To evaluate the diagnostic utility of salivary C-reactive protein (CRP) and its potential correlation with serum CRP levels in full-term neonates with late-onset sepsis (LOS).
MethodsThis cross-sectional study included 90 neonates assigned to three equal groups: culture proven LOS, clinical LOS and a control group. Clinical findings and routine laboratory data including complete blood pictures and blood culture results were documented. Highly sensitive serum CRP was measured according to hospital protocol, while salivary CRP levels were measured using enzyme-linked immunosorbent assay.
ResultsThe median serum CRP was significantly higher in septic neonates compared to controls (p < 0.001). For serum CRP, the optimum cut-off value for LOS diagnosis was found to be 7.2 mg/L with sensitivity, specificity, positive and negative predictive values of 91, 100, 100, and 85.7%, respectively. No significant difference was observed in levels of salivary CRP among the 3 study groups (p = 0.39). No correlation was found between the levels of salivary and serum CRP (r = 0.074, p = 0.49).
ConclusionSerum CRP, at a cut-off value of 7.2 mg/L, exhibited a high specificity and positive predictive value in LOS diagnosis, whereas salivary CRP levels weren’t significantly different between the 3 study groups nor did they predict abnormal serum CRP thresholds in newborns with sepsis.
Sepsis is one of the main causes of morbidity and mortality among newborns. According to its time of onset after birth, neonatal sepsis (NS) is classified into: early-onset sepsis variably defined as occurring within 48−72 hours after birth and late-onset sepsis (LOS) occurring thereafter.1,2 The clinical findings of NS are often subtle and nonspecific, in view of that, neonates suspected of sepsis are usually prone to empirical administration of broad-spectrum antibiotics until sepsis can be excluded, which favors the emergence of drug resistant strains.3 The diagnosis of NS is often based on clinical assessment in combination with laboratory findings. Blood culture remains the definitive standard for diagnosing NS, despite being time consuming. In addition, a relatively small blood sample or prior exposure to empirical antibiotics may cause false-negative results. Notably, a positive blood culture may reflect asymptomatic bacteremia or contamination.4
Many studies evaluated the sensitivity and specificity of the several markers in NS e.g. interferon-gamma, different interleukins and blood cell parameters, yet results vary extensively between studies.5,6
The clinical use of C-reactive protein (CRP) as a biomarker for sepsis in neonates has been well validated. Nevertheless, a limitation to its serial monitoring in newborns currently is its reliance on repeated blood draws putting this susceptible population at medical risk.7 Establishing a non-invasive method for its quantification could reduce side-effects and improve its clinical utility in newborns. Saliva contains systemic proteins, immunoglobulins, electrolytes, nucleic acids, microorganisms, toxins, and drugs, being an important reservoir of them. Therefore, it represents an ideal non-invasive alternative to serum screening for a variety of infectious processes.8 Several studies investigated the use of saliva as a substitute biofluid for serum CRP, yet results are inconsistent.8–10 The objective of the current study was to measure the levels of salivary CRP and to explore its potential correlation with serum CRP for LOS diagnosis in a cohort of full-term neonates.
MethodsStudy populationThis cross-sectional study included ninety full-term neonates who were admitted in the neonatal intensive care unit (NICUs) of Cairo University, Cairo, Egypt, between October 2018 and March 2019. The exclusion criteria from this study were pre-term neonates, neonates with early onset sepsis, respiratory distress syndrome, hypoxia, major congenital anomalies, metabolic disease, mechanical ventilation and neonates that underwent major surgical procedures. The patients were divided into three equal groups. Demographic and clinical data were collected and recorded. Newborns in the clinical LOS group were diagnosed on the basis of clinical suspicion, but their blood cultures were negative. Neonates in the culture proven LOS group were diagnosed clinically and had a positive blood culture. The controls were age and sex matched newborns with no clinical or laboratory findings of sepsis. The study protocol ethics was approved by the scientific committee of the Pediatrics department, Faculty of Medicine, Cairo University and followed the tenets of the Helsinki declaration. Informed consent was obtained from the parents.
