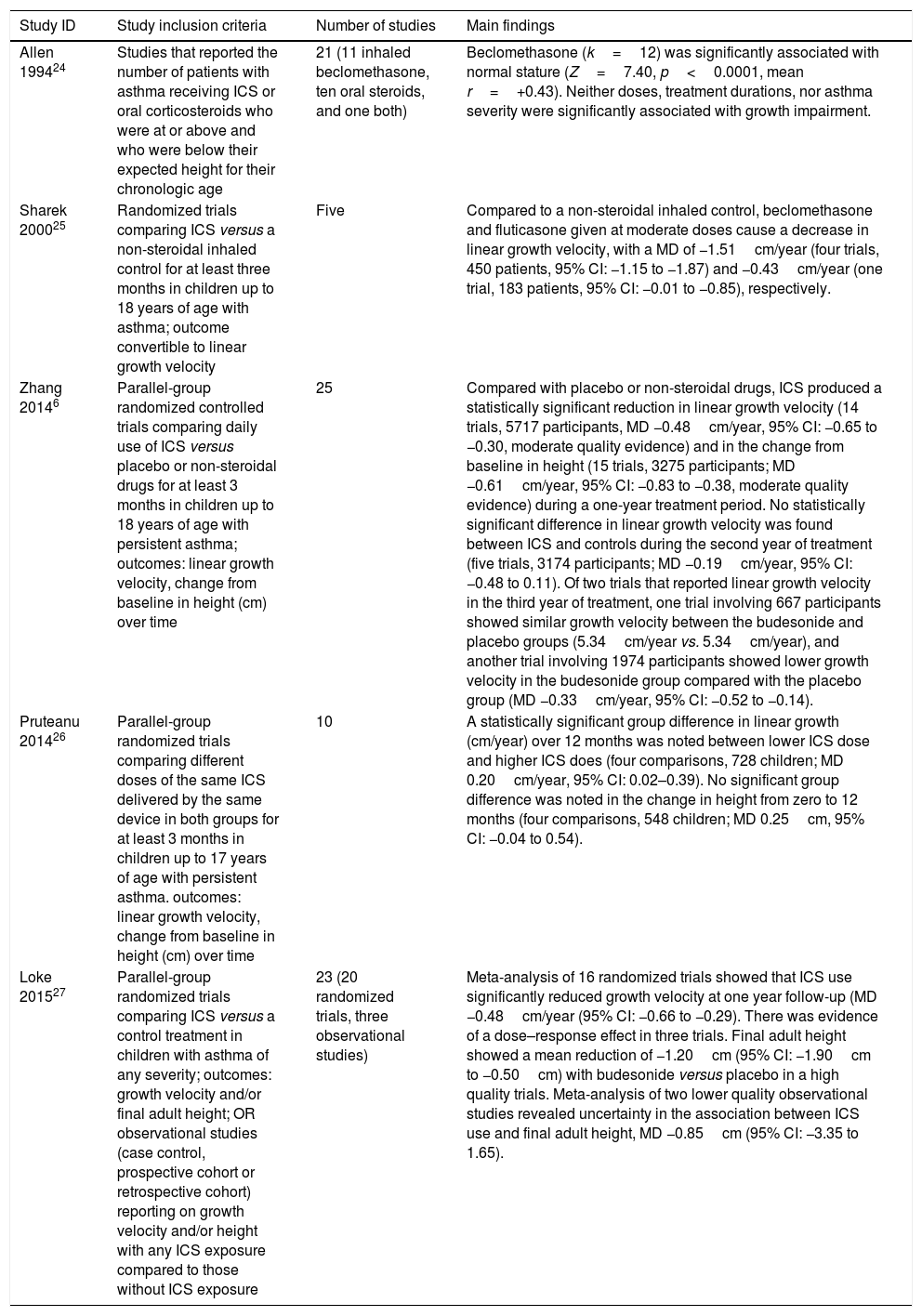
Five systematic reviews with meta-analysis that assessed the effects of ICS on growth in children with asthma were retrieved.6,24–27Table 2 summarizes the main findings of these five reviews. The first meta-analysis, published in 1994 by Allen et al.,24 included studies that reported the number of patients with asthma receiving inhaled or oral corticosteroids who were at, above, or below their expected height for age. These frequencies allowed the direct derivation of a one-sample chi-squared statistic, and was then transduced into the common metrics for significance levels and effect sizes to test the association between use of corticosteroids and growth. Such a statistical technique is no longer used for meta-analysis. This meta-analysis included 21 observational studies, of which 11 reported inhaled beclomethasone, ten reported oral steroids, and one reported both. The pooled data of 12 studies of becleomethasone showed that this medication was significantly associated with normal stature (Z=7.40, p<0.0001, mean r=+0.43). Neither doses, treatment durations, nor asthma severity were significantly associated with growth impairment. The pooled data of 11 studies of oral corticosteroids are presented in the review question 3.
