Mycoplasma pneumoniae pneumonia (MPP) is a common respiratory infection in children. Tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17), and IL-6 have correlation with Mycoplasma pneumoniae lung infection and MPP pathogenesis.
MethodmiRNAs participate in the pathogenesis of various diseases by regulating the development and differentiation of the immune cell. Blood was collected and total RNA was isolated. miRNA microarrays were performed to identify differentially expressed miRNAs in MPP patients. The levels of relative miRNAs and mRNAs were evaluated by qRT-PCR.
ResultsThere are 23 differentially expressed miRNAs in MPP children's plasma, 15 miRNAs had enhanced expression and 8 had depressed expression. MPP patients showed lower mir-1323 level in blood samples than healthy controls. MPP patients with pleural effusion had much higher Il6 and Il17a mRNA levels than those without pleural effusion. The expression level of Il6 had a negative correlation with miR-1323 level. In the human THP-1 cell line, the level of miR-1323 was significantly reduced through lipopolysaccharides treatment. In THP-1 cells, overexpression or silencing of miR-1323 significantly reduced or promoted Il6 expression.
ConclusionIn conclusion, miR-1323 targets the mRNA of Il6 and inhibits the expression of Il6. The pathogenesis of MPP inhibits the expression of miR-1323 in macrophages, triggers the overexpression of Il6, and enhances inflammation response.
The smallest free-living prokaryote is mycoplasma. In a pool of 16 different kinds of human mycoplasmas, 6 of them can cause disease, and the predominant pathogen is Mycoplasma pneumoniae.1Mycoplasma pneumoniae caused Mycoplasma pneumoniae pneumonia (MPP) is a common respiratory infection in children. According to statistical data, mycoplasma pneumonia accounts for 10%–40% of community acquired pneumonia (CAP) in children.2 The infection rate of Mycoplasma pneumoniae in the youth has elevated, and patients with severe mycoplasma pneumonia and refractory mycoplasma pneumonia have increased.3 It is currently known that Mycoplasma pneumoniae can continuously damage the respiratory epithelium and cilia through the induction of immune response, and the host cannot effectively clear the pathogen.4 Severe MPP cases will give rise to numerous complications and eventually develop into a severe life-threatening pneumonia.5
The human body produces a variety of inflammatory factors by activating immune cells after a Mycoplasma pneumoniae infection.6 The immune response mediates and regulates immune function and inflammatory response. The inflammatory factors accumulate locally and are gradually spread from upper respiratory tract to the lower respiratory tract.7 The clinical manifestations are bronchiolitis, pneumonia, etc.; in severe cases, the brain, heart, liver, and kidneys of the child may be involved, causing complications such as encephalitis, myocarditis, and hepatitis.8 Research showed that tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17) and IL-6 had correlation with Mycoplasma pneumoniae lung infections and MPP pathogenesis.9 However, the specific molecular mechanism of MPP stimulating inflammation is unknown.
In recent years, microRNA (miRNA) has been recognized by researchers as a new class of regulatory gene molecules. miRNA is a kind of evolutionarily conservative endogenous non-coding short chain small RNA. It has been shown that it can participate in the occurrence and development of various diseases. There are few reports of miRNA expression in MPP. miR-1323 is widely reported to participate in the pathogenesis of several different cancers, such as breast cancer, squamous cell carcinoma, and lung cancer.10–12 Based on our microarray analysis result, the expression of miR-1323 was depressed in children with MPP. Thus, this research aimed to investigate mirRNA-1323 expression and possible function in children with MPP.
MethodsPatient characteristicsFrom March 2019 to April 2020, 26 children with MPP were recruited in this research. Exclusion criteria include current immunomodulator and immunosuppressive agent usage, recurrent pneumonia, premature delivery, and immunodeficiency. 9 children in these participants had pleural effusion, and 31 healthy children were recruited as control in the same period. Inclusion criteria of healthy control include no respiratory tract infection, no chronic infectious disease, no immune system disease history, no allergies, and no other immunity-related disease. The healthy controls have also been tested to exclude those with potential MPP infection by PCR on nasal swabs. This research was approved by the ethics committee of Cangzhou Central Hospital and corresponding informed consents were signed by all the participants’ parents or guardians.
Mycoplasma pneumoniae infection was diagnosed by serologic testing and PCR from nasopharyngeal secretion. The clinical and demographic data of participants were collected through questionnaires. Peripheral blood samples were collected for laboratory examination.
THP-1 cell lineThe human THP-1 cell line, derived from the peripheral blood of a 1-year-old male with acute monocytic leukaemia, was purchased from ATCC. THP-1 cells were cultured in RPMI 1640 medium (Thermo Fisher, USA), supplemented with 10% fetal bovine serum (Invitrogen, USA), 100 U/mL penicillin-streptomycin (Sigma-Aldrich, USA), and 2 mM L-glutamine with 5% CO2 at 37 °C. 0.1 μg/mL LPS (Sigma-Aldrich) was employed for stimulating THP-1 cells during the relative period.
