To investigate the impact of recombinant human interferon α1b (rhIFNα1b) treatment in infants hospitalized with lower respiratory tract infections on subsequent wheezing.
MethodsThe clinical data of infants (n=540) with viral pneumonia, wheezy bronchitis, or bronchiolitis hospitalized in 19 Chinese hospitals from June 2009 to June 2015 were retrospectively analyzed. The parameters relevant to wheezing episodes within the last year were collected by telephone and questionnaires. The rhIFNα1b treatment group (n=253) and control group (n=287) were compared in terms of wheezing episodes within the last year. Moreover, the wheezing group (95 cases) and non-wheezing group (445 cases) were compared.
ResultsOut of 540 cases, 95 (17.6%) experienced wheezing episodes, 13.8% (35/253) cases treated with rhIFNα1b, and 20.9% (60/287) cases without rhIFNα1b experienced wheezing episodes within the last year. The rhIFNα1b treatment significantly improved wheezing episodes within the last year, compared with the control peers (p=0.031). Single-factor regression showed statistically significant differences between the wheezing and non-wheezing groups in terms of age, rhIFNα1b use, childhood and family history of allergy, housing situation, and feeding history (p<0.05). Binary logistic regression showed a childhood history of allergy (OR=2.14, p=0.004), no rhIFNα1b use (OR=1.70, p=0.028), and living in a crowded house (OR=1.92, p=0.012) might be risk factors of subsequent wheezing. Accordingly, breastfeeding (OR=0.44, p=0.008) and hospitalization age of ≤1-year-old (OR=0.58, p=0.024) were protective factors.
ConclusionsEarly use of rhIFNα1b in infants hospitalized with lower respiratory tract infections and breastfeeding could prevent subsequent wheezing. Living in a crowded house could promote subsequent wheezing.
Infants and young children, especially children less than 2 years of age, often develop lower respiratory tract infections such as pneumonia, wheezy bronchitis, and bronchiolitis, and the main pathogens of these infections are viruses.1–3 Strong evidence shows that viral infections during infancy or early childhood influence T lymphocyte subsets and related factors in infants and young children, leading to recurrent wheezing and asthma in later childhood.4–6 Among viral infections, only the influenza virus and cytomegalovirus have targeted drug therapy; but, treatments for other viral infections are still largely supportive and symptomatic. Interferon (IFN) is an important cytokine produced by the body after a viral infection. It has dual effects of anti-virus and immune regulation.7 Recombinant human IFN alpha 1b (rhIFNα1b, Hapgen®) is a type of genetic engineering IFN developed in China,8 and rhIFNα1b has been used for the treatment of chronic viral hepatitis B, hand, foot, and mouth disease and Epstein-Barr virus (EBV) associated nasopharyngeal carcinoma.9,10 In this study, we investigated the effects of rhIFNα1b and other factors on subsequent wheezing in infants diagnosed with viral pneumonia, wheezy bronchitis, and bronchiolitis.
MethodPatientsThe study population of this study consisted of infants younger than 3 years of age who were hospitalized in 19 hospitals with the diagnosis of viral pneumonia, wheezy bronchitis, or bronchiolitis and during the period from June 2009 to June 2015.
Study designAll the experimental and clinical procedures of this study were approved by the local ethics committee of Children's Hospital of Second Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, China (ethic code: KXY2018148), which were in complete accordance with the ethical standards and regulations of human studies of the Helsinki declaration (2014). Questionnaires were based on the available scientific literature on wheezing and the asthma epidemiology questionnaire.11 The hospital medical records were retrieved and age, gender, diagnosis (viral pneumonia, bronchiolitis or wheezy bronchitis), use of rhIFNα1b (a dose of at least 1μg/kg.d and treatment for at least 3 days) in hospital, age at follow-up, birth weight, gestation age, childhood and family history of allergy, feeding history (breastfeeding, mixed feeding, milk powder feeding), family environment (smokers in the home, pets, and housing situation), and wheezing episodes within the last year were obtained by telephone and from questionnaires.
According to the use of rhIFNα1b during hospitalization, the patients were divided into two groups including the rhIFNα1b treatment group and the control group. The two groups were compared in terms of wheezing episodes within the last year. Moreover, based on the frequency of wheezing episodes within the last year, the subjects were divided into two groups, which were the wheezing group and non-wheezing group, and the related factors of wheezing were studied.