Summary of systematic reviews regarding the effects of inhaled corticosteroids (ICS) on growth of children with asthma.
| Study ID | Study inclusion criteria | Number of studies | Main findings |
|---|---|---|---|
| Allen 199424 | Studies that reported the number of patients with asthma receiving ICS or oral corticosteroids who were at or above and who were below their expected height for their chronologic age | 21 (11 inhaled beclomethasone, ten oral steroids, and one both) | Beclomethasone (k=12) was significantly associated with normal stature (Z=7.40, p<0.0001, mean r=+0.43). Neither doses, treatment durations, nor asthma severity were significantly associated with growth impairment. |
| Sharek 200025 | Randomized trials comparing ICS versus a non-steroidal inhaled control for at least three months in children up to 18 years of age with asthma; outcome convertible to linear growth velocity | Five | Compared to a non-steroidal inhaled control, beclomethasone and fluticasone given at moderate doses cause a decrease in linear growth velocity, with a MD of −1.51cm/year (four trials, 450 patients, 95% CI: −1.15 to −1.87) and −0.43cm/year (one trial, 183 patients, 95% CI: −0.01 to −0.85), respectively. |
| Zhang 20146 | Parallel-group randomized controlled trials comparing daily use of ICS versus placebo or non-steroidal drugs for at least 3 months in children up to 18 years of age with persistent asthma; outcomes: linear growth velocity, change from baseline in height (cm) over time | 25 | Compared with placebo or non-steroidal drugs, ICS produced a statistically significant reduction in linear growth velocity (14 trials, 5717 participants, MD −0.48cm/year, 95% CI: −0.65 to −0.30, moderate quality evidence) and in the change from baseline in height (15 trials, 3275 participants; MD −0.61cm/year, 95% CI: −0.83 to −0.38, moderate quality evidence) during a one-year treatment period. No statistically significant difference in linear growth velocity was found between ICS and controls during the second year of treatment (five trials, 3174 participants; MD −0.19cm/year, 95% CI: −0.48 to 0.11). Of two trials that reported linear growth velocity in the third year of treatment, one trial involving 667 participants showed similar growth velocity between the budesonide and placebo groups (5.34cm/year vs. 5.34cm/year), and another trial involving 1974 participants showed lower growth velocity in the budesonide group compared with the placebo group (MD −0.33cm/year, 95% CI: −0.52 to −0.14). |
| Pruteanu 201426 | Parallel-group randomized trials comparing different doses of the same ICS delivered by the same device in both groups for at least 3 months in children up to 17 years of age with persistent asthma. outcomes: linear growth velocity, change from baseline in height (cm) over time | 10 | A statistically significant group difference in linear growth (cm/year) over 12 months was noted between lower ICS dose and higher ICS does (four comparisons, 728 children; MD 0.20cm/year, 95% CI: 0.02–0.39). No significant group difference was noted in the change in height from zero to 12 months (four comparisons, 548 children; MD 0.25cm, 95% CI: −0.04 to 0.54). |