Laboratory investigationsLaboratory investigations performed routinely on appearance of any signs suggestive of sepsis included CBC, serum CRP and blood culture. CBC was analyzed by the automated blood cell counter Sysmx xs-800i (Roche diagnostics) using 2 mL peripheral blood samples, anticoagulated with Ethylenediaminetetraacetic acid. For accurate mean platelet volume (MPV) calculation, samples were analyzed within 60 min after collection to avoid platelet swelling and false increase of MPV value. Differential count was analyzed by pathologists blinded to the infection status of these infants. Immature to total (I/T) neutrophils ratio was calculated by dividing the total number of immature neutrophils by the total neutrophilic count. Degenerative changes in neutrophils included vacuolization, toxic granulations and Dohle bodies. For blood culture, a 1 mL blood sample was withdrawn under strict aseptic measures and inoculated into a blood culture bottle. Bactec microbial detection system (Bactec 9050, Becton-Dickinson, New Jersey, USA) was used for blood culture. According to standard microbiological methods, positive cases were subjected to subculture. This was considered positive if the isolated organism was known to cause bacteremia or if the organisms isolated consecutively in 2 cultures within 7 days were known as skin contaminants. For determination of serum high sensitivity CRP (hs-CRP), 1 mL blood was collected into a plain vacutainer tube. Serum was separated as soon as possible to prevent hemolysis and measured on cobas automated analyzer (Roche diagnostics). Samples of saliva were collected within 4–12 h of clinically indicated serum CRP levels using a previously established protocol.11 Collection of samples occurred nearly 1 h before feeding to avoid milk or formula contamination by tilting the head forward to pool saliva in the floor of the mouth. Samples were obtained by using a 1-mL syringe, wings removed, attached to low wall suction (< 20 mmHg) maintained for 10–15 seconds, collecting around 0.5 mL. Samples were then placed in polypropylene tubes and stored in -20 °C until use. Estimation of salivary CRP level was performed using a quantitative enzyme linked Immunosorbent assay kit (Bioassay technology laboratory). Samples were plotted against standard curve for CRP quantification.
Statistical methodsSample size calculation was based on the sensitivity of measuring salivary CRP level in predicting neonatal sepsis among neonatal ICU cases. Prior data indicated that the sensitivity of salivary CRP in predicting neonatal sepsis ranged from 54% to 94%.7,8,12 A minimum of 24 neonates in each group was needed to reject the null hypothesis with 80% power setting type I error probability to 0.05. Data were described in terms of mean ± standard deviation (± SD), median and range, or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between the study groups was done using one-way analysis of variance (ANOVA) test with posthoc multiple 2-group comparisons. For comparing categorical data, Chi-square (X2) test was performed. Exact test was used instead when the expected frequency is less than 5. Correlation between quantitative variables was done using the Spearman-rho method. Receiver operator characteristic (ROC) analysis was used to define the optimum cut-off value for the studied diagnostic markers. P values less than 0.05 was considered statistically significant. Statistical calculations were performed using computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA) release 22 for Microsoft Windows.
ResultsDemographic features and clinical characteristicsThe current study included 90 full-term admitted neonates. There was no difference between the septic and control groups in regards to gender, weight or postnatal age (p > 0.05 for all) (Table 1). Among the recorded clinical features, poor suckling, impaired Moro reflex, feeding intolerance, abdominal distention and respiratory distress were significantly more frequent among septic as compared to the control group (p < 0.001).
Demographic characteristics of the neonates under study.a.
| Culture proven LOS(n = 30) | Clinical LOS(n = 30) | Control(n = 30) | p valueb | |
|---|---|---|---|---|
| Female/Male | 6/24 | 10/20 | 10/20 | 0.4 |
| Admission weight (kg) | 2.89 ± 0.52 | 3.05 ± 0.56 | 2.95 ± 0.58 | 0.21 |
| Postnatal age (days) | 12.68 ± 6.07 | 12.65 ± 6.48 | 10.27 ± 5.58 | 0.21 |
LOS, late-onset sepsis; n, number.
Positive blood cultures were obtained in 30 neonates. Methicillin-resistant Staphylococcus aureus was the most common isolate in the septic group (45%) followed by Klebsiella spp. (36%), coagulase-negative Staphylococci (10%) and other organisms including Acinetobacter, Pseudomonas and Candida spp. (3% each).