Microarray analysis of miRNAs in plasmaPlasma sample (200 μL) was mixed with Trizol (Ambion, USA) (750 μL) to isolate total RNA. Microarray hybridization, data generation and normalization were performed by the Kangchen Biological Engineering Co. Ltd. Human miRNA chip (miRCURY™, Exiqon, Denmark) with 3100 miRNA probes employed. Quantile algorithm was used for normalization. When a false discovery rate of ≤0.05 and p-value <0.05, miRNA was thought to be differentially expressed. If the expression of a miRNA had more than a two-fold difference between MPP children and healthy control, this miRNA was also thought to be differentially expressed.
qRT-PCRTotal RNA of the PBMCs from the children with MPP was isolated by Trizol (Invitrogen, USA). Reverse transcription PCR was performed by the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, USA). qRT-PCR was performed by the TaqMan Universal PCR Master Mix kit (Applied Biosystems). Actb was used as an endogenous control. Primers used in this experiment were the following:
human Il6 F: AGACAGCCACTCACCTCTTCAG
human Il6 R: TTCTGCCAGTGCCTCTTTGCTG
human Il17 F: CCGGACTGTGATGGTCAA
human Il17 R: CTCATTGCGGTGGAGATT
human Tnf F: CTCTTCTGCCTGCTGCACTTTG
human Tnf R: ATGGGCTACAGGCTTGTCACTC
human Il1b F: CCACAGACCTTCCAGGAGAATG
human Il1b R: GTGCAGTTCAGTGATCGTACAGG
human miR-1323 F: AAACTGAGGGGCATTTTC
human miR-1323 R: GAACATGTCTGCGTATCTC
human miR-98-5p F: GAGGTAGTAAGTTGTATTG
human miR-98-5p R: GAACATGTCTGCGTATCTC
human miR-152-3p F: TCAGTGCATGACAGAACT
human miR-152-3p R: GAACATGTCTGCGTATCTC
human Actb F: ACGTTGCTATCCAGGCTGTGCTAT
human Actb R: TTAATGTCACGCACGATTTCCCGC
Statistical analysisSPSS Statistics Version 22.0 software was employed to perform statistical analysis. Values were expressed as n (percentage, %) or mean ± SD. Data were statistically analyzed using two-sided Student's t-test and the Pearson Correlation test. The chi-square test was used for analyzing non-parametric data. p value less than 0.05 was considered to be statistically significant.
ResultsParticipants characteristicsClinical and demographic data in both MPP group and control group were collected and showed in the supplementary Table S1. When compared with children the in control group, those with MPP presented longer duration of fever, enhanced neutrophils number, and elevated lactate dehydrogenase and C-reactive protein levels. The lymphocytes subgroups were also examined and the CD19+ CD23+ cell proportion was dramatically elevated in children with MPP. The concentration of Mycoplasma pneumoniae specific IgG was also increased.
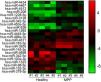
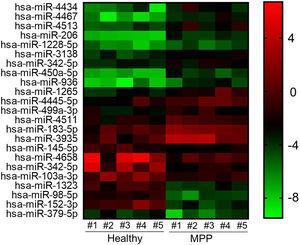
Altered miRNA profiles in the blood of MPP patientsThrough microarray analysis, miRNA expression in MPP patients’ blood samples were profiled and the heat map and cluster analysis are shown in Fig. 1. A total of 23 miRNAs had significantly altered expression in MPP patients, 15 miRNAs had enhanced expression and 8 had depressed expression. Based on the result of microarray analysis, the most down-regulated miRNAs were miR-1323, miR-98-5p, and miR-152-3p.
Total miRNAs profiling from microarray analysis.
Heat map and cluster analysis of miRNA expression. Individual patient samples are shown in columns and miRNAs. Individual patient samples are shown in columns and miRNAs in rows. Of all differentially expressed miRNAs, 15 miRNAs were up-regulated and 8 miRNAs down-regulated.
MMP, mycoplasma pneumoniae pneumonia.
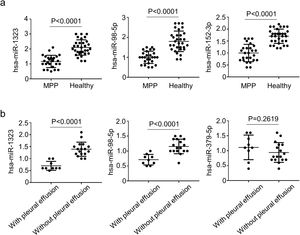
To further confirm the result of microarray analysis, the levels of miR-1323, miR-98-5p, and miR-152-3p were also evaluated through qRT-PCR. As shown in Fig. 2A, when compared with healthy controls, patients with MPP had dramatically lower levels of miR-1323, miR-98-5p, and miR-152-3p in blood samples. Among 26 children with MPP, 9 of them had pleural effusion. Meanwhile, compared with MPP cases lacking pleural effusion, the patients who did have pleural effusion presented lower mir-1323 and mir-98-5p levels; but mir-152-3p has no significant difference (Fig. 2B).