| Loke 201527 | Parallel-group randomized trials comparing ICS versus a control treatment in children with asthma of any severity; outcomes: growth velocity and/or final adult height; OR observational studies (case control, prospective cohort or retrospective cohort) reporting on growth velocity and/or height with any ICS exposure compared to those without ICS exposure | 23 (20 randomized trials, three observational studies) | Meta-analysis of 16 randomized trials showed that ICS use significantly reduced growth velocity at one year follow-up (MD −0.48cm/year (95% CI: −0.66 to −0.29). There was evidence of a dose–response effect in three trials. Final adult height showed a mean reduction of −1.20cm (95% CI: −1.90cm to −0.50cm) with budesonide versus placebo in a high quality trials. Meta-analysis of two lower quality observational studies revealed uncertainty in the association between ICS use and final adult height, MD −0.85cm (95% CI: −3.35 to 1.65). |
The second systematic review was based on the results of a Cochrane review published in 2000 by the same group.25 This review included four trials of beclomethasone involving 450 patients and one trial of fluticasone with 183 patients. Compared to a non-steroidal inhaled control, beclomethasone and fluticasone given at moderate doses had caused a decrease in linear growth velocity, with a mean difference (MD) of −1.51cm/year (95% CI: −1.15 to −1.87) and −0.43cm/year (95% CI: −0.01 to −0.85), respectively. Sensitivity analysis in the beclomethasone subgroup, which evaluated study quality, delivered devices, control medication, and statistical model, showed similar results.
In 1994, two Cochrane reviews assessed the effects of ICS in children with persistent asthma.6,26 The first Cochrane review6 included 25 trials, of which 21 provided data of medium to long-term effects (44 weeks to 4–6 years) of ICS on growth in children with mild-to-moderate persistent asthma. Compared to placebo or non-steroidal drugs, ICS significantly reduced both linear growth velocity (−0.48cm/years, 95% CI: −0.65 to −0.30) and mean height increase (−0.61cm, 95% CI: −0.83 to −0.38) during a one-year treatment period. Subgroup analysis showed that some first-generation drugs had a slightly larger suppressive effect on growth than newer drugs, with a mean reduction in linear growth velocity of −0.91cm/year (95% CI: −1.26 to −0.55), −0.59cm/year (95% CI: −0.73 to −0.45), −0.08cm/year (95% CI: −0.27 to 0.11), −0.47cm/year (−0.97 to 0.03), −0.22cm/year (95% CI: −0.63 to 0.18), and −0.39cm/year (95% CI: −0.63 to −0.15) for beclomethasone, budesonide, ciclesonide, mometasone, flunisolide, and fluticasone, respectively. Only five included trials examined the effects of ICS on growth beyond one year. The pooled data of these five trials showed that ICS-induced growth suppression seemed to be maximal during the first year of therapy and less pronounced in subsequent years of treatment. Decreased adherence to treatment in the ICS group and increased additional use of corticosteroids due to poor asthma control in the control group over time might attenuate long-term effects of ICS on growth.
The second Cochrane review26 included three RCTs that compared the effects of ICS at low (50–100μg) versus low to medium (200μg) doses of HFA-beclomethasone equivalent in 728 prepubescent school-aged asthmatic children. A small (0.20cm/year) but statistically significant difference in linear growth was observed over 12 months, with a lower growth velocity in the higher ICS dose group.
The systematic review and meta-analysis by Loke et al.27 in 2015 included 20 RCTs and threee observational studies. The meta-analysis of 20 RCTs yield a mean reduction of −0.48cm/year (95% CI: −0.66 to −0.29) in linear growth velocity, that was almost identical to the Cochrane review by Zhang et al.6 The pooled data of two lower quality observational studies did not reveal a significant association between ICS use and final adult height (−0.85cm, 95% CI: −3.35 to 1.65).