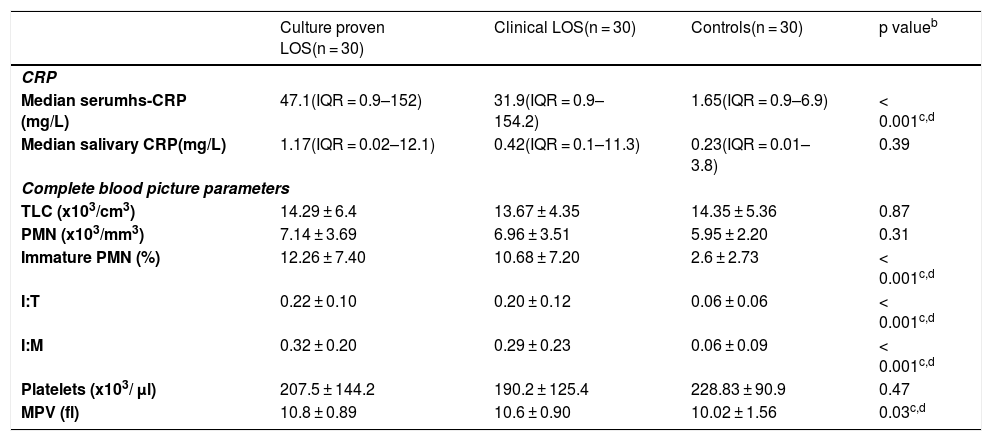
The median serum CRP was significantly higher in septic neonates compared to the aseptic group (p < 0.001) being 31.9 mg/L (IQR = 0.9–154.2) in the clinically septic, and 47.1 mg/L (IQR = 0.9–152) in culture proven septic neonates; while being 1.65 mg/L (IQR = 0.9–6.9) in controls. Though the median salivary CRP was 0.42 mg/L (IQR = 0.1–11.3) in clinically septic and 1.17 mg/L (IQR = 0.02–12.1) in culture proven septic neonates compared to 0.23 mg/L (IQR = 0.01–3.8) in the control group, yet, it didn’t reach statistical significance (p = 0.39). Furthermore, there was a no statistically significant correlation between serum and raw salivary CRP concentrations (r = 0.074, p = 0.49). Levels of serum and salivary CRP are listed in Table 2.
Laboratory parameters of the neonates under study.a.
| Culture proven LOS(n = 30) | Clinical LOS(n = 30) | Controls(n = 30) | p valueb | |
|---|---|---|---|---|
| CRP | ||||
| Median serumhs-CRP (mg/L) | 47.1(IQR = 0.9–152) | 31.9(IQR = 0.9–154.2) | 1.65(IQR = 0.9–6.9) | < 0.001c,d |
| Median salivary CRP(mg/L) | 1.17(IQR = 0.02–12.1) | 0.42(IQR = 0.1–11.3) | 0.23(IQR = 0.01–3.8) | 0.39 |
| Complete blood picture parameters | ||||
| TLC (x103/cm3) | 14.29 ± 6.4 | 13.67 ± 4.35 | 14.35 ± 5.36 | 0.87 |
| PMN (x103/mm3) | 7.14 ± 3.69 | 6.96 ± 3.51 | 5.95 ± 2.20 | 0.31 |
| Immature PMN (%) | 12.26 ± 7.40 | 10.68 ± 7.20 | 2.6 ± 2.73 | < 0.001c,d |
| I:T | 0.22 ± 0.10 | 0.20 ± 0.12 | 0.06 ± 0.06 | < 0.001c,d |
| I:M | 0.32 ± 0.20 | 0.29 ± 0.23 | 0.06 ± 0.09 | < 0.001c,d |
| Platelets (x103/ µl) | 207.5 ± 144.2 | 190.2 ± 125.4 | 228.83 ± 90.9 | 0.47 |
| MPV (fl) | 10.8 ± 0.89 | 10.6 ± 0.90 | 10.02 ± 1.56 | 0.03c,d |
hs-CRP, high sensitivity C-reactive protein; I:M, immature to mature neutrophils, I:T, immature to total neutrophils; IQR, interquartile range; LOS, late-onset sepsis; MPV, mean platelet volume; PMN, polymorphnuclear cells; TLC, total leucocytic count.