Comparisons of multiple miRNA between MPP cases and healthy controls.
a, Levels of miRNAs in in the PBMCs from the MPP patients and healthy controls; b, Levels of miRNAs in MPP patients with pleura effusion and MPP patients without pleura effusion. Error bars indicate standard error. All qRT-PCR data are presented as the fold induction relative to the Actb mRNA level.
MMP, mycoplasma pneumoniae pneumonia.
According to the Target Scan 7.1 database, Il6 mRNA is one of the direct targets of miR-1323 in the PBMCs (Fig. 3A). As shown in Fig. 3B, an activated inflammation response caused by MPP elevated Il17a and Il6 mRNA levels in MPP patients. Pleural effusion is an indicator and clinical manifestation of severe inflammation. Compared with children without pleural effusion, children with MPP and pleural effusion had much higher mRNA levels of Il6 and Il17a in blood samples (Fig. 3C). Through the Pearson Correlation test, the expression level of Il6 was confirmed to have a significant negative correlation with the level of miR-1323 (Fig. 3D).
Il6 is the targeted gene regulated by miRNA-1323.
a, The binding site of miR-1323 in the Il6 mRNA; b, Expression of Il6 and Il17a in the PBMCs from the children with MPP; c, Expression of Il6 and Il17a in MPP cases with and without pleural effusion; d, Correlation between the concentration of Il6 and miR-1323. All qRT-PCR data are presented as the fold induction relative to the Actb mRNA level.
MMP, mycoplasma pneumoniae pneumonia.
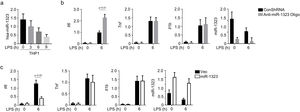
Macrophages play a crucial role in the excessive inflammation caused by pneumonia infection. In this study, LPS stimulation in vitro was used to mimic M. pneumoniae infection, as it is well recognized that membrane lipoproteins are immuno-stimulants exerting as lipopolysaccharides (LPS) and play a crucial role in the pathogenesis of inflammatory responses upon M. pneumoniae infection.13,14 In THP-1 cells, miR-1323 expression was inhibited by the LPS treatment, with the effect of LPS enhanced by increased time of treatment (Fig. 4A). As shown in Fig. 4B, the expression of Il6, Tnf, and Il1b were all enhanced by LPS, but only Il6 expression was elevated by the inhibition of miR-1323 expression (Fig. 4B). A decreased level of miR-1323 was also shown in Fig. 4B. Meanwhile, an enhanced expression of miR-1323 declined the mRNA level of Il6 after LPS treatment but had no influence on Tnf and Il1b expression (Fig. 4C). An elevated level of miR-1323 was also shown in Fig. 4C.
miR-1323 specially regulates Il6 expression in THP-1 cells.
a, Level of miR-1323 in THP-1 cells treated by LPS; b, THP-1 cells were treated by anti-miR-1323 oligo, mRNA levels of Il6, Tnf, Il1b, and miR-1323 were measure by qRT-PCR; c, miR-1323 was overexpressed in THP-1 cells, mRNA levels of Il6, Tnf, Il1b, and miR-1323 were measured by qRT-PCR. All qRT-PCR data are presented as the fold induction relative to the Actb mRNA level.
MMP, mycoplasma pneumoniae pneumonia; LPS, lipopolysaccharide.
It is currently believed that MPP is a combination of a direct pathogen invasion and immune injury. Studies demonstrate that the adhesion and proliferation of Mycoplasma pneumoniae to the host respiratory mucosal epithelial cells is the primary prerequisite for clinical symptoms.15Mycoplasma pneumoniae can firmly adhere and invade the epithelial cells to escape the body's immune mechanism or drug treatment. It can persist in the respiratory tract for several months, making the patient a chronically infected person or an asymptomatic carrier.16
MPP has become a common disease in children. Due to the long course of disease and severe symptoms, it can easily cause a variety of extrapulmonary complications.17 During the pathogenesis of MPP, inflammation mechanisms also play an important role.6 Immune cells and cytokines dominate in immune injury, and the molecular mechanism that causes pathological changes is not very clear and is the focus of current research.