Therefore, it remains unclear whether ICS use in childhood may have an impact on final adult height. An extended follow-up of the CAMP trial provides some insights into this question.43 Follow-up of the trial participants to the mean age of 25 years showed that children treated with budesonide (400μg/day) for a mean duration of 4.3 years during prepubertal age had a mean reduction of 1.20cm (95% CI: −1.90 to −0.50) in adult height compared to those treated with placebo. The effect size was similar to that seen after two years of treatment (−1.3cm, 95% CI: −1.70 to −0.90). These results suggest that the initial decrease in attained height related to ICS use in prepubertal age may persist as a reduction in adult height that is not progressive or cumulative.
Although RCTs are considered to be the gold standard of studying treatment effects, ICS growth trials have several limitations that may affect generalizability of the results. The majority of trials included prepubertal children with mild to moderate persistent asthma and used fixed doses of ICS. This might increase the likelihood of detecting possible undesirable effects of ICS because of the following reasons: this age group was likely to be most sensitive to the adverse effects of ICS on growth; patients were exposed to a relatively higher amount of drugs, as ICS were given at a fixed dose-regimen; and growth improvement due to good asthma control might be insignificant in these patients, because mild to moderate asthma itself has less adverse impact on growth. Thus, ICS growth trials provided the “worst case” estimate of the adverse effects of ICS on growth in children with asthma. The results from real-life observational studies appear to more closely reflect everyday clinical practice.
Latest recent review article by Camargos et al.29 was selected; it included 11 prospective real-life observational studies that assessed the effects of ICS on growth in children with asthma. Of 11 real-life studies, two were population-based, and the remaining were clinic- or hospital-based. Most studies did not show significant suppressive effects of ICS on growth, and two studies reported an initial growth reduction related to ICS which did not persist in subsequent years. One long-term prospective study showed that children with asthma who had received long-term treatment with budesonide attained normal adult height. This study followed-up the participants of another prospective controlled study in which 332 prepubertal children (270 budesonide, 62 controls) with asthma were enrolled. However, only 48% of original enrolled patients were included in the final analysis of adult height, which might have biased the results. Although observational studies reflect real-world clinical practice, confounding is the main limitation of such design.
Taken together, there is a substantial body of evidence suggesting that use of ICS in prepubertal children with asthma is associated with a small and molecule- and dose-dependent depression in growth in the first year of treatment, but no clinically relevant effect on adult height.
The mechanism of corticosteroid-induced growth impairment is not yet clearly understood. Corticosteroids are known to inhibit growth hormone (GH) secretion, insulin-like growth factor-1 (IGF-1) bioactivity, collagen synthesis, and adrenal androgen production. In addition to altering GH output, corticosteroids may reduce GH receptor expression and uncouple the receptors from their signal transduction mechanisms. Furthermore, corticosteroids may exert a direct growth-retarding effect on the growth plates. However, results regarding the association between ICS and alterations in production or activity of GH and IGF-1 have been inconsistent.6