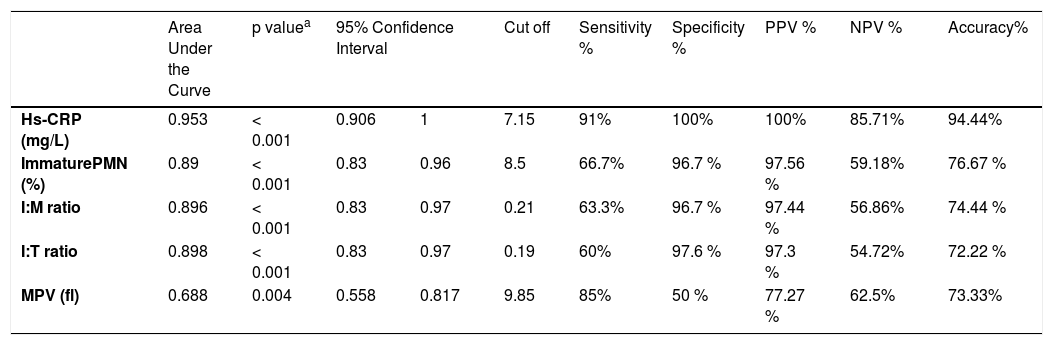
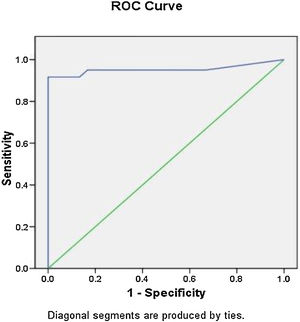
The noted findings in the complete blood picture which were mostly significantly higher in the septic groups that are listed in Table 2. There were no statistically significant differences between culture proven septic and clinical septic groups as regards noted findings in the complete blood picture, serum or salivary CRP. Their optimal cut-off values identified by ROC curves of culture proven and clinical sepsis versus controls, as well as their predictive abilities are presented in Table 3. The optimum cut-off value of serum CRP for LOS diagnosis was 7.2 mg/L with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 91, 100, 100, and 85.7%, respectively (Table 3; Fig. 1).
Validity and predictive outcomes of various laboratory parameters to differentiate between septic and control groups.
| Area Under the Curve | p valuea | 95% Confidence Interval | Cut off | Sensitivity % | Specificity % | PPV % | NPV % | Accuracy% | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Hs-CRP (mg/L) | 0.953 | < 0.001 | 0.906 | 1 | 7.15 | 91% | 100% | 100% | 85.71% | 94.44% |
| ImmaturePMN (%) | 0.89 | < 0.001 | 0.83 | 0.96 | 8.5 | 66.7% | 96.7 % | 97.56 % | 59.18% | 76.67 % |
| I:M ratio | 0.896 | < 0.001 | 0.83 | 0.97 | 0.21 | 63.3% | 96.7 % | 97.44 % | 56.86% | 74.44 % |
| I:T ratio | 0.898 | < 0.001 | 0.83 | 0.97 | 0.19 | 60% | 97.6 % | 97.3 % | 54.72% | 72.22 % |
| MPV (fl) | 0.688 | 0.004 | 0.558 | 0.817 | 9.85 | 85% | 50 % | 77.27 % | 62.5% | 73.33% |
hs-CRP, high sensitivity C-reactive protein; I:M, immature to mature neutrophils; MPV, mean platelet volume; NPV, negative predictive value; PPV, positive predictive value; PMN, polymorphonuclear cells.