Statistical methodsThe statistical package for social sciences (SPSS Inc. Chicago, Illinois, USA; Windows, version 17.0) was used for the statistical analyses. The quantitative variables were tested by the Kolmogorov-Smirnov test for normality, and two-tailed Student’s t-tests were used after the data fulfilled the criteria of normal distribution and equal variance; otherwise, Mann–Whitney U-tests were used. The Chi-square test or Fisher’s exact test for categorical variables were also used. Demographics and baseline characteristics were compared between the wheezing group and the non-wheezing group. If the outcome of the single factor comparison showed that P was <0.05, the indicators were analyzed via binary logistic regression (the method of selecting independent variables is forward LR). The receiver operator characteristic (ROC) curve was created to evaluate the prediction ability of the logistic regression model.
ResultsGeneral informationFollowing the screening of the medical data, 813 cases were determined with available follow-up data, of these, 273 cases were excluded from the further analysis because of incomplete questionnaires, rhIFNα1b dosages less than 1μg/kg.d or a treatment duration <3 days was inhaled, a date in hospital beyond the scope of the follow-up, or because of the hospitalization age of the subjects greater than 3 years old. Finally, 540 patients were included in the final analysis, including 312 cases (57.8%) of pneumonia, 132 cases (24.4%) of wheezy bronchitis, and 96 cases (17.8%) of bronchiolitis. A total of 253 cases were treated with conventional therapy plus rhIFNα1b and 287 cases were treated with only conventional therapy without rhIFNα1b.
Wheezing episodes within the last yearA total of 95 cases (17.6%) out of 540 cases experienced wheezing episodes within the last year; 35 (13.8%) out of 253 cases treated with rhIFNα1b and 60 (20.9%) out of 287 cases without rhIFNα1b treatment had wheezing episodes within the last year. The difference in the frequency of wheezing episodes within the last year between the rhIFNα1b treatment group and the control group was statistically significant (X2=4.64, p=0.031).
Analysis of related factors of wheezingComparison of demographics and baseline clinical characteristics between wheezing and non-wheezing groups
There were 540 cases, including 95 cases in the wheezing group and 445 cases in the non-wheezing group. The hospitalization age, birth weight, and age at follow-up were compared between the two groups via the Mann-Whitney test and other factors were compared by the Chi-square test. The single factor regression test indicated that the differences between the wheezing and non-wheezing groups in terms of the hospitalization age, the use of rhIFNα1b in hospital, childhood and family history of allergy, housing situation, and feeding history, were all statistically significant (p<0.05; Table 1).
Comparison of demographics and baseline characteristics of the wheezing and non-wheezing groups.
| Wheezing group | Non-wheezing group | X2/Z | p | |
|---|---|---|---|---|
| Male / female | 67/28 | 282/163 | 1.75 | 0.19 |
| Term / preterm birth | 91/4 | 408/37 | 1.88 | 0.17 |
| Age at the time of hospitalization, years (median) | 1.3 | 1.0 | −3.55 | 0.00 |
| Birth weight, kg (median) | 3.0 | 3.0 | −1.04 | 0.30 |
| Breast feeding/ mixed feeding/ milk powder feeding | 33/37/25 | 229/149/67 | 11.02 | 0.004 |
| Child history of allergy (yes/no) | 29/66 | 76/369 | 9.04 | 0.003 |
| Family history of allergy (yes/no) | 21/74 | 56/389 | 5.81 | 0.016 |
| Smokers in home (yes/no) | 51/44 | 256/189 | 0.47 | 0.49 |
| Pets in home (yes/no) | 11/84 | 37/408 | 1.03 | 0.31 |
| rhIFNα1b treatment (yes/no) | 35/60 | 218/227 | 4.64 | 0.031 |
| Viral pneumonia / bronchiolitis / wheezy bronchitis | 50/22/23 | 262/74/109 | 2.41 | 0.3 |
| Housing situation (spacious/crowded) | 25/70 | 179/266 | 6.44 | 0.011 |
| Age at follow-up, years (median) | 3.4 | 3.3 | −0.064 | 0.95 |
The factors that were significantly different in the univariate analysis (p<0.05) were further analyzed by binary logistic regression (Table 2). The binary logistic regression analysis showed that a childhood history of allergy, feeding history, the use of rhIFNα1b in hospital, hospitalization age, and the housing situation entered the final regression equation. A childhood history of allergy, no rhIFNα1b treatment, and crowded housing were risk factors; breastfeeding and hospitalization age ≤1-year-old were protective factors (Table 3).