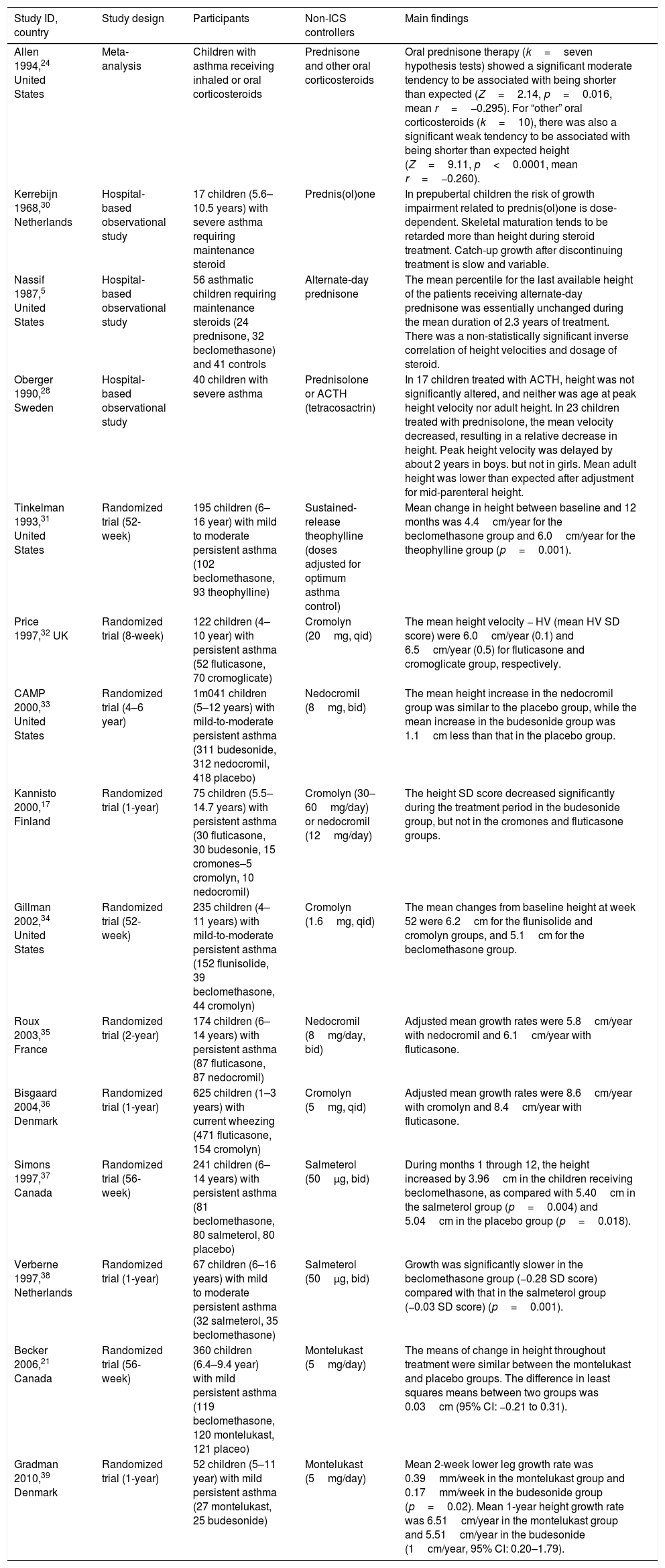
Effects of non-ICS asthma controllers on child's growthThe search retrieved 15 studies5,17,21,24,28,30–39 that provided data regarding the effects of non-ICS asthma controllers on child's growth (Table 3).
Summary of studies that provided data regarding the effects of non-ICS controllers on child's growth.
| Study ID, country | Study design | Participants | Non-ICS controllers | Main findings |
|---|---|---|---|---|
| Allen 1994,24 United States | Meta-analysis | Children with asthma receiving inhaled or oral corticosteroids | Prednisone and other oral corticosteroids | Oral prednisone therapy (k=seven hypothesis tests) showed a significant moderate tendency to be associated with being shorter than expected (Z=2.14, p=0.016, mean r=−0.295). For “other” oral corticosteroids (k=10), there was also a significant weak tendency to be associated with being shorter than expected height (Z=9.11, p<0.0001, mean r=−0.260). |
| Kerrebijn 1968,30 Netherlands | Hospital-based observational study | 17 children (5.6–10.5 years) with severe asthma requiring maintenance steroid | Prednis(ol)one | In prepubertal children the risk of growth impairment related to prednis(ol)one is dose-dependent. Skeletal maturation tends to be retarded more than height during steroid treatment. Catch-up growth after discontinuing treatment is slow and variable. |
| Nassif 1987,5 United States | Hospital-based observational study | 56 asthmatic children requiring maintenance steroids (24 prednisone, 32 beclomethasone) and 41 controls | Alternate-day prednisone | The mean percentile for the last available height of the patients receiving alternate-day prednisone was essentially unchanged during the mean duration of 2.3 years of treatment. There was a non-statistically significant inverse correlation of height velocities and dosage of steroid. |
| Oberger 1990,28 Sweden | Hospital-based observational study | 40 children with severe asthma | Prednisolone or ACTH (tetracosactrin) | In 17 children treated with ACTH, height was not significantly altered, and neither was age at peak height velocity nor adult height. In 23 children treated with prednisolone, the mean velocity decreased, resulting in a relative decrease in height. Peak height velocity was delayed by about 2 years in boys. but not in girls. Mean adult height was lower than expected after adjustment for mid-parenteral height. |
| Tinkelman 1993,31 United States | Randomized trial (52-week) | 195 children (6–16 year) with mild to moderate persistent asthma (102 beclomethasone, 93 theophylline) | Sustained-release theophylline (doses adjusted for optimum asthma control) | Mean change in height between baseline and 12 months was 4.4cm/year for the beclomethasone group and 6.0cm/year for the theophylline group (p=0.001). |
| Price 1997,32 UK | Randomized trial (8-week) | 122 children (4–10 year) with persistent asthma (52 fluticasone, 70 cromoglicate) | Cromolyn (20mg, qid) | The mean height velocity − HV (mean HV SD score) were 6.0cm/year (0.1) and 6.5cm/year (0.5) for fluticasone and cromoglicate group, respectively. |