Recently, studies have shown that the miRNA participates in the occurrence and development of diseases by regulating immune cell development and differentiation. In acute lung injury, the expression of multiple miRNAs in the lungs is dynamically regulated, miR-16 is up-regulated and miR-150 is down-regulated.18,19 In mice, overexpression of miR-181a promotes B lymphocyte differentiation and reduces circulating T lymphocytes.20 The transformation of T cells is promoted by miR-181a, miR-146a and miR-146b, leading to a pro-inflammatory response.21 miR-155 participates in regulating T helper cell differentiation and mediating T cells to participate in the immune response.22 In monocytes, miR-155 responds to viruses and bacteria infection and reduces the inflammatory response.23 The let-7 miRNAs inhibit the expression of IL-13 and regulate IL-13 secretion.24 In inflammation caused lung damage, the miR-127 expression is down-regulated, and an enhanced miR-127 expression can inhibit the release of cytokines by macrophages.25
In this study, microarray analysis found that there were differences in the expression of miRNA in the plasma of children with MPP, suggesting that miRNA may participate in the occurrence and development of MPP. There were 23 differentially expressed miRNAs in MPP children's plasma, 15 miRNAs had enhanced expression and 8 had depressed expression. The most down-regulated miRNAs were miR-1323, miR-98-5p, and miR-152-3p.
It is thought that a strong inflammatory reaction in children with severe MPP can trigger a systemic inflammatory reaction and pleural effusion.26 According to relevant reports, the infection rate of severe MPP with pleural effusion is as high as 50%.27 For children with severe MPP and pleural effusion, cephalosporin or penicillin are often used in clinical anti-infective treatment.28 In this research, among 26 children with MPP, 9 of them had pleural effusion. Levels of mir-1323 and mir-98-5p were significantly lower in MPP patients with pleural effusion than those with no pleural effusion.
Research showed that IL-17, IL-6 and TNF-α are indicators commonly used in clinics to reflect inflammation.29 It is widely used in infectious diseases. Altered Il17, Il6, and TNF-α expressions are important for the occurrence of Mycoplasma pneumoniae lung infection and MPP pathogenesis.9 Serum IL-17 is an inflammatory mediator that has a certain effect on the immune response of cells induced by Mycoplasma pneumoniae. IL-17 has played a defensive role in the lung infection of Mycoplasma pneumoniae, which can improve the body's ability to eliminate pathogens and promote neutrophils to aggregate.30 Serum IL-6 is a highly active immuno-regulatory factor that participates in pathological processes of lung inflammation. By detecting the expression of Il17 and Il6 levels in a patient's serum, the patient's condition can be accurately assessed.
Compared with controls, children with MPP had lower mRNA levels of Il6 and Il17a. Compared with children without pleural effusion, children with MPP and pleural effusion had much higher mRNA levels of Il6 and Il17a. According to the Target Scan 7.1 database, Il6 is one of the direct targets of miR-1323. The expression level of Il6 also showed a significant negative correlation with the level of miR-1323.
After Mycoplasma pneumoniae invades the lower respiratory tract, it stimulates epithelial cells and macrophages to release a variety of cytokines such as IL-1β, IL-4, IL-6, IL-8, IL-18, IFN-γ, activates specific and non-specific immune cells, and causes excessive immune inflammation in the pathogenesis of MPP.15 In severe cases, activated macrophages can cause damage to multiple tissues and organs of the body, causing hemophagocytic syndrome. Kurai et al. found that the levels of IL-17, IL-6, TNF-α and IL-4 in the alveolar lavage fluid of Mycoplasma pneumoniae infected mice increased, aggravating the lung inflammation caused by neutrophils.30
Based on these results, we used LPS to stimulate THP-1 cells and explored the expression of miR-1323, Il6 and various cytokines in LPS stimulated THP-1 cells. The expression of miR-1323 in THP-1 cells was inhibited by LPS treatment. After the administration of LPS, the expression of Il6, Tnf, and Il1b were all enhanced in THP-1 cells. However, only the expression of Il6 was inhibited by miR-1323 overexpression and promoted through the inhibition of miR-1323 expression.
Cytokines play an important role in the occurrence and development of MPP, and its in-depth study is conducive in exploring the specific pathogenic mechanism of MPP. In recent years, with the increase in the incidence of MPP and with more and more severe and refractory cases, the study of its pathogenesis is conducive to an early clinical identification and effective treatment.
ConclusionIn conclusion, miR-1323 targets the mRNA of Il6 and inhibits the expression of Il6. The pathogenesis of MPP inhibits the expression of miR-1323 in macrophages, triggers the overexpression if Il6 and enhances inflammation response. It should be noted that blood samples in the current study were not collected at any particular phase or stage of MPP, and it would be important to investigate the temporal expression profile of these miRNAs at different phases of the disease in the future. Also, the sample size is relatively small in this study, and more samples could be analyzed to verify the findings.
FundingThe study was supported by the Cangzhou Science and Technology Research and Development Program (cz151302039).
Conflict of interestThe authors declare no conflicts of interest.