Related factors and the assignment of wheezing within the last year.
| Factors | Assignment |
|---|---|
| Age at the time of hospitalization | ≤1year old=1; >1year old=0 |
| Childhood history of allergy | Yes=1; no=0 |
| Housing situation | Crowded=1; spacious=0 |
| The use of rhIFNα1b | No=1; yes=0 |
| Feeding history | Breast feeding=1; mixed feeding=2; milk powder feeding=3 |
| Wheezing episodes within the last year | Wheezing=1; non-wheezing=0 |
Logistic regression analysis of related factors of wheezing within the last year.
| B | SE | Wald | P | OR | 95%CI | |
|---|---|---|---|---|---|---|
| rhIFNα1b | 0.53 | 0.24 | 4.83 | 0.028 | 1.70 | 1.06∼2.74 |
| Age at the time of hospitalization | −0.54 | 0.24 | 5.08 | 0.024 | 0.58 | 0.37∼0.93 |
| Childhood history of allergy | 0.76 | 0.27 | 8.21 | 0.004 | 2.14 | 1.27∼3.59 |
| Feeding history | 7.07 | 0.03 | ||||
| Breast feeding | −0.82 | 0.31 | 7.02 | 0.008 | 0.44 | 0.24∼0.81 |
| Mixed feeding | −0.44 | 0.31 | 2.08 | 0.15 | 0.64 | 0.35∼1.17 |
| Housing situation | 0.65 | 0.26 | 6.26 | 0.012 | 1.92 | 1.15∼3.21 |
Age at the time of hospitalization; ≤1year old=1; >1year old=0, Childhood history of allergy; yes=1; no=0, Housing situation; crowded=1; spacious=0, Use of rhIFNα1b; no=1; yes=0, Feeding history; Breast feeding=1; mixed feeding=2 ; milk powder feeding=3, Wheezing episodes within the last year; wheezing=1; non-wheezing=0.
rhIFNα1b, Human Interferon α1b Treatment; CI, confidence interval; SE, standard errors; B, beta coefficient.
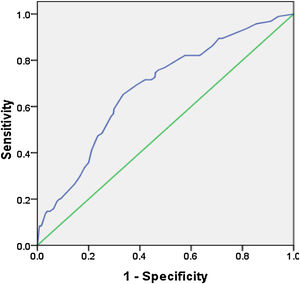
The ROC curve was created by using the predicted values of the model, and the area under the curve was 0.68, 95% CI (0.62∼0.74), p=0.00, which showed that the regression model had greater than medium diagnostic accuracy (Fig. 1).