| CAMP 2000,33 United States | Randomized trial (4–6 year) | 1m041 children (5–12 years) with mild-to-moderate persistent asthma (311 budesonide, 312 nedocromil, 418 placebo) | Nedocromil (8mg, bid) | The mean height increase in the nedocromil group was similar to the placebo group, while the mean increase in the budesonide group was 1.1cm less than that in the placebo group. |
| Kannisto 2000,17 Finland | Randomized trial (1-year) | 75 children (5.5–14.7 years) with persistent asthma (30 fluticasone, 30 budesonie, 15 cromones–5 cromolyn, 10 nedocromil) | Cromolyn (30–60mg/day) or nedocromil (12mg/day) | The height SD score decreased significantly during the treatment period in the budesonide group, but not in the cromones and fluticasone groups. |
| Gillman 2002,34 United States | Randomized trial (52-week) | 235 children (4–11 years) with mild-to-moderate persistent asthma (152 flunisolide, 39 beclomethasone, 44 cromolyn) | Cromolyn (1.6mg, qid) | The mean changes from baseline height at week 52 were 6.2cm for the flunisolide and cromolyn groups, and 5.1cm for the beclomethasone group. |
| Roux 2003,35 France | Randomized trial (2-year) | 174 children (6–14 years) with persistent asthma (87 fluticasone, 87 nedocromil) | Nedocromil (8mg/day, bid) | Adjusted mean growth rates were 5.8cm/year with nedocromil and 6.1cm/year with fluticasone. |
| Bisgaard 2004,36 Denmark | Randomized trial (1-year) | 625 children (1–3 years) with current wheezing (471 fluticasone, 154 cromolyn) | Cromolyn (5mg, qid) | Adjusted mean growth rates were 8.6cm/year with cromolyn and 8.4cm/year with fluticasone. |
| Simons 1997,37 Canada | Randomized trial (56-week) | 241 children (6–14 years) with persistent asthma (81 beclomethasone, 80 salmeterol, 80 placebo) | Salmeterol (50μg, bid) | During months 1 through 12, the height increased by 3.96cm in the children receiving beclomethasone, as compared with 5.40cm in the salmeterol group (p=0.004) and 5.04cm in the placebo group (p=0.018). |
| Verberne 1997,38 Netherlands | Randomized trial (1-year) | 67 children (6–16 years) with mild to moderate persistent asthma (32 salmeterol, 35 beclomethasone) | Salmeterol (50μg, bid) | Growth was significantly slower in the beclomethasone group (−0.28 SD score) compared with that in the salmeterol group (−0.03 SD score) (p=0.001). |
| Becker 2006,21 Canada | Randomized trial (56-week) | 360 children (6.4–9.4 year) with mild persistent asthma (119 beclomethasone, 120 montelukast, 121 placeo) | Montelukast (5mg/day) | The means of change in height throughout treatment were similar between the montelukast and placebo groups. The difference in least squares means between two groups was 0.03cm (95% CI: −0.21 to 0.31). |
| Gradman 2010,39 Denmark | Randomized trial (1-year) | 52 children (5–11 year) with mild persistent asthma (27 montelukast, 25 budesonide) | Montelukast (5mg/day) | Mean 2-week lower leg growth rate was 0.39mm/week in the montelukast group and 0.17mm/week in the budesonide group (p=0.02). Mean 1-year height growth rate was 6.51cm/year in the montelukast group and 5.51cm/year in the budesonide (1cm/year, 95% CI: 0.20–1.79). |