Neonatal sepsis (NS) contributes substantially to neonatal morbidity and mortality globally, despite recent health care advances13 reaching an incidence of 17.5% among term neonates in some Arab states in the Gulf region.14 Common clinical manifestations of LOS recorded in this work were poor suckling, impaired Moro reflex, feeding intolerance, abdominal distention and respiratory distress. Similarly, feeding intolerance, fever, hypotonia, neonatal jaundice and abdominal distension were also the most common manifestations of LOS in the work by Li et al.15
As non-specific signs/symptoms make it very challenging to formulate a timely diagnosis, blood culture is still considered the gold standard for diagnosis. The causative organisms vary in different countries.4 In the current study, Gram positive organisms accounted for 55% of culture proven sepsis which is in line with the study by Sorsa13 in which nearly 60% of LOS was caused by Gram + ve bacteria. Our results demonstrate that MRSA was the commonest isolate followed by Klebsiella and CoNS. Notably, a previous report showed that Staphylococci accounted for 59.4% of isolates in LOS followed by Klebsiella (17.5%).16 On the contrary, other investigators found that CoNS was the most common pathogen in LOS.14 A previous Egyptian study in the same hospital reported that gram-ve pathogens comprised 74 % of isolates in LOS, and that 41.9% of cases were caused by Klebsiella and only 6% were caused by MRSA.17 However, their study enrolled neonates of various gestational ages, which might account for the difference between their results and ours. It is of note that the distribution pattern of causative pathogens varies across regions and may even change over time within the same hospital.18
As the early detection of neonates susceptible to have sepsis may augment the therapeutic range and lead to better outcomes, different hematologic parameters and acute phase reactants have been previously explored to determine their utility for early recognition of sepsis.19
Saliva is a potential non-invasive alternative to assess microbial, immunologic, and molecular biomarkers20 with previous reports demonstrating its potential use as a marker of systemic inflammation including LOS,7,8,12 we aimed to investigate the role of salivary CRP as a diagnostic marker in septic neonates. We found no significant difference in salivary CRP levels between the septic and control groups (p = 0.39). As for serum hs-CRP, in the present work, we found significant difference in its levels between the sepsis groups and the control group (p < 0.001). The sensitivity and specificity of serum CRP cut-off level of 7.2 mg/L in diagnosis of LOS were 91 and 100 %, respectively while it had a PPV of 100 % and NPV of 85.7 %. Interestingly, the role of serum CRP in diagnosis of late-onset infection is debatable. Earlier studies demonstrate the usefulness of serum CRP to diagnose LOS.21,22 On the contrary, Brown et al.23 suggested that serum CRP may be insufficiently accurate to aid early diagnosis of LOS or select infants to undergo further investigations or antimicrobial therapy treatment.
In agreement with previous studies, salivary CRP levels in the present work didn’t correlate with serum CRP measurements.9,24 Remarkably, Pay and Shaw10 recently demonstrated that currently salivary CRP poorly reflects systemic inflammation and does not consistently and strongly correlate with serum CRP. It is of note that Iyengar et al.8 suggested that salivary CRP could predict abnormal serum CRP thresholds; nevertheless, their study enrolled all neonates with gestational ages 23–42 weeks who required serial CRP levels as part of their routine care in the NICU. In disagreement with our findings, a study in 2018 7 demonstrated significant differences in salivary CRP between full-term neonates with sepsis and controls. Another study proposed salivary CRP as a diagnostic marker of late-onset neonatal pneumonia.12 The discrepancy between our results and the aforementioned studies may be explained as suggested by Pay and Shaw,10 by changes in the CRP concentration caused by the influence of the oral environment including localized inflammation in the mouth, as well as lack of a standardized method for collecting a consistent salivary sample given the patient-dependent salivary flow rates.
Our results reveal that serum CRP exhibited the highest overall diagnostic accuracy of 94.4% compared to the recorded hematological parameters which had high specificities but low sensitivities except for MPV which had 85% sensitivity but 50% specificity at a cutoff of 9.9 fl. Interestingly, El-Mashad et al.25 proposed a lower diagnostic cut-off value of 7.9 fl, while another study reported a higher cut-off of 10.8 fl.26 On the contrary, other investigators found no significant difference as regards MPV between their study groups.27 These variations might be explained by the different gestational ages of recruited neonates and the timing of onset of sepsis.
In conclusion, our study demonstrated that salivary CRP levels were not significantly different between the 3 study groups nor did they predict abnormal serum CRP thresholds in newborns with sepsis. Serum CRP could accurately discriminate between septic and aseptic full-term neonates with LOS at a cutoff value of 7.2 mg/mL. Further studies that include a larger number of neonates with different gestational ages and weights are warranted to confirm our findings.
Conflicts of interestThe authors declare no conflicts of interest.