DiscussionRecombinant human interferon alpha 1b (rhIFN a 1b, Hapgen®) is a type of genetic engineering interferon developed in China, and rhIFNα1b has been used for the treatment of chronic viral hepatitis B, hand, foot, and mouth disease and the Epstein-Barr virus (EBV) infection.8 The main pathogens of lower respiratory tract infections during infancy or early childhood are viruses,2,3 and many studies have suggested that early viral infections in infants and young children are associated with subsequent wheezing or asthma.12–16 In a previous study conducted in 19 hospitals which enrolled 813 cases shows that the application of rhIFNα1b treatment for infants with lower respiratory tract infection has a protective effect on subsequent wheezing (without rhIFNα1b treatment OR 1.70, p=0.028), probably because exogenous rhIFNα1b can elevate the level of IFN, and IFNs are important modulators of the immune response.17
In order to study the effect of rhIFNα1b and other related factors on subsequent wheezing in infants hospitalized with lower respiratory tract infection, this study investigated 13 possible related factors. The results show that rhIFNα1b treatment, age of hospitalization, a childhood history of allergy, feeding history, and housing situation are associated with subsequent wheezing in 540 infants hospitalized with lower respiratory tract infection. Our findings show that infants with ≤1year old, in comparison with 2–3 years old age group, with a lower respiratory tract infection are not prone to wheezing (OR=0.58, p=0.024). However, further studies are needed to confirm this finding indicating a relationship between age and susceptibility to wheezing in infants with a lower respiratory tract infection. However, Feldman et al.18 recently studied the relationship between the etiology and timing of early childhood respiratory wheezing illnesses during the first 3 years of life and the development of asthma in children at the age of 6 and found that there was no correlation between childhood respiratory syncytial virus (RSV) infection at the age of 1 and 2 and the diagnosis of asthma at age 6, but wheezing RSV illnesses that occurred at the age of 3 were associated with a nearly 14-fold increase in asthma risk at 6 years of age. Wang et al. used the nebulized rhIFN α1b and found that the particle size of rhIFN α1b injection was small enough to be transmitted to the lung, and the total delivered dose and delivery rate showed a tendency of increase in turn in the neonatal, infant, and child breathing modes, indicating that the effective dose of the drug and the age of patients should be considered when formulating the clinical treatment plan.19 Therefore, although several studies have shown that early viral infection in infants and young children is associated with recurrent wheezing episodes, this issue is controversial.18,20 Therefore, the identification of the risk and protective factors between early viral infection and recurrent wheezing in infants and young children is the most effective strategy for the prevention of wheezing in children.
A childhood history of allergy is a risk factor for wheezing (OR=2.21, p=0.004). Compared with milk powder feeding, breastfeeding protects infants with lower respiratory tract infection from subsequent wheezing (OR=0.44, p=0.008). Studies have shown that early childhood sensitization to food and inhalant allergens may be the beginning of the “atopic march”, and may promote other allergic diseases such as respiratory allergies.21,22 Breast milk contains IgA, cytokines, and long-chain fatty acids that stimulate the development of the infants' immune system.23 Breastfeeding also activates the intestinal microflora in infants, which in turn activates T cells and enhances immune function.24 Therefore, breastfeeding and the extension of exclusive breastfeeding time will reduce the risk of subsequent wheezing in infants and young children.25 Living in a crowded house (p=0.012; OR=1.92) increases the chance of respiratory infection and the chance of exposure to allergens in the home and contributes to wheezing episodes.
IFN is a type of antiviral low molecular weight protein in the normal human body that can play antiviral and immunomodulatory roles in a variety of ways. According to the specificity of the receptor, IFN can be divided into type I IFNs, type II IFNs and type III IFNs. Type I IFNs include IFN-α, IFN-β, etc. Different subtypes of IFN play different immunomodulatory roles.26 This study shows that the application of rhIFNα1b treatment for infants with lower respiratory tract infection has a protective effect on subsequent wheezing (without rhIFNα1b treatment OR 1.70, p=0.028), probably because exogenous rhIFNα1b can elevate the level of IFN, and IFNs are important modulators of the immune response, particularly in the inhibition of viral replication within host cells, the activation of natural killer cells and macrophages, the increase in antigen presentation to lymphocytes, the induction of the resistance of host cells to viral infection, and the weakening of the damage of the virus to lung tissue.7,27 However, further studies with a large sample size would be helpful to countercheck that the application of rhIFNα1b treatment for infants with lower respiratory tract infection has a protective effect on subsequent wheezing. On the other hand, it may be related to the immunomodulatory functions of type I IFNs on T lymphocyte subsets.28
The outstanding characteristic of this study is that it demonstrated that the early application of rhIFNα1b therapy for infants with lower respiratory tract infection can protect infants from subsequent wheezing.
Ethics approval and consent to participateThis study was approved by the local ethics committee of Children's Hospital of Second Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, China (Ethic code: KXY2018148).
Consent for publicationThis manuscript has not been published and is not under consideration for publication elsewhere in whole or in part. No conflicts of interest exist in the submission of this manuscript, and the manuscript has been approved for publication by all listed authors.
Availability of data and materialAll the data used in this study are available from the corresponding author on reasonable request.
Conflicts of interestThe authors declare no conflicts of interest.
FundingNot applicable.
The authors would like to thank all those who contributed their time and resources to the development of this article.