One meta-analysis24 and three hospital-based observational studies5,28,30 assessed systemic corticosteroids. The meta-analysis by Allen et al.24 included 12 observational studies of inhaled beclomethasone and 11 observational studies of oral corticosteroids. The results of beclomethasone have been presented in the review question 2. The pooled data of 11 studies of oral corticosteroids showed that both prednisone and other corticosteroids were significantly associated with being shorter than expected height. Of three individual studies for systemic corticosteroids, two studies28,30 had documented growth impairment related to prednis(ol)on, and one30 of them showed that the effects of prednis(ol)on were dose-dependent. In contrast, no significant adverse effects of alternate-day prednisone on growth were observed in 24 asthmatic children over a mean period of 2.3 years of treatment.5
Six trials compared linear growth between asthmatic children treated with inhaled cromones and ICS.17,32–36 Only one trial used placebo,33 and showed that patients treated with nedocromil over a period of 4–6 years had a similar mean height increase than those treated with placebo. Four trials have consistently showed that prepubertal and preschool asthmatic children treated with cromones and fluticasone (100–200μg/day) had a similar linear growth velocity which is comparable to expected growth for age.32,34–36 Another trial17 showed normal growth in both cromones and fluticasone groups during a one-year treatment period, as compared to the population-based reference value.
Two trials assessed the effects of montelukast on growth in children with mild persistent asthma. One 56-seek placebo-controlled trial showed a similar mean change in height throughout treatment between the montelukast and placebo groups.21 Another one-year trial showed a mean linear growth rate of 6.51cm/year in the montelukast group which is comparable to expected growth for age, and higher than that in the budesonide group (5.51cm/year).39
Two trials assessed the effects of salmeterol on growth, of which one37 compared with placebo and another38 compared with beclomethasone. Both trials showed normal linear growth in patients treated with salmeterol, as compared to placebo or the population-based reference value.
One 52-week trial31 compared linear growth between oral sustained-release theophylline and beclomethasone, and showed a linear growth velocity of 6.0cm/year in the theophylline group which is comparable to expected growth for age. In contrast, patients treated with beclomethasone had a reduced linear growth velocity (4.4cm/year).
Final considerationsThe currently available evidence suggests that asthma per se, especially more severe and/or uncontrolled cases, can adversely impair child's growth. The perceived growth retardation in asthmatic children is likely to be due to pubertal delay, and the majority of them will attain a normal adult height.
Medium-to long-term trials show a small but statistically significant ICS-induced growth suppression (an average reduction of −0.48cm/year in linear growth rate) in the first year of treatment in prepubertal children with mild to moderate persistent asthma, and the effects tend to be less pronounced in the subsequent years of treatment. ICS-induced growth suppression appear to be molecule-dependent, with some first-generation drugs having a slightly larger suppressive effect than newer drugs. There is also some evidence of a dose–response relationship. Limited evidence suggests that the initial decrease in attained height related to ICS in prepubertal age may persist as a reduction in adult height that is neither progressive nor cumulative.
ICS growth trials may provide the “worst case” estimate of the deleterious effects of ICS on growth in children with asthma because of the inclusion of most sensitive age group (prepubertal children), with less severe disease (mild-to-moderate- persistent asthma), and exposed to a higher amount of ICS (fixed dose-regimen). A smaller ICS-related growth suppressive effect may be expected in asthmatic children seen in everyday clinical practice, as shown by real-life observational studies. In turn, anecdotal but well-documented reports of severe growth failure caused by ICS given at doses normally considered to be safe indicate idiosyncratic responses (increased sensitivity to the systemic effects of ICS) in the individual patient.44
The maintenance treatment with systemic corticosteroids can cause dose-dependent growth suppression in children with severe asthma. Systemic corticosteroids are also associated with other major adverse effects, such as adrenal suppression, osteopenia, immunosuppression, hypertension and psychosis.45 Despite that long-term use of systemic corticosteroids is indicated only for a very small proportion of children who have difficult-to-control asthma, overprescribing of oral corticosteroids is still common in children with asthma.46 The safety of repeated bursts of oral corticosteroids in children with asthma has not yet been well established.47–49 However, a recent population-based nested case-control study in 19420 children with asthma showed that systemic corticosteroids, but not ICS, were significantly associated with increased odds of fracture.50 The multivariable regression results did not show a significant association between first fracture after asthma diagnosis and current use (odds ratio [OR] 1.07, 95% CI: [0.97–1.17]), recent use (OR 0.96, 95% CI: [0.86–1.07]), or past use (OR 1.00, 95% CI: [0.91–1.11]) of ICS, compared with no use, while adjusting for sociodemographic factors and other medication use. However, at least 1 prescription of a systemic corticosteroid in the 1-year lookback period resulted in greater odds of fracture (OR 1.17, 95% CI: [1.04–1.33]), compared with no prescription. There was also evidence of a potential dose effect of systemic corticosteroids, as only those children in the highest dose category had increased odds of fracture compared with no use.
Other non-ICS asthma control medications, such as cromones, montelukast, salmeterol, and sustained-release theophylline, have no significant adverse effects on growth in children with asthma. However, they are less effective than ICS in controlling asthma, and usually used as add-on medications when asthma is not well controlled by low-dose ICS.
The above-presented evidence regarding the effects of asthma and its treatments on child's growth allows the authors to make the following recommendations:
- 1)
Asthma should be controlled as well as possible given that severe and/or uncontrolled asthma not only adversely impair child's growth, but also cause significant morbidity and mortality. The well-established benefits of ICS in controlling asthma outweigh the potential risks of a relatively small and non-cumulative suppression in linear growth in children with persistent asthma. Fear of such small growth reduction should not be a reason to withhold or withdraw this highly effective therapy in asthmatic children. Good communication between healthcare professionals and parents is essential to reduce steroid phobia and improve treatment adherence.
- 2)
The minimally effective ICS doses should be used for the treatment of children with asthma. Addition of an add-on control medication, especially leukotriene receptor antagonist or long-acting beta-agonist, is preferable to increasing the dose of steroid when asthma is not well controlled by low-dose ICS.
- 3)
Some first-generation drugs may have slightly larger suppressive effect on growth than newer drugs, but the clinical relevance of these small differences is questionable. ICS selection should be based on the efficacy, overall safety profile, delivery device, ease of use, availability, and cost.
- 4)
Regular monitoring of height during ICS therapy in children with asthma is recommended to detect growth deviation, especially idiosyncratic response to ICS. The authors also recommend to assess pubertal stage, mid-parental target height, and parental perception on child's growth.
- 5)
Healthcare providers should carefully assess the indication of corticosteroid therapy, given that both oral and inhaled corticosteroids are commonly prescribed for the treatment of wheezing in the first year of life in Brazil.51
- 6)
A considerable number of these infants may have episodic viral wheeze rather than asthma, and there is no current evidence to support the use of maintenance ICS in these patients.52 In this case, the risks of corticosteroids may overweigh the benefits.
- 7)
Finally, it is still valid to say that “asthma is a killing disease, but nobody died of being short”.53
The authors declare no conflicts of interest.
Linjie Zhang is currently a Research Productivity (PQ level 2) fellow of Brazilian National Council for Scientific and Technological Development (CNPq).
Please cite this article as: Zhang L, Lasmar LB, Castro-Rodriguez JA. The impact of asthma and its treatment on growth: an evidence-based review. J Pediatr (Rio J). 2019;95:S10–S22.